-
Life on Earth, ranging from single-celled organisms to more complex mammals, has evolved an internal circadian clock system that allows living organisms to coordinate their physiological processes and biological activities with the environmental day-night cycle[1]. Therefore, the circadian clock system plays a critical role in maintaining internal physiological functions such as the immunity, metabolism, and mental health of mammals[2]. However, there is increasing evidence that circadian rhythm disorders can lead to a series of related pathophysiological consequences for mammals[3-5].
Stress refers to the method of protecting organisms from any kind of stressors (including traumatic events, challenges, or demands), and is typically divided into two categories: acute stress and chronic stress. Acute stress can occur in an organism due to exposure to a traumatic event for a short period of time. Acute stress may induce a severe emotional response, but it is highly manageable within the individual. In contrast, chronic stress is defined as the response of an organism to a challenge for an extended period of time, which may cause serious medical consequences, and particularly contributes to nutritional imbalance, immunological diseases, and depression. In general, stress exposure can lead to physiological changes that involve immediate activation of the sympathetic-adrenomedullary system and delayed activation of the hypothalamic-pituitary-adrenal (HPA) axis[6]. The survival of the organism requires continuous adaption to various challenges, and the secretion of glucocorticoids (GCs) by the adrenal gland plays an important role in stress adaption[7]. Briefly, GCs facilitate the immediate response of catecholamines after the sympathetic-adrenomedullary system is stimulated, which prepares the organism for various challenges, such as by the increasing blood pressure, heart rate, and glucose levels. Moreover, GCs participate in energy storage to optimize stress adaption via promoting lipolysis and hepatic glycogenolysis. However, an excessive level of GCs can cause many side effects, such as metabolic, immune, and mental disorders[8]. Undoubtedly, regulation of the HPA axis is essential for preventing the complications of chronic stress.
It has been verified that light can stimulate the secretion of GCs by the adrenal gland, which is independently associated with activation of the HPA axis[9, 12]. Additionally, light-induced GCs can influence circadian gene expression in the peripheral tissues, suggesting that there is extensive crosstalk between GCs and the circadian clock[10, 11].
In this paper, we review current studies on the interaction of the circadian clock and chronic stress. Finally, we critically discuss the consequences of this interaction on the HPA axis, immunity, metabolism, and mental disorders.
A comprehensive search of the MEDLINE/PubMED database was conducted in January to March 2018 to identify related papers published in English. In electronic databases, we used the following key word combinations: 'circadian clock' AND 'chronic stress', 'circadian clock' AND 'HPA axis', 'HPA axis' AND 'chronic stress', 'chronic stress' AND 'immunity', 'circadian clock' AND 'immunity', 'chronic stress' AND 'metabolism', 'circadian clock' AND 'metabolism', 'chronic stress' AND 'mental disorders', 'circadian clock' AND 'mental disorders'. To propose a successful review for recent advancements, the publication date of the articles ranged from 2000 to 2018.
Primary studies were included in the review to demonstrate the latest work, and both narrative reviews and meta-analyses were selected for full-text reading. In addition, genetic studies were included to explain the association of related diseases and the HPA axis with core clock genes. We used the reference management software, EndNote, to perform sorting and classification of related papers.
-
The term zeitgebers refers to external time cues, such as light and food, that synchronize the internal clocks of organisms to the day-night cycle on Earth. However, the endogenous circadian rhythm is still present even in the absence of zeitgebers, free-running rhythms that last much longer, but they have a period of approximately 24 h[12]. The circadian clock system receives external zeitgebers and adjusts the intrinsic circadian rhythm to correspond to environmental changes via phase resetting effects[1, 2]. The circadian system of an organism is mainly composed of the master pacemaker in the hypothalamic suprachiasmatic nucleus (SCN) and subordinate clocks in the peripheral tissues. The SCN is a tiny pacemaker neuron region of the anterior hypothalamus, and is situated directly above the optic chiasm[13]. It is essential for coordinating the circadian rhythm of physiological processes and biological behavior, including hormone levels, athletic performance, metabolism, and immune function, throughout the entire body. The dominant Zeitgeber for the master pacemaker of the SCN is the light/dark cycle. SCN neurons receive direct photic input from retinal ganglion cells, and the pineal gland then delays the transmission of temporal information to the peripheral tissue in the form of melatonin secretion driven by the SCN. In mammals, the final part of the neuronal route from the SCN to the pineal gland is the β1 adrenergic receptor, and the main neurotransmitter regulating melatonin production is norepinephrine (NE). Therefore, the SCN stimulates melatonin production via the release of NE for the duration of darkness. Upon exposure to light, melatonin production is suppressed due to lack of NE stimulation[14]. The subordinate clocks in the peripheral tissues receive temporal information from the master pacemaker via the melatonin rhythm, which in turn coordinates the circadian rhythm of the effective physiological system, such as the immune system, blood pressure, bone metabolism, and many others[15, 16]. With the influence of other zeitgebers, the subordinate clocks in the peripheral tissues are regulated by the master pacemaker via a variety of autonomic signals and humoral factors to anticipate the environmental day-night cycle and prevent decreased expression of the subordinate clock gene in the peripheral tissues. In the presence of food cues, several subordinate clocks can be synchronized independently of the master pacemaker[17]. However, the regulatory mechanism of the master pacemaker for subordinate clocks remains unknown[18].
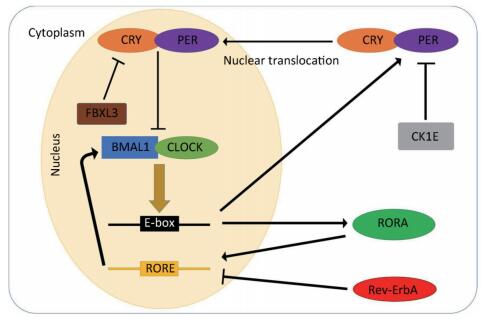
In general, the mammalian circadian system consists of a central oscillator with a period of approximately 24 h, input pathways conveying external time cues to the central oscillator, and output pathways synchronizing the circadian oscillators present in almost all cells of the body with environmental changes[19]. The molecular mechanism of the circadian clock is currently described by transcriptional-translational feedback loops (TTLs)[20]. The major TTL is composed of the circadian locomotor output cycle genes kaput (Clock), Casein kinase-1 δ/ε (CK1δ/ε), Cryptochrome (Cry1/2), and Period (Per1-3) as well as the brain and muscle arnt-like (Bmal1) genes. Briefly, two transcription factors, CLOCK and BMAL1, form the heterodimers that bind to promoter element E-boxes to enhance transcription of the Per and Cry genes. As their transcripts are translated, PER and CRY proteins slowly accumulate in the cytoplasm throughout the day. After reaching a certain concentration, they interfere with each other and form complexes, which in turn relocate to the nucleus to suppress the activity of the CLOCK/BMAL1 heterodimers. However, the accumulated PER protein in the cytoplasm can be phosphorylated by CK1E; this phosphorylation results in their ubiquitination and degradation, which is accelerated by CK1δ. Similarly, F-box/LRR-repeat protein 3 (FBXL3) is one component of the E3 ubiquitin ligase complex, which mediates ubiquitination and subsequent degradation of CRY1 and CRY2 proteins in the nucleus. As PER and CRY proteins are degraded, the repression of the CLOCK/BMAL1 heterodimers disappears and the next cycle begins[21, 23].
Another type of TTL is the accessory feedback loop, which is involved in the fine tuning of the major loop. This feedback loop consists of reverse erythroblastoma (Rev-Erb-α) and the retinoic acid receptor-related orphan receptor (RORα). CLOCK/BMAL1 heterodimers can also drive the transcription of Rev-Erb-α and RORα. Rev-ErbA proteins are negative regulators of Bmal1 transcription, while RORA proteins play a critical role in enhancing the transcription of Bmal1. Both are essential for the oscillation of BMAL1 proteins[22]. The molecular pathways of the transcription and translation feedback loops are presented in Figure 1.
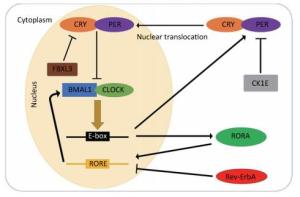
Figure 1. Schematic of the transcriptional-translational feedback loops. The transcriptional-translational feedback loops constitute the molecular circadian clock. In the major loop, CLOCK and BMAL1 form the heterodimers that bind to promoter element E-boxes to enhance the transcription of the Per and Cry genes. Excessive CRY/PER protein complexes can inhibit CLOCK/BMAL1-mediated transcription in the nucleus and CK1E, leading to the degradation of PER proteins. In the accessory feedback loop, CLOCK/BMAL1 heterodimers promote the transcription of Rev-Erb-α and RORα. Rev-ErbA proteins suppress the transcription of bmal1, while RORA proteins enhance it.
In mammals, the formation of the biological circadian rhythm depends on the joint action of clock genes and transcription factors. Our study demonstrated that core clock genes are rhythmically expressed not only in the SCN, but also in several central and peripheral tissues[23]. In vitro studies of peripheral tissues have also demonstrated that almost all tissues express the core clock genes[24, 25]. Subordinate clocks play a crucial role in individual peripheral tissues, as they drive the rhythmic expression of specific genes involved in many physiological functions[26]. Several studies have revealed that the subordinate clocks in peripheral tissues are not SCN-independent and require signal output from the SCN to become entrained to the rhythmicity[27, 28].
HPA AXIS-Mediated Regulation of Chronic Stress Prolonged external or internal challenges that continually disrupt the homeostasis of organisms cause chronic stress. Exposure to chronic stress yields a wide variety of adverse medical outcomes, such as metabolic disorders, suppression of the immune system, and deterioration of mental health. This phenomenon apparently has the potential to induce damage across the entire lifespan[30]. However, there are numerous defensive pathways driven by the HPA axis that prevent organisms from experiencing chronic stress throughout their life.
The HPA axis is a neuroendocrine system that mainly consists of the hypothalamus, pituitary gland, and adrenal gland. It participates in the regulation of neuroendocrine responses to stress stimuli. In the presence of chronic stress, the brainstem and limbic forebrain receive related stress signals from the sensory system across the entire body and relay these signals to the paraventricular nucleus (PVN) of the hypothalamus to stimulate corticotrophin- releasing hormone (CRH) and arginine vasopressin (AVP) secretion. These signals are essential for activation of the HPA axis[29-31]. CRH is the major regulator and AVP strengthens the positive stimulatory effect of CRH on the secretion of adrenocorticotrophic hormone (ACTH). Both CRH and AVP are transported to the related receptors in the posterior pituitary corticotrophs via the hypophyseal portal system to promote the secretion of ACTH[33]. ACTH is then transported by the blood to bind to the melanocortin type-2 receptors (MC2Rs) in the zona fasciculata of the adrenal cortex in the adrenal gland, where the secretion of GCs is stimulated. GCs are characterized by negative feedback that inhibits the secretion of CRH and ACTH[32]. GCs mediate their biological effects via mineralocorticoid receptors (MRs) or glucocorticoid receptors (GRs), which are widely expressed in almost all cells in the body with the exception of the SCN[33]. Briefly, the intracellular glucocorticoid receptor (iGR) is a ligand-dependent transcription factor. After a specific ligand binds to iGRs, the activated iGRs bind to glucocorticoid response elements in the nucleus to regulate the transcription of target genes[35, 36].
In mammals, acute stress-induced activation of the HPA axis plays an important role in resisting destructive stimuli. Briefly, high levels of GCs lead to an increased availability of glucose, a stable lysosomal membrane, and suppressed activation of neutrophils to facilitate the 'fight or flight' response[34]. Experiments in mammals have demonstrated that even minimally destructive stimuli can cause the death of mammals without a bilateral adrenal gland, while mammals with the adrenal cortex of an adrenal gland can survive for a long time[8]. For instance, previous studies have demonstrated that adrenalectomized mice exhibit an enhanced susceptibility to organ injury, endotoxemia, and even sleep deprivation[37-39]. Currently, stress-induced death in patients with cortisol replacement remains a problem. This evidence supports the notion that GCs are necessary for stress resistance. In addition, some hormones, including catecholamines, maintain homeostasis of mammals only in the presence of GCs, the so-called permissive action of GCs.
In addition to the activation of the HPA axis, the ultimate biological effect is commonly associated with GC sensitivity. GC sensitivity can reflect the tissue responsiveness to GCs, which is determined by multiple factors including the expression and affinity of GRs. On exposure to chronic stress, persistently elevated circulating GCs can result in reduced GC sensitivity or even resistance. GC resistance refers to the inability of the tissue to respond to the limited GC response, consequently resulting in excess secretion of GCs via constant activation of the HPA axis to maintain the balance between the biological effect and secretion[40]. It is well known that GCs are highly effective in inhibiting inflammatory responses and immune disorders. Therefore, GC resistance aggravates the chronic inflammatory states of chronic stress-related diseases including rheumatoid arthritis (RA), allergy diseases, obesity, diabetes mellitus, and depression[41]. The GC resistance linking to each disease will be fully described later in this review. In the following, we will discuss the interaction of GCs and the circadian clock in mammals.
-
It is well known that the secretory rates of GCs are high in the early morning but low at midnight. This biological behavior results from cyclical fluctuations in the signals from the master pacemaker in the SCN that stimulates GC secretion. Moreover, the activation of iGRs is accompanied by the peak level of GCs[42]. These phenomena indicate that there is a relationship between iGR activation and the circadian rhythm. In contrast, MRs can be constantly activated by GCs even at low circulation levels due to the higher affinity. This comparison means the circadian rhythm of GCs is predominantly mediated by GRs. Studies in humans have demonstrated that GCs can influence the output signals of the master pacemaker by markedly inhibiting the expression of the core clock gene of the master pacemaker in the SCN and subordinate clocks in the peripheral tissues[43]. For example, Balsalobre et al. have confirmed that injection of dexamethasone as a GC agonist at different times induces a phase shift of the subordinate clocks in mice, such that GC signaling can modify the internal circadian clock network. In addition, dynamic feeding times uncoupled the subordinate clocks from the master clock in wild-type mice, while this phenomenon disappeared in dexamethasone- injected mice[44]. In summary, GCs facilitate the coupling of the subordinate clocks and master clock and stabilize the rhythm of the subordinate clocks against external perturbation.
A circadian oscillation with a peak in the GC circadian rhythm before the active phase is displayed during the day in humans and, conversely, during the night in rodents[45]. Furthermore, this rhythm overlays a stronger ultradian pattern for the secretion of GCs and ACTH driven by a negative feedback loop. All components of the HPA axis, including CRH, ACTH, and GCs, can detect the appearance of a circadian oscillation[47]. Therefore, the secretion of GCs is typically episodic rather than smoothly continuous, and is regulated by the SCN via the HPA axis or alternative pathways. Briefly, the SCN activates the ANS to alter the ACTH sensitivity of the zona fasciculata, which coordinates the secretion of GCs with the environmental day-night cycle. In addition, the adrenal peripheral clock participates in the ACTH signal pathway and subsequent GC secretion via circadian expression of the related core clock genes[46]. The core clock genes Per, Bmal1, and Cry play a critical role in maintaining the clock rhythm in the negative feedback loop. This notion is supported by the evidence of an abnormal circadian rhythm in the biological behavior of mice deficient of Bmal1 and Cry. Time-independent and low ACTH sensitivity occurred in the Bmal1 knockout mice but not in the wild-type mice throughout the day. It is well established that GR sensitivity is necessary for negative feedback of the HPA axis. However, CRY1 and CRY2 predominantly occupy the ligand binding sites of the GR during its peak at night in order to inhibit GC activation. As a result of this specificity, Cry-knockout mice are characterized by impaired negative feedback and constantly elevated levels of GCs[48]. In addition, the circadian rhythms of Per1 knockout mice cannot be induced by light, and the accuracy and stability of the circadian rhythm of GC secretion are significantly suppressed. Genetic deletion of both Per1 and Per2 leads to the disappearance of the circadian rhythm of GC secretion[49]. In summary, the integrity of the circadian clock is necessary for the circadian rhythm of GC secretion.
The literature review on the core genes and GC secretion is presented in Table 1.
Studies Participants Measure Results Gómez-Abellán et al.[43] Human adipose tissue treated with dexamethasone (n = 6) and control adipose tissue (n = 6) Rhythm calculation and analysis of gene expression Circadian pattern of Clock, Bmal1, and Per2 expression is altered by dexamethasone exposure Balsalobre et al.[44] Dexamethasone-phosphate injected and wild-type mice Expression level of Per1 Dexamethasone led to phase shifts in circadian gene expression in the peripheral tissue, aside from the SCN Lamis et al.[48] Cry-deficient and wild-type mice Corticosterone and ACTH measurements Genetic deletion of Cry resulted in reduced suppression of the HPA axis Yang et al.[49] mPer2−/− mice (n = 4) and wild-type mice (n = 4) Locomotor activity and corticosterone measurements Abnormal circadian rhythm of activity and corticosterone production in mPer2−/− mice Note. SCN: suprachiasmatic nucleus, ACTH: adrenocorticotrophic hormone, HPA: hypothalamic-pituitary-adrenal. Table 1. Summary of Studies on Clock Genes and Glucocorticoid Secretion
-
In mammals, the immune system may be largely influenced by stress. Acute stress strengthens the ability of the immune system to protect the body from pathogens by stimulating immunological cells. In contrast, chronic stress participates in the inhibition of immune system via activation of the HPA axis. Therefore, there is a strong connection between immunity and the HPA axis, which involves the process of maintaining homeostasis of the internal environment. It is well established that an immune challenge can stimulate activation of the HPA axis via multiple pathways[50]. When an immune insult occurs in the body, the rapid production of numerous inflammatory mediators, including tumor necrosis factor-alpha (TNF-α), interleukins (IL-1/2/6), and interferons (IFN-α/β/γ), may trigger activation of the HPA axis[51, 52]. Briefly, inflammatory mediators are transported by the peripheral circulation system to regulate neuronal circuits that project to the PVN via an impaired blood-brain barrier in order to stimulate the release of CRH[53].
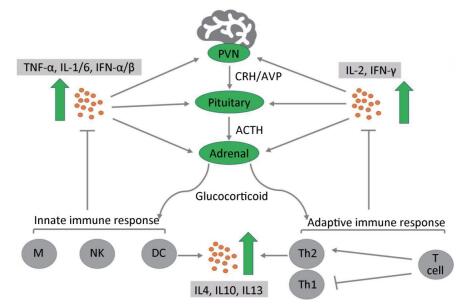
During activation of the HPA axis, high levels of GCs are released into the body to exert anti-inflammatory and immunosuppressive effects. GCs can block the progression of inflammation and are responsible for rapid resolution of the inflammation[54]. During the early stage of inflammation, GCs stabilize the lysosomal membrane, decrease the permeability of capillaries, and decrease the migration of white blood cells into the inflamed area. When inflammation has been well established, GCs enhance the rate of healing. Despite the phase of inflammation, the primary anti-inflammatory mechanism suppresses the synthesis of inflammatory mediators and enhances the anti-inflammatory cytokines of multiple types of immune cells, including both T lymphocytes and B cells; this process is termed immunosuppression[55]. When GCs adhere to immune cells, they suppress the expression of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB cells), which is an important regulator involved in the release of proinflammatory mediators that promote the immune response[56]. Therefore, the level of proinflammatory cytokines that stimulate the HPA axis is markedly decreased[58]. Among them, the most important mediator is IL-2, which promotes the proliferation of T helper cell type 1 (Th1), which is essential for cell-mediated immunity. In addition, GCs regulate the adaptive immune responses by inducing a shift in T helper (Th) cell differentiation toward a predominance of T helper cell type 2 (Th2) (Figure 2). This biological behavior of GCs inhibits the release of proinflammatory cytokines and increases the synthesis of anti-inflammatory cytokines, including IL-4, IL-10, and IL-13. Regardless of the precise mechanism by which the anti-inflammatory and immunosuppressive effects occur, GCs play a crucial role in combating certain types of disease, such as graft-versus-host disease, RA, and allergy diseases[57]. These diseases are characterized by chronic inflammation and a severe immune response. However, prolonged use of GCs may not treat these diseases, but instead exacerbate the chronic inflammatory states. For instance, RA patients exhibit a reduced expression of GR and increased level of inflammatory cytokines such as TNF-α, thus causing GC resistance[59, 60].
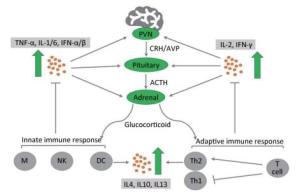
Figure 2. Cross-interaction between the hypothalamic-pituitary-adrenal axis and the immune system. Immune cells release proinflammatory cytokines (TNF-α, IL-1/2/6, IFN-α/β/γ) via the innate immune response and adaptive immune response, which activate three levels of the hypothalamic-pituitary-adrenal axis to secrete glucocorticoids (GCs). Conversely, GCs bind to specific GC receptors on immune cells to suppress the release of proinflammatory cytokines and enhance a shift in T helper (Th) cell differentiation toward a predominance of T helper (Th2) cells. This biological behavior stimulates the production of anti-inflammatory cytokines (IL-4, IL-10, IL-13) to prevent damage due to serious inflammatory reactions[49]. ACTH, adrenocorticotropic hormone; AVP, arginine vasopressin; CRH, corticotropin-releasing factor; PVN, paraventricular nucleus of the hypothalamus; M, macrophage; NK, natural killer cells; DC, dendritic cells.
There is considerable evidence in the literature indicating that the circadian clock is essential for regulation of the immune system and allows the organism to adapt to daily environment-induced changes in the immune response. The major regulatory mechanism of the circadian clock with respect to the immune system is influenced by the function of immune cells[61]. The secretion of proinflammatory cytokines by macrophages follows a circadian rhythm, whereas this circadian secretion was disrupted in core clock gene (Bmal1, Cry, Rev-Erb-α) knockout mice[64]. Several studies have demonstrated that both BMAL1/CLOCK and Rev-ErbA proteins enhance the migration of macrophages to infectious and inflammatory sites by promoting or inhibiting the expression of chemokine ligand 2 (CCL2)[62, 63, 65]. In addition, CLOCK proteins regulate the activity of NF-κB, which is supported by the evidence that inhibition of human CLOCK proteins deactivates the NF-κB signal pathway[66, 68]. However, subjects who have experienced long-term sleep deprivation have been reported to possess a larger number of immune cells (monocytes, NK cells, and lymphocytes) at night and higher levels of proinflammatory cytokines (IL-6 and TNF-α) during the day than healthy individuals[67]. The above studies revealed that the immune responses in organisms are characterized by the circadian rhythm, and, thus, disruption of the circadian clock is associated with immune disorders and chronic inflammatory diseases. In turn, the severity of the immune response also influences the circadian rhythm. It has been reported that the circadian rhythm of mice was altered in response to lipopolysaccharide treatment[69]. For instance, expression of the Per2 gene was suppressed in the livers of rats with turpentine oil-induced inflammation[70].
The immune system protects organisms from diseases via recognition of foreign pathogens and induces immune resistance; therefore, it can lead to an immune disease when the immune balance is off or the immune system is compromised[71]. The circadian clock plays a crucial role in the homeostatic maintenance of the immune system; thus, there is a cross-interaction between circadian clock disorders and immune diseases, particularly for RA and allergy diseases. RA is a long-term autoimmune disorder of unknown etiology, which typically results in nonspecific inflammation of the peripheral joints[72]. The symptoms of RA, namely, pain and stiffness, follow a circadian rhythm and are more severe in the early morning than at other times of the day. In addition, the level of the proinflammatory cytokine IL6 has been identified as strongly rhythmic in patients with RA, with peak values in the morning. Studies have demonstrated that the circadian rhythm of IL-6 may explain the specific diurnal variation of RA symptoms, along with the inadequate circadian secretion of cortisol[73]. Interestingly, the circadian expression of Per2 in synovial cells in RA patients changed significantly, with high levels expressed in the morning, whereas healthy individual exhibited peak values at night[74]. It is believed that the inflammation associated with RA cross-interacts with the circadian clock. The circadian clock controls the pathological rhythms by regulating the expression of inflammatory mediators, which play a critical role in the inflammation of RA. This regulation is supported by evidence indicating that both the number of activated T lymphocytes and the release of TNF-α are increased in Cry1/2 knockout mice[75]. However, related inflammatory mediators can also provide feedback on the expression of core clock genes. Recent studies have revealed that TNF-α can upregulate the expression of Bmal1 and Cry1, while downregulating the expression of Per2[76]. Several allergic diseases, including allergic rhinitis, exhibit circadian rhythm variations. Nakamura et al. found that Clock mutations in mast cells lead to circadian variation in immunoglobulin E-mediated allergic reactions[77].
The literature review on core genes and immunity is presented in Table 2.
Studies Participants Measure Results Sato et al.[62] Murine macrophage cell line RAW264 Chemotaxis assay of migratory activity and relative expression of the inflammatory molecular element, CCL2 Rev-Erb-α inhibits the inflammatory infiltration of macrophages by suppressing CCL2 expression Tang et al.[68] HUVECs Western blot analysis of hCLOCK knockdown HUVECs Inhibition of hCLOCK deactivates the NF-κB signal pathway Susan et al.[70] Rat treated with TURP (n = 4) and healthy controls (n = 4) Relative expression of Per2 in the liver TURP suppress the expression of peripheral clock gene Per2 Yoshida et al.[74] RA patient synovial cells Relative expression of Per2 TNF-α alters the Per2 circadian rhythm by suppressing the expression of Per2. Hashiramoto et al.[75] Cry1/2 knockdown mice (n = 12) and wild-type mice (n = 12) Relative expression of TNF-α The expression of TNF-α is under control of Cry in RA Nakamura et al.[76] Wild-type and Clock-mutated BMCMCs Quantitative analysis of IgE/mast cell-mediated allergic reactions Clock mutations in mast cells lead to circadian variation in IgE-mediated allergic reactions Note. HUVECS: Human Umbilical Vein Endothelial Cells, BMCMCs: bone marrow-derived cultured mast cells, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells, TURP: turpentine oil, TNF-α: tumor necrosis factor α, RA: rheumatoid arthritis, IgE: immunoglobulin E. Table 2. Summary for Studies Included for Clock Genes and Immunity
-
It has been previous described that acute stress can induce immediate emotional response, and thus cause anorexia in humans. In contrast, studies in mammals strongly support the notion of chronic stress having non-negligible undesirable effects in humans consuming constant high calorie food, and one well-documented consequence is that hyperphagia may contribute to the development of obesity and diabetes mellitus[78]. The main regulatory mechanism is the HPA axis, which regulates appetite in humans and animals during stress. It has been demonstrated that stress causes anorexia if high calorie food is not available, while inducing hyperphagia if high calorie food is available[79]. Under normal physiological conditions, GCs participate in the stimulation of gluconeogenesis in the liver, where there is a marked increase in glycogen storage. In addition, GCs also reduce the rate of glucose utilization in the peripheral tissues[80]. Both increased rates of gluconeogenesis and decreased rates of glucose utilization result in elevated blood glucose levels and stimulate insulin secretion. On exposure to stress, excessive GCs are released throughout the body and alter homeostasis, resulting in multiple metabolic diseases. Acute stress causes a temporary elevation in blood glucose levels in order to suppress the appetite, which leads to weight loss in humans who do not consume constant high calorie food. In contrast, chronic stress enhancement of the appetite in humans to achieve prolonged high calorie food intake may lead to hyperglycemia[79]. Moreover, it has been reported that an increased level of plasma insulin is ineffective in maintaining blood glucose levels under GC resistance-induced high levels of circulating GCs due to the reduced sensitivity of many tissues, and so-called insulin resistance is responsible for diabetes mellitus[81]. Reagan et al. confirmed that diabetes may also serve as a metabolic model of chronic stress[82]. This research further demonstrated the view that there is a close relationship between chronic stress and the metabolic pathway of glucose. Another physiological effect of GCs on adipose tissue involves the mobilization of fatty acids via lipolysis. Chronic stress induces excessive GC secretion and hyperphagia, thereby leading to an increase in the concentration of free fatty acids in the plasma and excess deposition of fat in the chest and head regions of the body, which may cause obesity. This is supported by evidence from obese rats with elevated plasma GC concentrations compared to healthy rats[83]. Additionally, GC resistance also occurs in obese patients via reduced expression of GRs in adipose tissue compared to that in skeletal muscle[84].
It is likely that the circadian clock of organisms is involved in most metabolic processes during periods of chronic stress. The circadian system is essential for maintaining the balance of glucose metabolism at multiple levels[85]. Bmal1 knockout mice are characterized by impaired insulin secretion and insulin resistance during the fasting phase. Once supplanted with the exogenous Bmal1 gene, the dysregulated insulin action is restored to normal[86]. Both the PER and CRY proteins are negative feedback elements of the circadian system, and CRY regulates fasting glucose levels via the induction of gluconeogenesis[87]. Stamenkovic et al. found that the expression of Per2/3 and Cry circadian genes was reduced in diabetes mellitus patients[88]. The clock gene, Rev-Erb-α, regulates islet β cell function. In the impaired glucose-induced insulin secretion of the murine model, the expression of REV-ERBα was significantly reduced, resulting in suppressed proliferation of β cells. A high fat diet influences the expression level of Rev-ErbA and disrupts the diurnal secretion of insulin. This experiment indicated that Rev-ErbA can facilitate the adaption of β-cells to different challenges[89]. Furthermore, the core clock genes, Bmal1 and clock, modulate the expression of liver enzymes, thereby affecting the homeostasis of glucose and lipid metabolism. Mice with a liver-specific genetic deletion of Bmal1 tended to appear hypoglycemic in the fasting phase[90]. In type Ⅱ diabetes mellitus and hyperlipidemic mouse models, BMAL1 and CLOCK may directly participate in the regulation of phosphoryl carboxykinase activity in the liver, thus yielding circadian rhythmic changes[91]. Ubiquitin-specific protein is activated by 11β-hydroxysteroid dehydrogenase 1 to regulate liver gluconeogenesis. The activation of the liver peroxisome proliferator-activated receptor (PPARγ) in aromatics receptor-mediated insulin resistance is an important pathway[92]. Studies have indicated that the PPARγ is also involved in the association between the circadian system and lipid metabolism. Briefly, the PPAR-α binds to the Bmal1 promoter to improve its expression, while PPARγ function is regulated by PER2[93, 94].
The literature review on core genes and metabolism is presented in Table 3.
Studies Participants Measure Results Sadacca et al.[86] Wild-type mice (n = 9) and Bmal1 knockout mice (n = 9) Glucose and insulin tolerance tests, measurement of glucose-stimulated insulin secretion Severe glucose intolerance and defective insulin production occur in the Bmal1 knockout mice Stamenkovic et al.[88] Islets from type Ⅱ diabetes mellitus patients (n = 9) and healthy Individuals (n = 50) Expression analysis of the core clock genes Per2, Per3, and Cry2 Type Ⅱ diabetes mellitus led to reduced expression level of the clock genes Per2, Per3, and Cry2 Vieira et al.[89] Mice were fed with a chow diet (n = 4) and a high fat diet (n = 4) Relative expression of Rev-Erb-α in pancreatic -cells Leptin up-regulates Rev-Erb-α expression whereas a high fat diet disrupts Rev-Erb-α expression Grimaldi et al.[94] Per2 knockout and wild-type mice Lipidomic analyses of mice, relative expression of PPAR-α in mouse embryo fibroblasts Per2 alters lipid metabolism by suppressing PPARγ to inhibit adipogenesis Note. PPARγ: peroxisome proliferator-activated receptor. Table 3. Summary of Studies on Clock Genes and Metabolism
-
Both stress and the circadian rhythm play a critical role in daily alterations of the light-dark cycle, lifecycle, and emergence of psychological diseases, namely, major depressive disorder (MDD), posttraumatic stress disorder (PTSD), schizophrenia (SCZ), anxiety disorder, and attention deficit hyperactivity disorder (ADHD)[95]. The HPA axis is not only stimulated in response to stress, but also in response to negative feedback regulated by limbic structures including the amygdala and hippocampus. Both the amygdala and the hippocampus express GRs; thus, GC feedback acts on these regions to participate in the inhibition of stress responses, formation of memories, or even structural changes. Acute stress, such as a traumatic event, promotes memory formation accompanied by high circulating GC levels. For chronic stress, the prolonged increase in GCs ultimately led to neuronal atrophy and impaired memory[96]. An important experimental study has demonstrated that a disrupted circadian rhythm and delayed sleep time can have significant impact on mood, cognitive function, and behavior[97].
MDD is characterized by the presence of depression that persists for at least 2 weeks. Depressed patients exhibit a disrupted circadian rhythm and GC resistance due to impaired HPA axis feedback, which often lead to abnormalities in related brain regions (the amygdala and hippocampus)[98]. Whether a disrupted circadian rhythm leads to depression, or whether depression can cause a disruption in the circadian rhythm, remains unknown[99]. The symptoms of depression include a disrupted circadian rhythm and early morning awakening, which suggests an alteration in the time phase of the circadian system pacemaker[100]. It is common that circadian rhythm disorders are accompanied by depression, but the etiology remains unclear. Bahk et al. confirmed that the circadian rhythm closely interacts with suicide in depressed patients. Significantly more suicidal thoughts are reported by evening-type depressed patients than by morning-type patients[102]. In addition, depressed patients expressed markedly higher mRNA levels of Bmal1, Clock, and Per1 than did non-depressed people[101]. Recent neuroendocrinology findings suggested that abnormalities followed the onset of depression and that patients manifested abnormal secretion of melatonin[103]. Furthermore, the literature also indicates that there is no significant difference in the level of plasma melatonin in depression patients without circadian rhythm disorders compared to those of normal people, while depressed patients with circadian rhythm disorders express a significantly higher level of plasma melatonin than do normal people[104]. These results demonstrate that a reduced level of plasma melatonin is the physiological basis for depression in patients with an altered circadian rhythm.
Experiencing a traumatic event or chronic exposure to abuse may facilitate the development of long-term mental disorders, namely, PTSD. Previous research has demonstrated a relationship between hippocampal atrophy and PTSD[106]. Briefly, the hippocampus is a rich region of GRs that are sensitive to the damaging effects of secreted GCs in the presence of circadian rhythm alterations and chronic stress[105]. Therefore, there is an interaction between the circadian rhythm and PTSD. It has been reported that irregular rhythmicity of the HPA axis and the autonomic and immune systems results in circadian disruption in PTSD[107]. Circadian disruption in PTSD was also supported by evidence in rats exposed to chronic stressors, which led to rhythm dysregulation of core body temperatures[108]. Interestingly, genome-wide association studies (GWASs) have identified a correlation between PTSD and the core clock gene, RORα[109]. The protein RORA protects the hippocampus and other sensitive tissues against the damaging effects of GCs, thus changes in RORA levels may confer a risk for PTSD[110]. In contrast, animal model studies have demonstrated that PTSD also alters the expression of core clock genes such as Per1 and Per2 in central clock tissues, including the SCN[111].
A recent study revealed that all patients with SCZ exhibited disrupted circadian rhythms compared to those of healthy controls, particularly with respect to alterations of sleep-wake and melatonin cycles[112, 113]. Genetic studies have identified numerous proteins associated with SCZ risk. Even common single nucleotide polymorphisms (SNPs) may result in SCZ, which has been confirmed by GWASs with large patient samples. For example, Neuregulin 1 (Nrg1) has been identified as an SCZ risk gene. The protein Nrg1 is involved in a series of neural processes, such as synapse formation. Therefore, Nrg1-deficient mice can be used as SCZ-relevant mouse models, which exhibit significant circadian abnormalities[114]. In contrast to GWASs, copy number variant studies have identified risk genes that contribute to SCZ via deleted or duplicated DNA[115]. It has been well documented that duplication of the vasoactive intestinal peptide receptor 2 (Vipr2) gene may lead to SCZ. The Vipr2 gene is involved in the stability of circadian rhythmicity in the SCN. Moreover, disrupted rest/activity patterns were observed in Vipr2 knockout mice[116].
Anxiety is the normal response in individuals faced with stress, but an excessive stress response may cause anxiety disorder co-occurring with disruption of the circadian rhythm. Furthermore, clinical data have indicated that variants in the expression of the circadian clock-related gene, Per3, might contribute to human anxiety disorder[117]. Beyond this study, Cissé et al. have evaluated the anxiety-like behavior of mice when exposure to dim light at night (dLAN) is associated with two core clock genes, Clock and Rev-Erb. Chronic exposure to dLAN induces anxiety-like behavior in mice, which enhances Clock gene expression in the hypothalamus, whereas Rev-Erb gene expression is suppressed[118]. Additionally, Cry1/2 deficient mice exhibited increased anxiety behavior compared to wild-type mice[119].
ADHD is a common childhood onset mental disorder with symptoms of impulsivity, inattention, and hyperactivity, which may extend into adulthood. In addition to these symptoms, ADHD patients frequently experience sleep disturbances, both children and adults[120]. A recent study reported a greater occurrence of insomnia during the night and sleepiness in the morning in ADHD children than in healthy children[121]. This phenomenon has also has been found in adults using functional linear modeling, suggesting that adults with ADHD have lower sleep quality than do normal adults[122]. Given that ADHD is strongly heritable, these results indicate that ADHD may involve the circadian clock or clock gene variants. For instance, many studies have advocated that an SNP (rs1801260) at the 3'-untranslated region (3'-UTR) region of the Clock gene has been identified as a predisposing risk factor for adult ADHD patients[123, 124]. This polymorphism is also associated with ADHD-related traits in healthy adults without evening preference, particularly in the male population[125]. It is well known that both core clock genes, Bmal1 and Per2, are linked to the internal circadian rhythm; Baird et al. conducted a case-control study and found decreased rhythmic expression of the Bmal1 and Per2 genes in ADHD patients compared to that in healthy individuals[126].
The literature review on core genes and mental disorders is presented in Table 4.
Studies Participants Measure Results Gouin et al.[101] Individuals with a history of depression (n = 30), non-depressed individuals (n = 30) Relative expression of clock gene mRNA: Clock, Bmal1, Per1, and Per2 in peripheral blood leukocytes Individuals with a history of depression were associated with higher Clock, Bmal1, Per1, and Per2 mRNA levels Logue et al.[110] Individuals with PTSD (n = 295), healthy controls (n = 196) Genotyping and statistical analysis of the core clock gene, RORα RORα is significantly associated with PTSD Koresh et al.[111] Individuals with PTSD (n = 8), healthy controls (n = 8) Relative expression of clock gene mRNA: Per1 and Per2 PTSD may alter the circadian pattern of Per1 and Per2 expression in the SCN Johansson et al.[112] Schizophrenia patients (n = 11) and healthy controls (n = 11) Relative expression of clock genes: Clock, Bmal1, Per1, Per2, Cry1, Cry2, Rev-Erbα Schizophrenia patients exhibit a loss of the rhythmic expression of Cry1 and Per2, and decreased expression of Clock, Per2, and Cry1 Liberman et al.[117] Individuals with anxiety disorder (n = 310) and healthy controls (n = 70) Genotyping of SNPs of the Per3 gene Per3 SNP (rs228697) is significantly associated with anxiety Cissé et al.[118] Adult mice with dLAN-induced anxiety (n = 63) and healthy control (n = 60) Relative expression of clock genes: Clock and Rev-Erbα Adult mice with dLAN-induced anxiety had increased levels of Clock gene expression and decreased levels of Rev-Erb gene expression in the hypothalamus Kissling et al.[123] Individuals with ADHD (n = 143), healthy individuals (n = 143) Genotyping and analysis of SNPs (rs1801260) of the Clock gene The Clock SNP (rs1801260) is significantly associated with ADHD Baird et al.[126] Adult ADHD patients (n = 13) and healthy controls (n = 19) Clock gene analysis of Bmal1 and Per2 ADHD patients exhibited decreased rhythmic expression of the Bmal1 and Per2 genes Note. PTSD: posttraumatic stress disorder, SCN: suprachiasmatic nucleus, dLAN: dim light at night, SNP: single nucleotide polymorphism. Table 4. Summary of Studies on Clock Genes and Mental Disorders
-
The significance of the interplay between the circadian clock and chronic stress is an important topic. Understandably, it is difficult to fully describe this interaction, but an increasing number of studies have revealed that the circadian clock regulates chronic stress via the HPA axis, while chronic stress affects the regulation of the circadian clock. Accordingly, this interaction extends to multiple physiological aspects of the body, such as immunity, metabolism, and mental health. The molecular components of the circadian clock also participate in these interactions. Unfortunately, in modern societies, prolonged high levels of stress result in numerous people developing an irregular work-rest schedule. Therefore, a disrupted circadian rhythm leads to a variety of further diseases.
Future studies should provide a more complete assessment of the common regulatory mechanism of both the circadian clock and chronic stress. We anticipate that these studies targeting circadian HPA axis regulation will provide a broader perspective for the development of successful therapeutic interventions for clinical diseases associated with chronic stress and circadian rhythm disruption.
Author Contributions Conceived and designed the manuscript: LI Dong Shui, ZHU Hai Yan; Searched and read papers: KUANG Xiao Dong, XIN Bo Yu; Wrote the manuscript: KUANG Xiao Dong, XIN Bo Yu, CAO Yuan; Critically revised the manuscript: XIN Bo Yu, ZHU Hai Yan; Provided final approval of manuscript to be published: LI Dong Shui, ZHU Hai Yan.
Competing Interests None of the authors have competing interests to declare.
Interaction between the Circadian Clock and Chronic Stress
doi: 10.3967/bes2018.093
the National Natural Science Foundation of China 81670467
- Received Date: 2018-04-12
- Accepted Date: 2018-08-30
| Citation: | KUANG Xiao Dong, YU Xin Bo, CAO Yuan, LI Dong Shui, ZHU Hai Yan. Interaction between the Circadian Clock and Chronic Stress[J]. Biomedical and Environmental Sciences, 2018, 31(9): 686-699. doi: 10.3967/bes2018.093 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: