HTML
-
Tuberculosis (TB) is a chronic infectious disease seriously endangering human health worldwide. Millions of people continue to fall sick with TB each year. According to a 2017 report by the World Health Organization, 10.4 million people fell ill with TB and 1.3 million people died from this disease, including 0.4 million patients co-infected with TB and human immunodeficiency virus[1].
Since its inception, rapid diagnosis, prompt treatment, and vaccination have been vital in eradicating TB. However, the lack of a rapid and reliable diagnostic method is a limitation in controlling TB. The immune tests based on cytokines and antibodies have several advantages over other conventional diagnostic tools such as sputum smear microscopy, bacterial culture-based methods, and chest radiography. It is a rapid, economical, and user-friendly diagnostic method. GeneXpert MTB was a rapid molecular biological diagnosis methods, but can only identify TB patients with bacteria positive similar to the traditional diagnostic tools. While immunological diagnosis targeted for both bacteria positive and negative patients. A vast array of mycobacterial-specific antigens, such as early secretory antigenic target 6 (ESAT-6), culture filtrate protein 10 (CFP-10), Rv3615c, Rv0934, and Rv2351c, have been identified for TB diagnosis[2, 3]. However, the accuracy of the existing assays is modest, and these tests have not been clinically applied[4, 5]. A combination of different antigens can improve the diagnostic performance; thus, identifying as many specific antigens as possible that can effectively distinguish healthy individuals from patients should continue.
Another limitation is the variable protection of Bacillus Calmette-Guérin (BCG) vaccine, which is the only TB vaccine presently used in human beings. BCG vaccination in childhood provides protection for only 10-20 years[6]. Moreover, the protective effect of BCG against pulmonary TB ranges from 0% to 80%[7, 8]. Clearly, developing new TB vaccines that are more effective than BCG is imminent to reach the targets of TB elimination. Twelve new potential vaccines exist in different stages of clinical trials, among which only 11 mycobacterial antigens are used as part of recombinant BCGs, viral-vectored booster vaccines, or protein and adjuvant combinations[9-13]. However, whether these leading vaccine candidates can pass through the phase Ⅲ clinical trials and finally register cannot be assured. Hence, the discovery of new potential antigens is still indispensable for developing a more effective TB vaccine.
Advances in bioinformatics have contributed to the discovery of novel antigens in a short time and at a low cost, including the identification of immunological epitopes[14]. Rv0674 is an essential gene for Mycobacterium tuberculosis (M. tuberculosis) growth, but its molecular function is unknown at present[15]. And Rv0674 was identified from culture filtrates by a combination of 2-DE/MALDI-TOF-MS and LC-MS/MS and was characteristic as a secreted proteins[16]. Its' 3D structure was released on PDB database though its' detailed information was not published yet. The secondary structure showed that Rv0674 contains 7 beta sheet and 11 alpha helix. In a previous study, the human B-cell epitopes of Rv0674 were predicted using the SEPPA2.0 software based on its' 3D structure, and the epitopes were synthesized to conduct antibody testing in patients with TB and healthy donors. A total of six human B-cell epitopes were identified[17]. Keeping this in view, attempts were made to characterize Rv0674 in humoral and cellular immune response in the present study.
-
This study was approved by the Ethics Committee of the National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention. The patients included in this study provided written informed consent before participating. The animal experiments were approved by the Ethics Committee of the National Institute for Communicable Disease Control and Prevention at the Chinese Center for Disease Control and Prevention. Efforts was made to minimize suffering during animal immunization and surgery procedures.
-
A total of 204 crowd sera samples were collected for enzyme-linked immunosorbent assay (ELISA). Among these, 95 samples formed the negative group, including 44 patients with non-TB lung diseases (non-TB) and 51 healthy donors, and 109 patients comprised the case group, including 51 smear-positive and 58 smear-negative TB. The inclusion criteria were as follows: for healthy control, healthy individuals who did not have any TB clinical symptoms and signs and exposure to M. tuberculosis; for patients with non-TB diseases, those who had other pulmonary diseases (such as pneumonia and bronchitis) but not TB; and for patients with TB, those who met with the national criteria for the diagnosis of TB in China[18]. Detailed information of the participants was available on the patient profile (Table 1).
Characteristics TB Non-TB HD Participants for humoral immunity evaluation Total gender 109 44 51 Male 76 30 12 Female 33 14 39 Age, years 42.72 ± 18.11 54.09 ± 14.95 28.33 ± 7.49 Participants for cellular immunity evaluation Total gender 61 66 64 Male 48 39 15 Female 13 27 49 Age, years 44.10 ± 16.00 55.14 ± 15.45 29.19 ± 8.37 Note. TB, Patients with TB; Non-TB, patients with non-TB lung disease; HD, non-TB and health donors. Table 1. Demographic Characteristics of TB and non-TB Patients and Healthy Donors Enrolled in Present Study
And a total of 191 participants, including 61 patients with TB, 66 patients with non-TB diseases, and 64 healthy individuals for enzyme-linked immunospot (ELISpot) assay (Table 1). The inclusion criteria were similar to those described above.
-
The sequence of Rv0674, Rv0934 and Rv1886c were obtained by polymerase chain reaction (PCR) from H37Rv (ATCC 27294) genome DNA template. The recombinant expression plasmid pET-32a, which contained the gene Rv0674, Rv0934 or Rv1886c, was successfully constructed and transformed into Escherichia coli BL21 (DE3). The protein expression was induced by adding 1 mmol/L isopropyl β-D-thiogalactoside (IPTG), and the cells incubated for 3 h at 37 ℃ with shaking were harvested by centrifugation (7, 000 rpm, 15 min, 4 ℃). The cell pellets were resuspended in 30 mL of buffer A (25 mmol/L Tris-HCl, pH 8.0, 500 mmol/L NaCl, 8 mol/L urea, and 10 mmol/L imidazole), purified using a column packed with Ni Sepharose High Performance (GE Healthcare, Pittsburgh, PA, USA), and eluted with buffer B (25 mmol/L Tris-HCl, pH 8.0, 500 mmol/L NaCl, 8 mol/L urea, and 300 mmol/L imidazole). The purified protein was pooled and dialyzed into 25 mol/L Tris-HCl (pH 8.0) and then concentrated using a 30K Ultrafiltration tube (Amicon, Massachusetts, USA). The concentrated protein was quantified using a bicinchoninic acid kit (Transgen Biotech, Beijing, China) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Endotoxin removal from the recombinant proteins was done by using the ToxinEraserTM endotoxin removal kit (Genscript Biotechnology, USA) and the endotoxin level of the recombinant proteins at the concentration of 0.5 mg/mL was determined by using the ToxinSensorTM Chromogenic LAL Endotoxin Assay Kit (Genscript Biotechnology, USA). The amount of endotoxin in all proteins was less than 1 EU/mL.
-
The humoral immune antigenicity of Rv0674 protein was detected and evaluated by ELISA testing serum immunoglobulin G (IgG) antibodies. The antigen was coated in a 96-microwell ELISA plate with a final concentration of 20 μg/mL at 4 ℃ overnight. The plates were washed with 0.01 mol/L phosphate-buffered saline PBS (pH 7.4) containing 0.05% Tween-20 using an automatic washer. Blocking was achieved by adding 200 μL of 3% BSA to each well, and the plates were incubated at 37 ℃ for 2 h. Then, 100 μL of serum (100 times dilution with PBS buffer) was incubated at 37 ℃ for 1 h. Next, horseradish peroxidase (HRP)-labeled goat anti-human IgG antibodies made with PBS were added and incubated at 37 ℃ for 50 min. TMB (TIANGEN, Beijing, China) substrate was then added, and the color reaction was terminated by adding 2 mol/L H2SO4. Finally, the absorbance was read at 450 nm in 10 min. The experiment was repeated twice.
-
The cellular immune antigenicity of Rv0674 protein was detected and evaluated using ELISpot assay. The peripheral blood mononuclear cells (PBMCs) were separated from the whole blood by Ficoll-Hypaque density gradient centrifugation. Then, PBMCs were incubated at a density of 2 × 105 cells/well and stimulated with Rv0674 protein (10 μg/mL) or a mixture of CFP-10, ESAT-6, and Rv3615c provided in the clinical QB-SPOT kit (Quanbo, Beijing, China)[19]. Following a 20-h incubation at 37 ℃ in 5% CO2 incubator, the assay was conducted as described in the kit's instructions. The individual spots were counted and analyzed according to the manufacturer's instructions.
-
The immunogenicity of Rv0674 protein was conducted and evaluated with animal immunity vaccination on BALB/c mice. The female BALB/c mice (6-8 weeks) were randomly divided into different groups with six mice in each group. Dimethyl-dioctyldecylammonium bromide (DDA) /Poly I:C/ Rv0674 was prepared by emulsification. Poly I:C was dissolved in 25 mmol/L Tris-HCl (pH 8.0) to 0.5 mg/mL. DDA was suspended in 25 mmol/L Tris-HCl (pH8.0) by heating to 80 ℃ for 10 min with a final concentration of 2.5 mg/mL, and then cooled to room temperature (RT) and emulsified with antigens. Poly I:C. Rv0674 was used at two doses: 50 μg/mouse (low dose) and 100 μg/mouse (high dose). Ag85B was used as a positive control. Negative controls were immunized with 200 μL of 25 mmol/L Tris-HCl (pH 8.0) or 200 μL of Poly I:C/DDA without antigens. The mice were injected subcutaneously in the back three times at 10-day intervals. The mice were sacrificed for immunogenicity detection 1 week after the last immunization.
-
The blood of immunized mice was acquired by retro-orbital puncture and naturally coagulated at RT for 2 h. Then, the sera were obtained by centrifugation at 2, 500 rpm for 10 min. The test procedure was carried out as described earlier in the step of human humoral immunity detection and evaluation. Next, 96-well ELISA plates were coated with the antigens with a final concentration of 20 μg/mL at 4 ℃ overnight. Sera were diluted tenfold from 1:10 to 1:107. A second ELISA was performed to determine a more accurate IgG titer, and the sera were double-diluted based on the results.
-
The spleen of immunized mice was removed surgically and added into a 15 mL conical tube with 5 mL of RPMI 1640 (Gibco, Massachusetts, USA). The spleen and media were then poured into a 60-mm Petri dish covered with a cell strainer, on which the spleen was crushed using a gentle circular motion until no solid tissue remained. The cell suspensions were added back into a 50 mL conical tube and concentrated by centrifugation at 1, 000 rpm for 5 min. The erythrocytes were lysed with 10 mL of ACK lysis buffer (0.15 mol/L NH4Cl, 10 mmol/L KHCO3, 0.1 mmol/L Na2EDTA, pH 7.2) at 37 ℃ for 10 min, and the reaction was terminated using an equivalent volume of RPMI 1640. Then, the splenocytes were washed with RPMI 1640 and diluted to 1 × 107 cells/mL in the RPMI 1640 medium with 10% fetal bovine serum (FBS), 100 μg/mL penicillin-streptomycin, 25 mmol/L hydroxyethyl piperazine ethylsulfonic acid (HEPES), and 2 mmol/L L-glutamine.
Then, 100 μL splenocytes was stimulated with 20 μg/mL Rv0674 or Rv1886c. Phytohaemagglutinin (PHA) (5 μg/mL) and RPMI 1640 were used as positive and negative controls, respectively. The splenocyte culture supernatant was collected after co-culture with respective recall antigens for 48 h. The BD OptEIA ELISA kits were used for detecting the cytokines interferon (IFN)-γ, interleukin (IL)-2, IL-4, IL-6, and IL-10. Briefly, microwells were coated with 100 μL of capture antibody and incubated overnight at 4 ℃. The plates were blocked with 200 μL of 10% FBS and incubated at RT for 1 h. Then, the wells were aspirated and washed five times with 300 μL of wash buffer. Subsequently, 100 μL of standard, samples, and control were pipetted into appropriate wells, respectively, and incubated for 2 h at RT. A 100-μL working detector (detection antibody and streptavidin-HRP reagent) was added to each well and incubated for 1 h at RT. Finally, 100 μL of substrate solution was added to each well and incubated for 30 min at RT in the dark. Finally, 50 μL of 2 mol/L H2SO4 was added to terminate the reaction, and the absorbance was read at 450 nm within 30 min.
-
The sensitivity, specificity, and diagnostic efficiency were calculated using the MedCalc statistical software, version 9 (Belgium). All statistical analyses of the results were performed using the GraphPad Prism software, version 5 (GraphPad Software, California, USA). The immunological data were compared employing a two-tailed t test. A two-sided alpha level of 0.05 was used to determine statistical significance in all of the analyses.
Ethics Statement
Patients Collection
Cloning, Expression, and Purification of Recombinant Rv0674 Proteins
Detection of Rv0674 Specific IgG from TB Patients
Evaluation of Rv0674 Specific ELISpot from TB Patients
Antigen Preparation and Animal Immune Evaluation
Effect of Rv0674 on Humoral Immunity to Mice
Effect of Rv0674 on Cellular Immunity to Mice
Statistical Analysis
-
A 723-bp fragment was cloned into a pET-32a plasmid and identified as Rv0674 by PCR and DNA sequencing (Figure 1A). The His-tagged recombinant Rv0674 protein was expressed in the form of secretory protein, purified by Ni Sepharose High Performance, and identified by SDS-PAGE; The pET-32a(+) plasmid contains His tag, Trx tag and S tag, which accounted for a basic 20.4 kD protein. So the recombinant Rv0674 was with a theoretical molecular weight around 47 kD. While its' apparent molecular weight was around 48 kD on the SDS-PAGE electrophoretogram (Figure 1B).
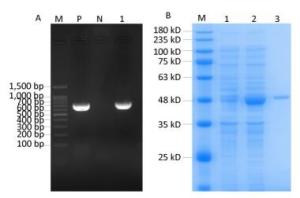
Figure 1. (A) The construction of recombinant Rv0674 by agarose gel electrophoresis analysis: Lane M, marker; lane P, H37Rv as positive sample; lane N, Distilled water as negative sample; lane 1, E.coli BL21 (DE3) CFU contains pET32a(+)-Rv0674 plasmids. (B) SDS-PAGE analysis of the purified recombinant Rv0674 protein: Lane M, marker; lane 1, pET32a(+)-Rv0674 before induction; lane 2, pET32a(+)-Rv0674 after induction; lane 3, purified recombinant Rv0674.
-
The humoral antigen features and serological diagnostic value of the Rv0674 were evaluated by detecting serum IgG antibodies in patients with TB using ELISA (Table 2). The antibody response level of Rv0674 in patients with TB was significantly higher than that in the negative control (P < 0.001), while the antibody response showed no difference between patients with non-TB diseases and healthy donors (P > 0.05).
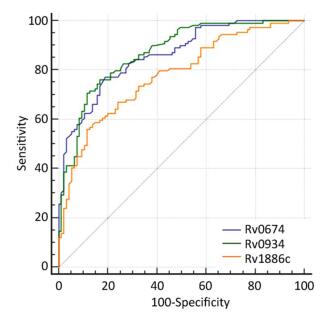
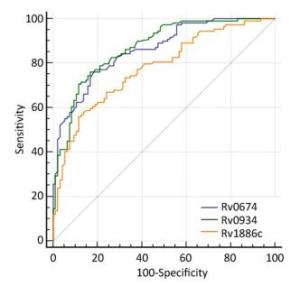
Group The Median OD450 Value AUC Sensitivity (%) Specificity (%) Youden Index TB Non-TB HD Rv0674 0.52*** 0.35 0.36 0.86 77.06 81.05 0.58 Rv0934 0.44*** 0.23 0.23 0.87 76.10 83.20 0.59 Rv1886c 0.53*** 0.43 0.37 0.78 55.96 88.42 0.44 Note. AUC: Area under the receiver-operator characteristic curve; ***P < 0.0001 (OD value between TB patients and negative controls). Table 2. The Reflect of the Potential of Serological Diagnosis Based on Rv0674
Rv0934 and Rv1886c, which were reported to induce antibody responses in patients with TB[20, 21], was used as a positive control to evaluate the serological diagnostic value of Rv0674. Rv0674 and Rv0934 had similar diagnostic sensitivity and specificity (77.06% and 81.05% vs. 76.10% and 83.20%), while Rv1886c had a low sensitivity of 55.96% and high specificity of 88.42%. And Rv0674 and Rv0934 showed a bigger area under the curve and Youden index than Rv1886c which can also be observed in the ROC plots (Figure 2). Among the patients with TB, Rv0674 showed a lower sensitivity in the smear-positive specimen compared with Rv0934 (84.31% vs. 92.16%), whereas Rv0674 showed a higher sensitivity in the smear-negative specimen compared with Rv0934 (70.69% vs. 62.07%). However, a combination of Rv0674 and Rv0934 caused an increase in the sensitivity to 87.16% and a decrease in the specificity to 71.58%.
-
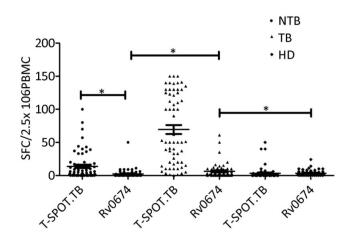
The cellular immunity and diagnostic performance of Rv0674 were investigated using ELISpot by detecting the IFN-γ levels from PBMCs produced by antigen stimulation. The cell level of IFN-γ produced by Rv0674 was significantly higher in patients with TB than in controls (P < 0.05) (Figure 2). Further evaluation of the diagnostic performance revealed that Rv0674 had a weaker sensitivity but a better specificity than that of the peptide cocktail of Rv3615c, ESAT-6, and CFP-10 used in the QB-SPOT kit. The sensitivity and specificity of Rv0674 using the ELISpot test were 26.23% (16/61) and 87.69% (114/130), respectively, while the sensitivity and specificity using the T-SPOT.TB kit were 90.16% (51/61) and 70.0% (91/130), respectively. However, the specificity of Rv0674 in patients with non-TB diseases was higher than that of peptide cocktail used in the QB-SPOT kit (95.45% vs. 53.03%).
-
The cellular immunogenicity of Rv0674 was investigated by detecting the cytokines levels in BALB/c mice (Figure 3).
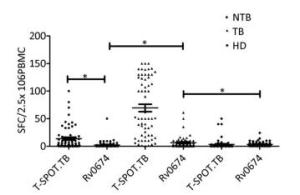
Figure 3. IFN-γ SFCs between different crowds. The comparison of IFN-γ spot-forming cells (SFCs) in non-TB patients (NTB), TB patients (TB) and healthy donors (HD). Each symbol represent the result obtained with an individual PBMC. The median value of each group is indicated by horizontal solid line. P was calculated by t-test to evaluate the statistically significant differences. *P < 0.05.
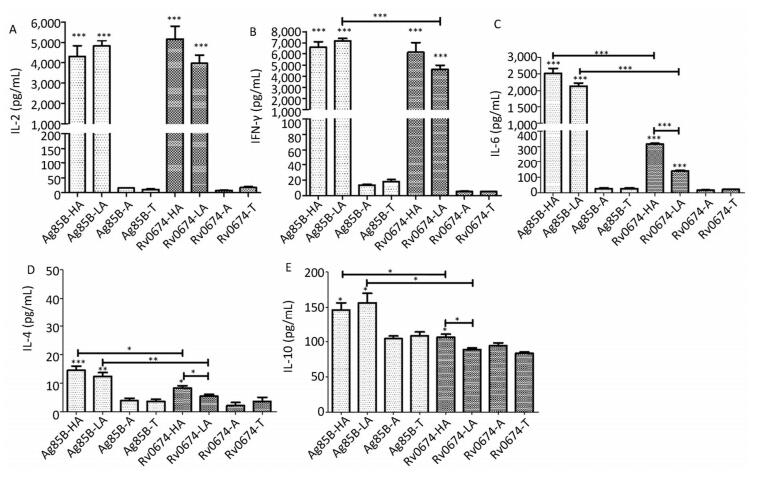
The results showed that both low-dose and high-dose Rv0674 antigens could induce the mice to produce high levels of IL-2 with a mean concentration of (5, 167 ± 1, 255) pg/mL and (3, 973 ± 817) pg/mL, respectively. No significant difference existed between the two (P > 0.05). Also, no significant difference in the concentration of IL-2 was found between the mice immunized with Rv0674 and Ag85B.
Another important Th1-type cytokine IFN-γ was also produced by the immunized mice with Rv0674 in both high- and low-dose groups, and the mean concentration was not significantly higher in the high-dose group (6, 148 ± 1, 737 pg/mL) than in the low-dose group (4, 601 ± 762 pg/mL) (P > 0.05). No significant difference in the concentration of IFN-γ was found between the mice vaccinated with the high-dose Rv0674 and Ag85B, while in the low-dose group, Ag85B elicited a higher level of IFN-γ compared with Rv0674 (P = 0.001).
Besides, significantly higher levels of IL-6 was induced in Rv0674 and Ag85B groups than negative controls (P < 0.001). And in both high- and low-dose groups, Ag85B elicited a markedly higher level of IL-6 compared with Rv0674.
On the contrary, low levels of Th2-type cytokine IL-4 and IL-10 were elicited by Rv0674 and Ag85B. The data showed that IL-4 and IL-10 were slightly lower in Rv0674 than in Ag85B in both high- and low-dose groups (P < 0.05). No remarkable alteration in the expression of either Th1 or Th2 cytokines was revealed in the negative groups.
-
Rv0674-specific antibody IgG was tested in the sera of the immunized mice with ELISA to evaluate the ability of humoral immunity. In contrast with the negative groups, the results clearly depicted that either Rv0674 or Ag85B generated a significantly high-level antibody response of IgG, IgG1, and IgG2a (Figure 4).
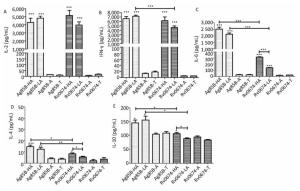
Figure 4. Cellular immune response in Rv0674 and Ag85B immunized BALB/c mice. Lymphocyte isolated from BALB/c mice immunized with high dose Ag85B (Ag85B-HA), low dose Ag85B (Ag85B-LA), Poly I:C/DDA adjuvants(Ag85B-A), Tris-HCl (Ag85B-T), high dose Rv0674 (Rv0674-HA), low dose Rv0674 (Rv0674-LA), Poly I:C/DDA adjuvants (Rv0674-A) and Tris-HCl (Rv0674-T) were stimulated with recall antigens, and the concentration of three different cytokines (A) IL-2, (B) IFN-γ, (C) IL-6, (D) IL-4, and (E) IL-10 were determined after a 48 hours stimulation. Asterisk (*) above the bar shows the difference between the experimental group and negative controls. Capped line with asterisk (*) indicates the difference between Rv0674 groups and & or Ag85B groups. P was calculated by t-test to evaluate the statistically significant differences. *P < 0.05, **P < 0.01, ***P < 0.001.
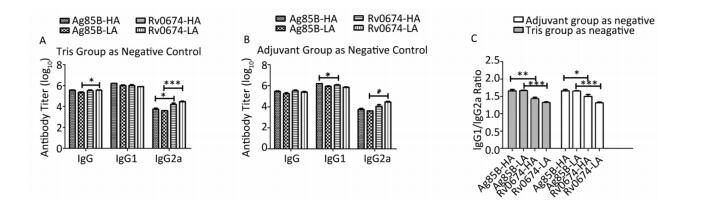
Consider mice immunized with Tris-HCl as negative group, Rv0674 evoked a slightly higher IgG and IgG2a antibody response compared with Ag85B in the high-dose vaccinated groups (P < 0.05) (Figure 5). Moreover, in the low-dose vaccinated groups, Rv0674 also induced significantly higher levels of IgG2a antibodies compared with Ag85B (P < 0.001). However, no obvious difference in IgG1 antibodies was detected between Rv0674 and Ag85B. For both the high- and low-dose vaccinated groups, the IgG1/IgG2a ratios in Rv0674 were 1.43 and 1.33, respectively, which were inferior to those (1.66 and 1.67) in the Ag85B groups.

Figure 5. Antibody response in Rv0674 and Ag85B immunized BALB/c mice. (A) Antibody titer of IgG, IgG1 and IgG2a observed in mice serum. The data are shown as geometric mean ± SD (log10). (B) Antibody isotype switching in response to Rv0674 groups and Ag85B was analyzed by IgG1/IgG2a ratio. The data analyzed by t-test to evaluate the statistically significant differences. *P < 0.05, **P < 0.01, ***P < 0.001.
Regard adjuvant group as a negative control, Rv0674 still induced higher levels of IgG2a antibodies than the Ag85B in low dose groups (P < 0.05). However, Rv0674 expressed a slightly lower IgG1 antibody response than Ag85B (P < 0.05). The IgG1/IgG2a ratio in the Rv0674 groups was still second to the Ag85B groups.
The Recombinant Rv0674
Humoral Immune Antigenicity Response of the Rv0674 Protein in Human
Cellular Immune Antigenicity Response of the Rv0674 Protein in Human
Cellular Immunogenicity Analysis of Rv0674 in BALB/c Mice
Humoral Immunogenicity Analysis of Rv0674 in BALB/c Mice
-
TB remains a worldwide health problem with 23% of the world's population estimated to have a latent TB infection[1]. One of the major factors leading to the slowly falling incidence of TB is the lack of rapid and accurate diagnostic methods at an early stage, thus TB patients cannot receive early and effective treatment, which ensures TB spread by prolonging the time of patients being unintentional infection sources. So accurate diagnosis is needed in the early stage of the disease for prompt treatment and reduction in transmission risk. Cytokine- and antibody-based immune tests are rapid, economical, and user-friendly for identifying patients with TB. Due to the variable sensitivity and specificity of antibody detection-based diagnostic tests, it is strongly recommended by WHO that these tests not be used for the diagnosis of TB. While targeted further research to identify new/alternative serological tests with improved accuracy is also strongly encouraged by WHO[22]. Recently, efforts toward discovering novel specific antigens and better methodological strategies is ongoing for efficient antibody-based immune diagnosis[23-25]. For example, Rv0934 (38 kD) is a widely studied MTB antigen and its high sensitivity and specificity in TB diagnosis has made itself been used in lots of commercial kits[5, 20]. In this study, we used Rv0934 as a reference to evaluate humoral immunity and serological diagnosis efficacy of Rv0674, and the results turned out that these two antigens had similar sensitivity and specificity. Moreover, Rv0674 had better performance than Rv0934 in identifying patients with smear-negative TB. This indicated Rv0674 could be a good potential diagnostic antigen for the diagnosis of TB.
Using single antigen to detect antibodies usually has a low sensitivity for the diagnosis of TB. This is because that recognition of mycobacterial antigens by the host is considered as heterogeneous among individuals[26]. So far no antigen had shown sensitivity higher enough to be used in a commercial test individually, so in the attempt to develop new TB diagnostic kit, it is more important to find fusion proteins or antigen cocktails which have a higher diagnostic sensitivity. In this study, a combination of Rv0674 and Rv0934 increased the serological diagnostic sensitivity to 87.16%. This combination study shows that Rv0674 and Rv0934 has a synergy effect in detecting TB infection which provides a solid basis for the probability to combine these two antigens in the TB diagnostic kit to achieve a higher sensitivity. A reduction in specificity (71.6%) was also observed when we combine these two antigens, it is because the improvement of ability to catch actual cases always comes with a fairly higher rate of false positive.
IFN-γ is the most important cytokine marker in T cell stimulation assays and is involved with protective immunity in the host towards mycobacterial antigens, as it can activate macrophages in conjunction with TNF-α to facilitate the killing of intracellular mycobacteria. The role of IFN-γ in M. tuberculosis infection also has been demonstrated in a murine model, patients with active TB, and those with latent TB. Recently, in vitro assays that measures IFN-γ in response to mycobacterial-specific antigens is a rapid and specific way to detect TB infection[27]. A vast array of mycobacterial-specific antigens, such as ESAT-6, CFP-10, Rv3615c, Rv2645, and Rv2351, have been identified to have the ability of stimulating PBMCs to express high levels of IFN-γ[2, 3, 28]. To verify the possibility of Rv0674 for IFN-γ release, we detected its ability of inducing human PBMCs to release gamma interferon by ELISpot in TB patients. Rv0674 displayed a weaker sensitivity but a better specificity than the reference reagent (QB-SPOT kit). The reasons for poor sensitivity of Rv0674 might be as follows: (1) a single antigen was used in this study while the commercial kit contained three antigens (ESAT-6, CFP-10, and Rv3615c); (2) the candidate antigens (ESAT-6, CFP-10, and Rv3615c) were from region of differentiation (RD), but Rv0674 was a non-RD protein; and (3) the prediction of B-cell epitopes indicated its antigenic properties for detecting antibodies. Therefore, Rv0674 might be a potential diagnostic antigen for detecting antibodies, besides IFN-γ determination.
After evaluating the diagnostic performance, the vaccine potential of Rv0674 was assessed in this study. As mentioned above, IFN-γ play a central role in M. tuberculosis infection by the activation of macrophages. An antigen that can induce strong IFN-γ production is regarded as a potential candidate for vaccine construction. For example, Ag85B, which has the capacity of inducing high levels of IFN-γ, has been used in the development of various new TB vaccines[29]. In this study, Rv0674 induced high level of IFN-γ as Ag85B did, suggesting that Rv0674 has a potential role in protective immunity against TB. Rv0674 immunized mice also produced very high levels of IL-2 as well as IFN-γ. IL-2 was also positively correlated with vaccine-induced protection against TB[30]. Signaling through IL-2 induces the activation of pathways that lead to the proliferation, survival and cytokine production of effector T cells[31].
The pathogenesis of M. tuberculosis infection is not mediated by the pathogen alone, but also by inducing the immune pathologic inflammatory response of the host[32]. Thus, the balance between pro-inflammatory and anti-inflammatory allows the patients to eradicate bacilli without suffering extensive tissue damage. IL-6 was known to function as pro-inflammatory cytokines and it played an important role in defense against M. tuberculosis. IL-6 knockout mice resulted in an early increase in bacterial load and a delay of IFN-γ induction when infected with M. tuberculosis[33]. In our study, we have found high levels of IL-6 had been induced by Rv0674 immunization in mice, but not as strong as Ag85B did. Meanwhile, slightly high IL-4 and IL-10 in Rv0674 high-dose groups were observed. IL-10 plays an important role in balancing immunity and limiting inflammatory responses. It is originally believed that IL-10 and Th1 cytokines are antagonistic, however, there are growing evidence suggesting that they act in a complementary form[34, 35]. A previous study by Murray PJ group shows that IL-10 knockout mice did not show increased IFN-γ production after they had been infected with M. tuberculosis[36]. Additionally, IL-10 can also enhance some inflammatory mediators expression, especially in an environment that is rich in IFN-γ[37]. Together, Rv0674 could generated potent cellular immunity by inducing IFN-γ, IL-2, IL-6, and IL-10.
Rv0674 also mediates a strong humoral immune response by eliciting high IgG antibody responses in BALB/c mice. This was consistent with the good performance of Rv0674 in humoral immunity in humans. When cellular-mediated immunity was considered the main protective response in M. tuberculosis infection, B cells also have a significant role in the defense against M. tuberculosis infection. An experiment done by John Chan group showed that B-cell-deficient mice are more susceptible to TB[38]. Furthermore, antibody isotype was investigated, and the results depicted that Rv0674 presented a preponderance of IgG1 over IgG2a, which was in favor of Th2 response. IgG2a is identified in a Th1 response, characterized by increased expression of IL-2, IFN-γ, and so on. However, IgG1 is part of a Th2 response enriched by IL-4 and IL-10[39]. Notably, a significantly high level of IL-2, IFN-γ, IL-6 and a feeble IL-4 and IL-10 were elicited by Rv0674 immunized mice. Therefore, the observed immune response profile of Rv0674 could be a Th1/Th2-mixed-type protective immunity with the predominance of Th1 cytokines.
Low immunizing doses have been shown to favor a cell-mediated response, and higher doses favor antibody production[40]. However, stronger levels of IFN-γ, IL-2, and IL-6 were detected in the high-dose group. Moreover, no significant difference in IgG titer was observed between low- and high-dose groups. Several factors combinedly determine the type and strength of immune responses, such as the route of vaccine delivery, adjuvant type, dose, and vaccine trait. In this study, mixed Poly I:C/DDA was used as an adjuvant. DDA, as a cationic liposome, was used as a repository to slowly release antigen to gain a sustained immunity. Moreover, DDA could induce a mixed Th1/Th2 response when it is associated with recombinant fusion protein[41]. Poly (I:C) is a mimic of double-stranded RNA with the ligation of Toll-like receptor 3, and it strongly drives a cell-mediated immunity[42]. The Poly I:C/DDA adjuvant might explain the immune response profile of Rv0674, a Th1/Th2-mixed-type protective immunity with the predominance of Th1 cytokine.
In conclusion, a novel M. tuberculosis antigen, Rv0674, was evaluated for its efficacy as a TB diagnosis marker and a vaccine candidate. The findings suggested that detecting IgG antibody against Rv0674 might help in the diagnosis of TB. Moreover, the immunological outcome of a profound expansion of Th1 cytokine and IgG suggested that Rv0674 might be a potential candidate in designing a new TB vaccine.
-
The funders had no role in the study design, data collection and analysis, manuscript preparation, or decision to publish. The authors especially thank the staff members of the Fuzhou Pulmonary Hospital and Beijing Changping Tuberculosis Institute for their outstanding contribution to this study. They also thank all the staffs of the Laboratory Animal Center, Chinese Center for Disease Control and Prevention, for their help in the animal experiments. They have no conflicts of interest to declare.







 Quick Links
Quick Links
 DownLoad:
DownLoad: