-
Lead exposure is a known potential risk factor for neurodegenerative diseases such as Alzheimer's disease (AD). Exposure to lead during the critical phase of brain development has been linked with mental retardation and hypophrenia in later life. This study was aimed to investigate the effects of lead exposure of pregnant mice on the expressions of insulin-degrading enzyme (IDE) and nerve growth factor (NGF) in the hippocampus of their offspring. Blood samples were collected from the tail vein, and after anesthetizing the pups, the brain was excised on postnatal day 21. Lead concentrations were determined by graphite furnace atomic absorption spectrophotometry, and the expressions of IDE and NGF were determined by immunohistochemistry and Western blotting. Results showed that the reduction in IDE and NGF expression in the hippocampus of pups might be associated with impairment of learning and memory and dementia induced by maternal lead exposure during pregnancy and lactation.
Exposure to lead, a ubiquitous environmental contaminant, occurs primarily by inhalation and ingestion. Although more than 90% of the body lead burden is deposited in the skeleton, the brain is a key target organ of lead toxicity. Membrane proteins such as N-methyl-D-aspartatereceptors (NMDAR) and calcium-permeable acid-sensing ion channel (ASICs) have been considered as principal targets in lead-induced neurotoxicity. The functioning of the cholinergic system is also affected by Pb2+ intoxication. Furthermore, exposure to lead has been shown to be related to with the outbreak of sporadic Alzheimer's disease (SAD), which is a well known type of dementia[1]. In fact, environmental lead contamination, especially during crucial periods of brain development, has been proposed to be a major risk factor for Alzheimer's disease (AD).
AD is a common neurodegenerative disorder. Neurofibrillary tangles (NFT) of hyperphosphorylated tau and amyloid depositions in the brain are biomarkers of AD. β-Amyloids (Aβs)have been proposed as causes of dementia such as the dementia in AD[2]. Studies have also linked lead exposure to AD[1], the accumulation of Aβs, and the hyperphosphorylation of tau.
Insulin degrading enzyme (IDE) is a metalloprotease encoded by the IDE gene, which plays a central role in the clearance of Aβs, as well as other β-sheeted substrates, including insulin, and insulin-like growth factor (IGF-1), in vivo. IDE is highlyexpressed in the brain, liver, kidney, and muscle tissues and clears brain Aβs through both degradation and oligomerization of the peptide. Lack of IDE has been shown to cause an elevated-level of brain Aβs in IDE -/- mice[3].
Nerve growth factor (NGF) is a neuropeptide primarily involved in the regulation of growth, maintenance, proliferation, and survival of neurons. Like other growth factors, NGF plays a pivotal role in axonal growth, promoting axonal branching, and elongation. A reduction in NGF expression has been linked with synaptic dysfunction and neuronal malformations. NGF was found to be decreased with aging, which might contribute to an age-dependent decline in cognitive function, including AD[4]. The present study focuses on the expression of NGF and IDE in the hippocampus of the mouse brain after lead exposure in utero and via breast milk, aiming totest the hypothesis that lead exposure early in life decreases the expressions of NGF and IDE in the hippocampus.
All procedures using animals were conducted in strict accordance with international standards of animal care and were approved by the local Care of Experimental Animals Committee. Pregnant females at embryonic day (E0) were randomly assigned to one of four groups and caged separately. Lead acetate was dissolved in distilled water at four different concentrations, 0%, 0.1%, 0.2%, and 0.5% indicating control, low, moderate, and high concentrations, respectively. Lead exposure of pregnant mice was initiated from the beginning of gestation and lasted until weaning (the 21st postnatal day). Twenty-one pups from the control and high (0.5%) concentration groups (regardless of male and female) were randomly selected at birth, and eighteen pups from low (0%) and moderate (0.2%) concentration groups were randomly selected at birth, making sure that at least one pup per litter was culled for the experiment. Eight pups from each group were used for Western blotting, and ten were used for determination of lead concentrations in the blood and hippocampus. Three pups from the control and high (0.5%) concentration groups were used for immunohistochemical assay (IHCA) and immunofluorescence assay to analyze the effects of lead exposure on the expressions of hippocampal IDE and NGF, the distribution of hippocampal IDE, and the viability of neurons.
All data were expressed as mean ± standard deviation (SD). One-way ANOVA and Bonferroni test were used to compare the expressions of IDE and NGF and lead concentrations in the blood and hippocampus tissue in the four groups. SPSS21.0 software (SPSS Inc., Chicago, USA) was used for statistical analysis. P <0.05 were considered to be statistically significant.
Lead concentrations in both blood and hippocampus were significantly increased by maternal lead exposure during pregnancy and lactation (F = 391.650, P < 0.05; F = 242.737, P < 0.05; Table 1), and the concentrations of lead were positively associated with drinking water lead concentration.
Group n Blood Lead Concentration Hippocampus Lead Concentration C 10 13.1480 ± 2.0183 0.1198 ± 0.0285 L 10 42.3628 ± 3.2675 0.3202 ± 0.0246 M 10 63.1563 ± 4.7271 0.4345 ± 0.0478 H 10 84.2114 ± 8.0969 0.6096 ± 0.0570 F 391.650 242.737 P < 0.05 < 0.05 Note. Data are expressed as mean ± SD (n = 10). Values differ significantly between the groups (P < 0.05). C, L, M, and H refer to control, 0.1%, 0.2%, and 0.5% Pb groups, respectively. Table 1. Blood and Hippocampus Lead Concentrations of All Chosen Pups (μg/dL)
Exposure tolead during critical periods of brain development increases the risk for both cognitive and behavioral deficits, especially in the case of childhood lead exposure. Childhood lead poisoning leads to all types of neurobehavioral disorders, as well as intellectual impairments[5]. Women with a history-of-childhood-lead exposure share a risk of spontaneous abortions or stillbirths and may have children who manifest significant learning disabilities. A study by Needleman showed that children born with a blood lead concentration of 10 μg/dL displayed a significant reduction in IQ and impairment in learning and memorizing in later life[6], illustrating that the development of the nervous system and the functioning of learning and memorizing are significantly affected by Pb2+ exposure.
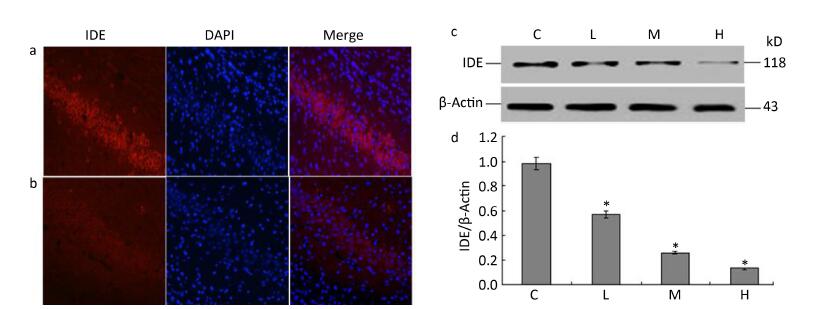
To determine whether lead exposure could affect the activity of IDE, we measured IDE expression and the localization of neuronal nuclei through an immunofluorescence analysis. The expression of IDE was significantly reduced in the 0.5% PbAc-treated group compared to that in the control group, as was the viability of neurons (Figure 1a-b). These results were consistent with data from Western Blot test, as the expression of IDE was negatively associated with lead exposure in a concentration-dependent manner (Figure 1d; F = 41.36, P < 0.05).
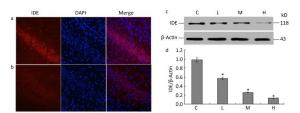
Figure 1. Change in IDE protein expression in the hippocampus of mouse pups induced by maternal lead exposure. (a) Immunofluorescence analysisof control group. (b) Immunofluorescence analysis of 0.5% PbAc group. Immunocytochemical staining was performed using a mouse anti-IDE antibody (red); nuclear staining was performed using DAPI (blue) (40 × 10 magnification) (n = 3). (c) Western blot analysis of hippocampal IDE of pups in different groups. (d) IDE protein expression levels analyzed by Western blot in each group. The values represent the ratio of IDE/β-actin intensity (n = 8).
The 110 kD thiol zinc metalloendopeptidase-IDE is present in cytosol, peroxisomes, and endosomes, and on cell surfaces and appears to be a master regulator of β-pleated sheet proteins such as Aβ, APP, bradykinin, and somatostatin. Metal ions such as Cu2+, Cu+, and Ag+ have been proven to play an inhibitory role in IDE activity by triggering allosteric effects. In our study, exposure to lead resulted in down-regulation of the expression of both IDE and NGF, suggesting that conformational changes in IDE might also be involved in Pb2+-induced down-regulation of IDE. Consistent with our assumption, a novel, catalytically inefficient form of IDE, IDE 15b, was identified in brain and non-neural tissues, suggesting that a mutated form of IDE might exist that could be related to AD and T2DM[7], supporting the idea that conformational changes in IDE may lead to the outbreak of certain neurodegenerative diseases such as AD and T2DM. Moreover, a diabetic mice model with a high risk of AD (C57BL/6) showed enhanced levels of liver (by 36%) and striatum (by 42%) IDE, but reduced level of brain IDE (by 20%-30%), suggesting that deficits in brain IDE may be associated with the development of SAD in the elderly, especially in diabetics[8]. To summarize, down-regulation of brain IDE expression and reduction of brain IDE (standard form of IDE) activity, which could be caused due to lead exposure during a critical time period, could increase the risk of metabolic neurodegenerative diseases by promoting brain Aβ accumulation such as in the case of AD and T2DM.
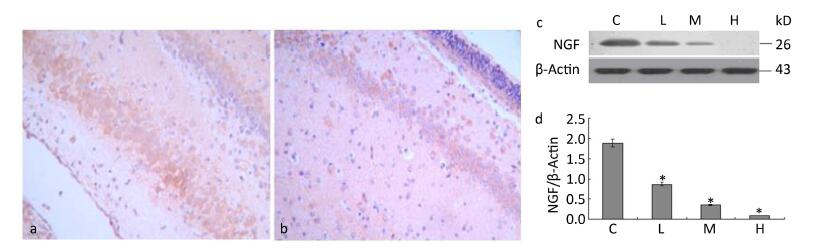
We examined the effects of maternal lead exposure on the expression of hippocampal NGF. Immunohistochemical analysis showed that NGF expression was decreased in the 0.5% PbAc-treated group compared to that in control mice (Figure 2a-b), and the distribution of NGF was also affected by maternal lead exposure. The results of Western blotting indicated that maternal exposure to PbAc during pregnancy and lactation substantially reduced NGF expression in the hippocampus of pups compared to that in control pups (Figure 2d; F = 57.23, P < 0.05).
Figure 2. Change in IDE protein expression in the hippocampus of mouse pups induced by maternal lead exposure. (a) NGF immunoreactivity in the CA1 region of the hippocampus in the control group. (b) NGF immunoreactivity in the CA1 region of the hippocampus in the 0.5% PbAc group (40 × 10 magnification) (n = 3). (c) Western blot analysis of hippocampal NGF of pups in different groups. (d) NGF protein expression levels analyzed by Western blot in each group. The values represent the ratio of NGF/β-actin intensity (n = 8) (*P < 0.05). C, L, M, and H refer to control, 0.1%, 0.2%, and 0.5% Pb groups, respectively.
NGF acts upon several classes of central and peripheral neurons, promoting cell survival, encouraging neurite outgrowth, and regulating cell differentiation. NGF is required in the maintenance of both the sympathetic and sensory neurons. Administration of NGF in vitro has been proven to rescue the sensory neurons from axotomy-induced cell death[9]. In fact, NGF plays a critical role in neuronal development and functioning of the mammalian nervous system. Reduction in NGF expression has been associated with decreased cholinergic activity and death of post frontal and temporal cortex in patients with AD[10]. NGF cooperates with TrkA receptors that promote receptor dimerization and tyrosine residue phosphorylation, and initiates the ERK/MAPK pathway in physiological conditions. Reduction in brain NGF expression may result in impairments of long-term potentiation (LTP) induction and synaptic plasticity, as well as deficits in cell (neuron) proliferation and differentiation and cell cycle progression. The dimeric form of NGF is joined by disulfide bonds, twisted in β-strands, forming a cystine-knot, which resembles t he active form of Aβ oligomer. Therefore, whether the active form of NGF β is also a substrate for IDE and the mechanism of the possible binding still need to be elucidated, and we are planning to explore these aspects in subsequent experiments.
Decline in cognition ability is a typical clinical feature of AD, the etiology of which may lie in the impairment of ERK/MAPK transduction caused by the extracellular accumulation of Aβs. It is well known that the ERK/MAPK signaling pathway plays an important role in the development of hippocampus, as well as in neural processing and the development of learning and memory. Moreover, the structural and functional impairments of the hippocampus are common pathologic changes of early AD. Therefore, several researchers believe that dysfunctions in the hippocampal ERK/MAPK signaling pathway may lead to the outbreak of some neurodegenerative diseases such as AD, PD, and ALS. Our findings have shown that neurons in the 0.5% PbAc treatment group are less distributed compared to those in the control group (Figure 1a-b; DAPI), suggesting that neurotrophic functioning of NGF and its signaling pathways may be damaged by lead intoxication.
Previous studies have shown that maternal lead exposure during gestation and lactation led to increased expressions of hippocampal p-Tau, total Aβ, and cortex Aβ40 of pups and reduced the expressions of hippocampal IGF1/IGF2, cortex IDE, and IGF2, while inducing dysfunctions in learning and memory capacity[11]. Our results indicate that maternal lead exposure could affect the expressions of IDE and NGF, which may lead to the accumulation of brain Aβs, neural dysfunctions, and malformations, as well as impairments of the signaling pathways. Further studies are being planned to investigate the mechanism of metal ion-induced neuropathy. Moreover, transcriptional levels of IDE, NGF, and other AD-related genes remain to be investigated.
Maternal Lead Exposure Induces Down-regulation of Hippocampal Insulin-degrading Enzyme and Nerve Growth Factor Expression in Mouse Pups
doi: 10.3967/bes2017.029
Post Doctoral Foundation of China 2015M572109
This research was supported by the National Natural Science Foundation of China (NSFC) 31201878, 81172716,and U1204804
Post Doctoral Fund of Henan province 2014049
- Received Date: 2016-11-11
- Accepted Date: 2017-03-02
| Citation: | LI Xing, LI Ning, SUN Hua Lei, YIN Jun, TAO Yu Chang, MAO Zhen Xing, YU Zeng Li, LI Wen Jie, John D BOGDEN. Maternal Lead Exposure Induces Down-regulation of Hippocampal Insulin-degrading Enzyme and Nerve Growth Factor Expression in Mouse Pups[J]. Biomedical and Environmental Sciences, 2017, 30(3): 215-219. doi: 10.3967/bes2017.029 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: