-
Aflatoxin B1 (AFB1), a secondary metabolite produced mainly by fungi, Aspergillus flavus and Aspergillus parasiticus, is one of the most toxic carcinogens known to date[1]. Areas with warm, humid climates and abundant rain provide optimal growth conditions for these molds. Improper storage of food leads to AFB1 contamination easily, and foods such as peanuts, corn, rice, sorghum, milk, and oil are susceptible to AFB1 contamination[2]. Once the food is contaminated by AFB1, it is difficult to remove it[3]. The exposure of AFB1 due to contamination is difficult to be realized, resulting in hepatocellular carcinoma after a long-term process[4]. Therefore, it is necessary to develop effective methods to prevent AFB1-induced hepatotoxicity.
In the mammalian body, the liver is the main target organ for AFB1 toxicity. AFB1 can be absorbed into the body through the skin, digestive tract, and respiratory tract. Upon entering the body, AFB1 is mainly metabolized to AFB1-exo-8, 9-epoxide (AFBO), aflatoxin M1, and aflatoxicol by the cytochrome P450 enzyme system and cytoplasmic reductase enzymes in the liver[5]. AFBO, which binds to DNA resulting in the formation of AFB1-DNA adducts, leads to mutations and carcinogenesis[6, 7]. It has also been reported that AFB1 can lead to liver injury via oxidative damage[8-10], inflammation[11], apoptosis[12].
Natural phytochemicals have gained great attraction for properties of chemical toxicity resistance by exerting multiple biological activities in recent years. Grape seed procyanidin extract (GSPE), rich in polyphenols, plays a protective role in liver injury induced by environmental toxicants, such as AFB1[13], lead[14, 15], and fluoride[16]. As one of the main components of GSPE, procyanidin B2 (PCB2) shows a similar positive impact on the liver. It is reported that PCB2 ameliorates acute hepatic injury and liver fibrosis caused by carbon tetrachloride (CCl4)[17, 18]. PCB2 has also been shown to reduce hepatic steatosis through transcription factor EB-mediated lysosomal and redox state[19]. Moreover, PCB2 protects against nonalcoholic fatty liver disease by modulating gut microbiota[20]. Taken together, these findings suggest that PCB2 exhibits a hepatoprotective effect, but whether PCB2 has a protective effect on AFB1-induced acute liver injury remains unknown.
The present study aimed to determine the protective effect of PCB2 on acute liver injury induced by AFB1 through in vivo experiments. The results of this study would lay a foundation for further research on the prevention of hepatocellular carcinoma and provide a theoretical basis for future dietary guidance to prevent the negative effects of AFB1.
-
PCB2 [purity > 98.0% high-performance liquid chromatography (HPLC)] was purchased from Chengdu Biopurify Phytochemicals Ltd. (Chengdu, China). AFB1 (purity > 98.0% HPLC) was purchased from Sigma Aldrich (USA). Dimethyl sulfoxide (DMSO, purity ≥ 99.9%) was purchased from MP Biomedicals (USA). Superoxide dismutase (SOD), catalase (CAT), glutathione (GSH), and malondialdehyde (MDA) assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Bicinchoninic acid (BCA) protein assay kit was purchased from MULTI SCIENCES (Hangzhou, China). Eastep® Super Total RNA Extraction Kit, GoScript™ Reverse Transcription Mix, and GoTaq® quantitative polymerase chain reaction (qPCR) Master Mix were purchased from Promega Corporation (USA). Interleukin-6 (IL-6) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) enzyme-linked immunosorbent assay (ELISA) kits were purchased from Jiangsu Meimian Industrial Co., Ltd. (Jiangsu, China). Primary antibodies against bcl-2 and bax were purchased from Cell Santa Cruz Biotechnology (USA); the β-actin antibody was purchased from Cell Signaling Technology Inc. (USA). The secondary goat anti-rabbit and goat anti-mouse horseradish peroxidase-conjugated antibodies were purchased from Cell Signaling Technology Inc. Western Lightning Plus enhanced chemiluminescence (ECL) was purchased from PerkinElmer Inc. (USA).
-
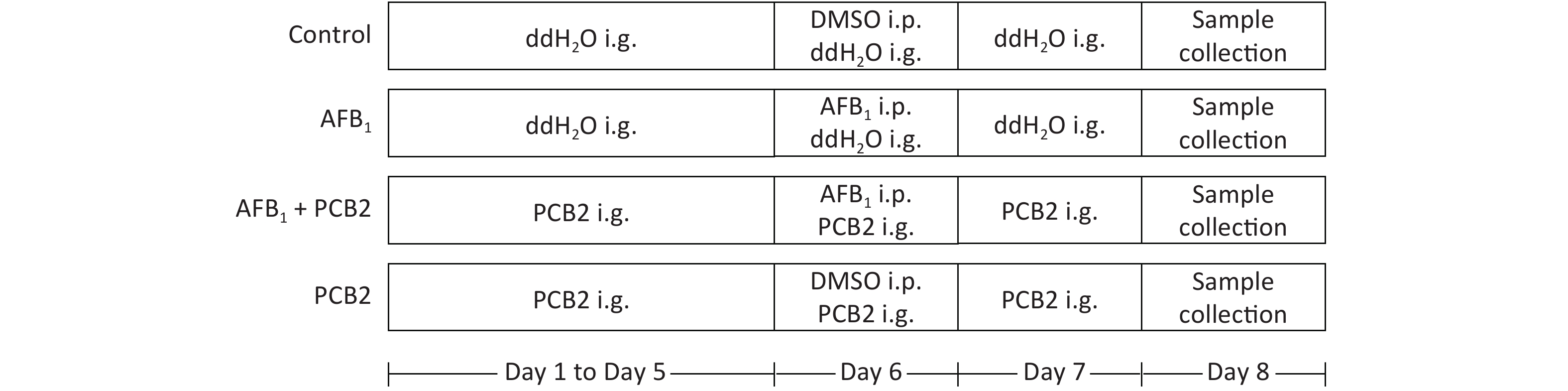
Six week-aged male Sprague Dawley (SD) rats were purchased from Guangxi Medical University Laboratory Animal Center (Nanning, China). All rats were housed in standard animal cages with free access to food and water under a strict 12 h light-dark cycle. They were acclimated to the animal facility environment for 1 week before experiments. SD rats were randomly divided into four groups (n = 10 each). The control and AFB1 groups were given double distilled water by gavage for 5 d consecutively. The AFB1 + PCB2 and PCB2 groups were administered PBC2 by gavage for 5 consecutive days (30 mg/kg, dissolved in double distilled water). After 5 d of gavage, the AFB1 and AFB1 + PCB2 groups were given single intraperitoneal injections of AFB1 (2 mg/kg, dissolved in DMSO) on the sixth day, whereas the control and PCB2 groups intraperitoneally received equal volume of DMSO. The AFB1 + PCB2 and PCB2 groups received daily intragastric administration of PCB2, whereas the other two groups received daily intragastric administration of double distilled water continually until the end of experiment. On the eighth day, the animals were euthanized. Blood samples were collected, and the serum was separated immediately. The liver tissue was isolated from each rat, washed in ice-cold saline, and stored at −80 °C for further experimental analyses. The dose, administration way, and exposure time of AFB1 mentioned earlier were based on study of Cui et al.[21] and Wang[22]. All experimental procedures were approved by the Animal Ethics Committee of Guangxi Medical University (Nanning, China). The whole procedure of animal treatment can be seen in Figure 1.
-
The body mass of all rats were weighed at a fixed time every morning. The whole liver, spleen, and kidney were weighed after being isolated from body, rinsed by ice-cold saline, and dried by filter paper. Weight gain was calculated as body weight after experiment minus the body weight before experiment. Liver coefficient, spleen coefficient, and kidney coefficient were calculated as liver mass/body mass, spleen mass/body mass, and kidney mass/body mass, respectively. The calculation result was expressed as g/100 g.
-
The whole blood remained still for 1 h at 4 °C after collection, and then, the serum was separated by centrifugation at 3,000 rpm for 15 min at 4 °C. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), direct bilirubin (DBIL), and alkaline phosphatase (ALP) were detected by Hitachi 7600-020 automatic biochemical analyzer, following the manufacturer’s protocol.
-
The excised liver tissue was fully fixed in 4% paraformaldehyde and then dehydrated, paraffin-embedded, sectioned, and stained with hematoxylin and eosin. The sections of liver tissue were observed with EVOS FL Auto Cell Imaging System.
-
Liver tissue was homogenized in ice-cold saline at a ratio of 1:9 (g:mL) and centrifuged at 2,500 rpm for 10 min at 4 °C. The supernatant was used for hepatic CAT, GSH, and SOD detection. The serum was used to measure MDA concentration. The measurements were performed according to the manufacturer’s instructions.
-
The serum level of interleukin-6 (IL-6) and hepatic level of 8-OHdG were measured by ELISA kits. Samples were prepared before performing ELISA test. For 8-OHdG determination, the required supernatant was obtained by homogenizing liver tissue in phosphate-buffered saline at a ratio of 1:9 (g:mL) and centrifuging at 5,000 rpm for 15 min. The serum was directly used to detect IL-6 concentration. The operation steps of ELISA include as follows: preparing standard solution for standard curve establishment, loading samples, washing plate, rendering color, terminating reaction, and determining the optical density value. All procedures were performed following the manufacturer’s instructions of ELISA kits.
-
Total RNA was extracted from the liver tissue by Eastep® Super Total RNA Extraction Kit. RNA reverse transcription was conducted by GoScript™ Reverse Transcription Mix, and qPCR was performed using GoTaq® qPCR Master Mix. The primers used in this study are listed in Table 1. PCR amplification was carried out by StepOne™ Real-Time PCR System for 40 cycles; the procedures included prevariation at 95 °C for 10 min, denaturation at 95 °C for 15 s, annealiation at 55 °C for 30 s, and extension at 72 °C for 30 s.
Gene Primer sequences (5′-3′) Product length (bp) IL-6 Forward: AGTTGCCTTCTTGGGACTGA 126 Reverse: CCTCCGACTTGTGAAGTGGT bcl-2 Forward: GACTGAGTACCTGAACCGGCATC 135 Reverse: CTGAGCAGCGTCTTCAGAGACA bax Forward: AGACACCTGAGCTGACCTTGGA 196 Reverse: TTGAAGTTGCCATCAGCAAACA β-actin Forward: GGAGATTACTGCCCTGGCTCCTA 150 Reverse: GACTCATCGTACTCCTGCTTGCTG Table 1. Polymerase chain reaction primers and the amplified product length
-
The excised liver tissue was homogenized in RIPA lysis buffer [1% Triton X-100, 1% deoxycholate, 0.1% sodium dodecylsulphate (SDS), and 1 mmol/L phenylmethylsulfonyl fluoride]. The lysate was centrifuged at 14,000 g for 10 min. The supernatant was used for protein quantification by BCA protein assay kit to ensure equal loading of total protein of each sample on a 12% SDS-polyacrylamide gel electrophoresis gel. The proteins were transferred to polyvinylidene fluoride membranes. Membranes were incubated with blocking buffer (20% skim milk) for 30 min at room temperature and then incubated with primary antibodies for bcl-2, bax, and β-actin overnight at 4 °C. Subsequently, the membranes were washed three times using 1× Tris-buffered saline with Tween buffer and incubated with secondary antibodies for 1 h at room temperature. The proteins on membrane were visualized by Western Lightning Plus ECL. The intensity of each protein band was quantified by densitometry using Image J software[23].
-
All data were expressed as mean ± standard deviation. Data were analyzed by one-way analysis of variance and followed by a least significant difference test. Statistical analysis was performed using SPSS 20.0 software. P < 0.05 was identified as statistically different.
-
The liver, spleen, and kidney index coefficients were elevated in rats exposed to AFB1 compared with the control group (all P < 0.01). PCB2 lowered the liver and kidney coefficients in AFB1-treated rats (all P < 0.01). The weight gain was inhibited by AFB1 (P < 0.01), while PCB2 could not significantly prevent it as shown in the AFB1 + PCB2 group versus the AFB1 group (P > 0.05; Figure 2A–D).
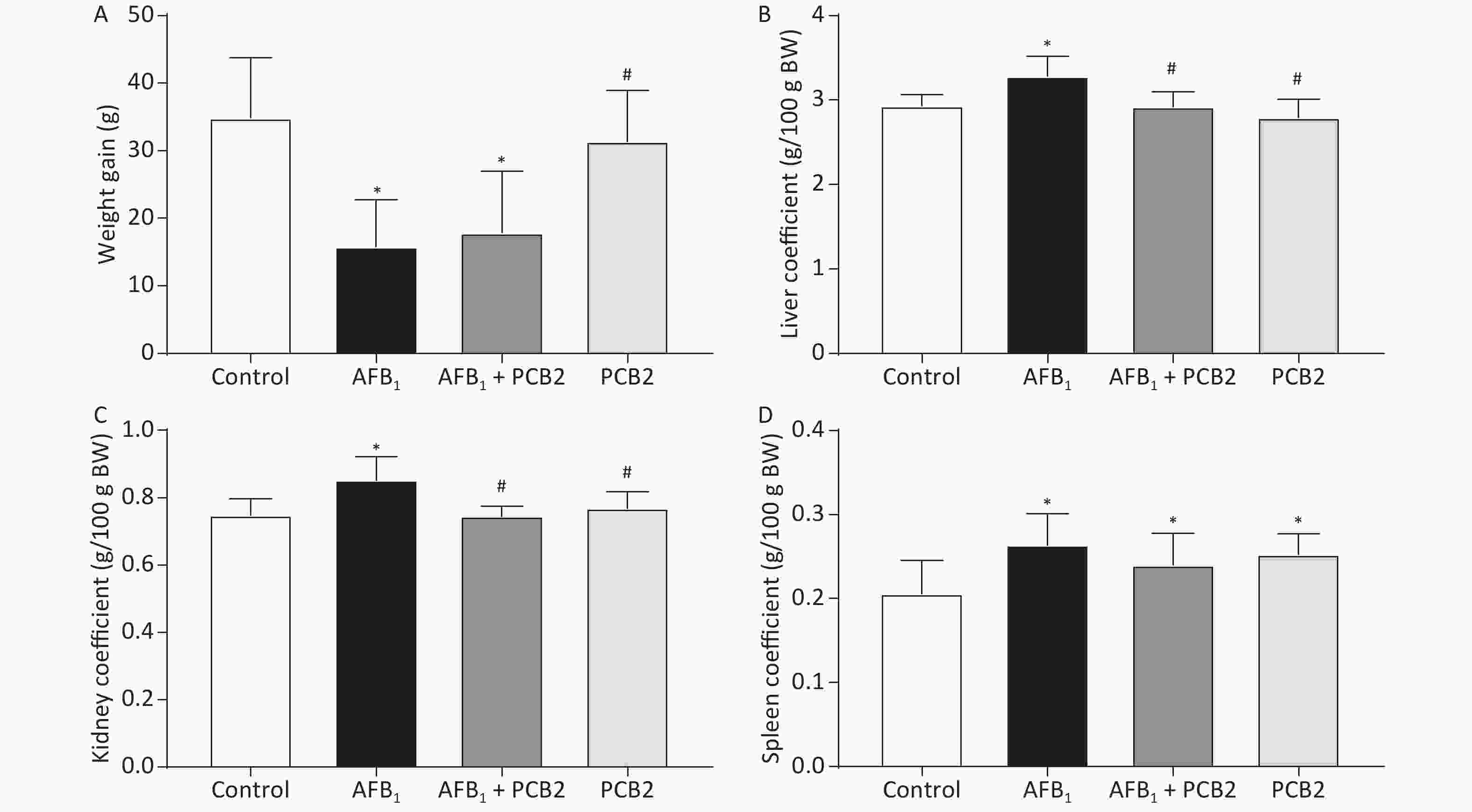
Figure 2. PCB2 reduced the abnormal AFB1-induced organ coefficient. (A) Total weight gain during the experiment, (B) liver coefficient, (C) kidney coefficient, and (D) spleen coefficient. The data are expressed as mean ± standard deviation. *P < 0.05 vs. the control group; #P < 0.05 vs. the AFB1 group. SD, standard deviation; AFB1, aflatoxin B1; PCB2, procyanidin B2.
-
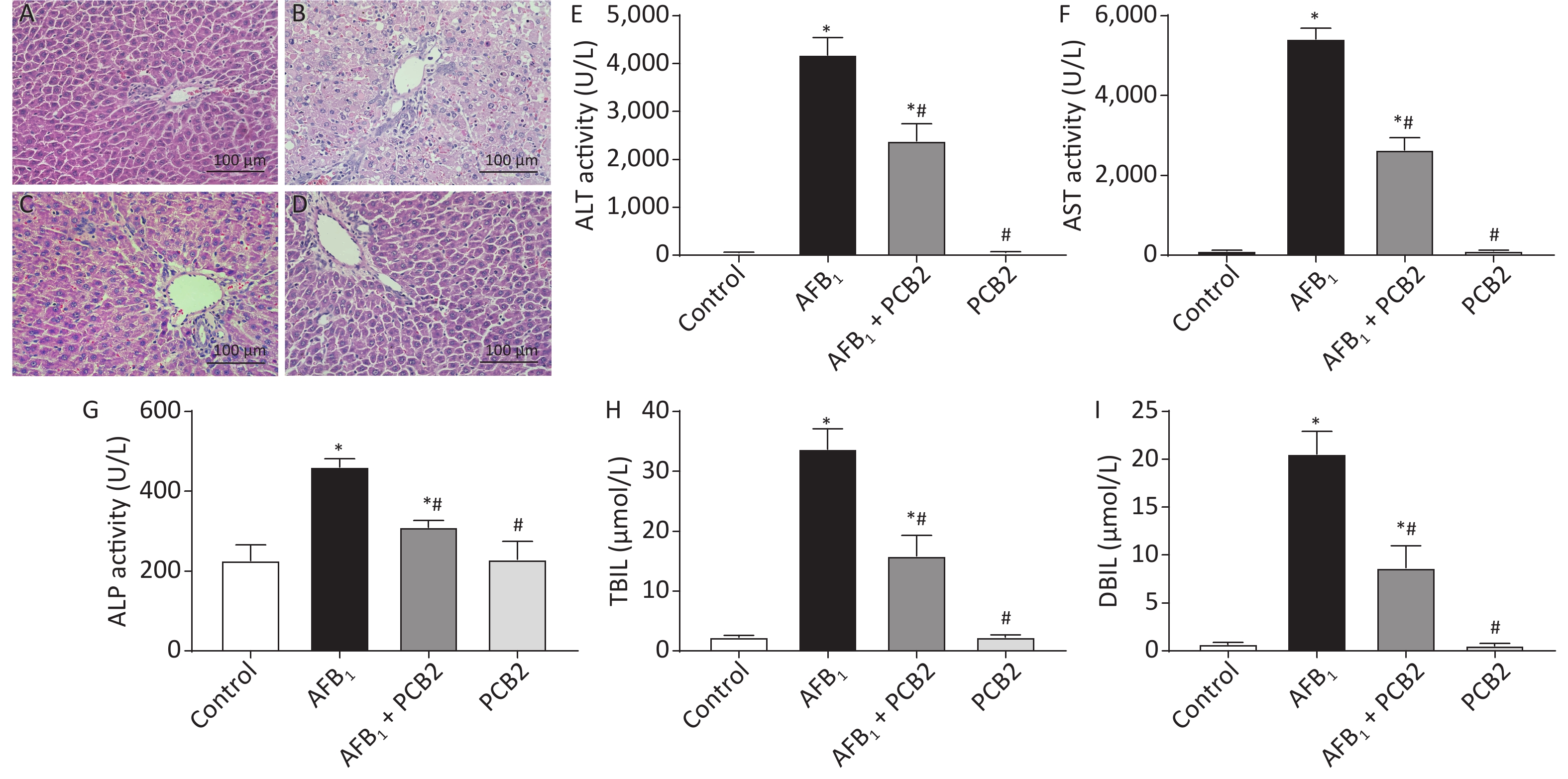
Histopathological change of liver sections was performed under the life EVOS FL Auto Cell Imaging System. The control and PCB2 groups showed normal liver architecture with even texture and polygonal hepatocytes. Liver injury in the AFB1 group was characterized by rough texture, spotty necrosis, ballooning degeneration of liver cells, and inflammatory cell infiltration, whereas the AFB1 + PCB2 group showed a reduction in these negative histopathological changes. The result indicated that the administration of PCB2 might have a protective effect on AFB1-induced histopathological damage (Figure 3A–D).
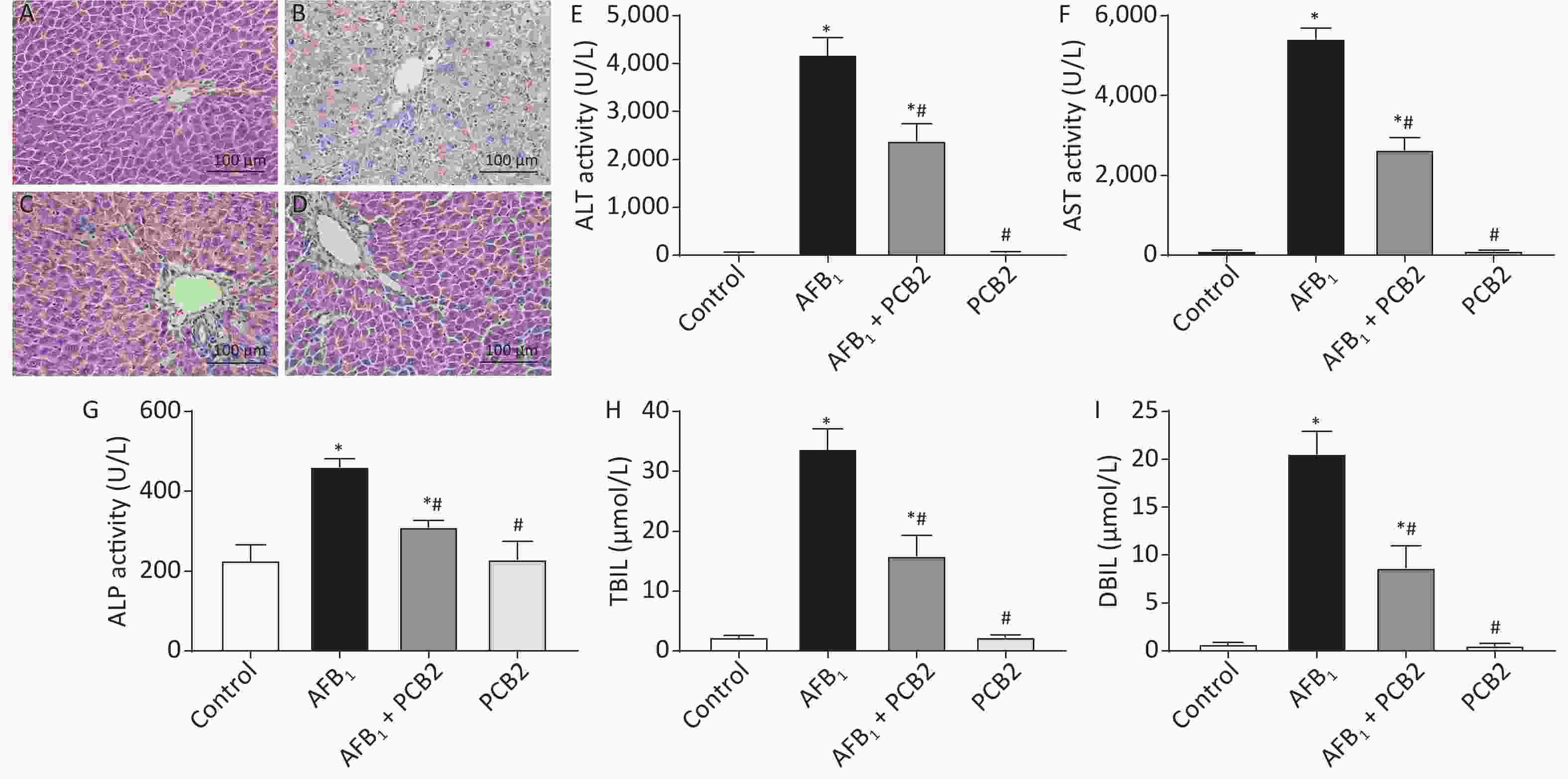
Figure 3. Procyanidin B2 (PCB2) ameliorated aflatoxin B1 (AFB1)-induced hepatic histopathological damage and abnormal liver function. Hematoxylin and eosin staining of the liver in the (A) control group, (B) AFB1 group, (C) AFB1 + PCB2 group, and (D) PCB2 group. (E) Serum alanine aminotransferase (ALT), (F) aspartate aminotransferase (AST), (G) alkaline phosphatase (ALP) activities, (H) total bilirubin (TBIL), and (I) direct bilirubin (DBIL) were measured. Scale bars: 100 μm. The data are expressed as mean ± standard deviation. *P < 0.05 vs. the control group; #P < 0.05 vs. the AFB1 group.
-
After exposure to AFB1, rats exhibited higher serum levels of ALT, AST, TBIL, DBIL, and ALP (all P < 0.01). PCB2 treatment decreased the serum levels of ALT, AST, TBIL, DBIL, and ALP (all P < 0.05); however, the levels detected were still above baseline (all P < 0.01). There was no statistical difference between the control and PCB2 groups (all P > 0.05). The results suggest that PCB2 could ameliorate AFB1-induced liver damage (Figure 3E–I).
-
Hepatic activities of CAT, SOD, GSH, 8-OHdG, and serum MDA were measured to access the extent of oxidation. CAT, GSH, and SOD levels were decreased in the AFB1 group compared with that in the control group (all P < 0.01), whereas the MDA and 8-OHdG levels were increased in the AFB1 group compared with that in the control group (both P < 0.01). PCB2 treatment could significantly reverse the decreased CAT, GSH, and SOD levels (all P < 0.05) and increased MDA and 8-OHdG levels as shown in the AFB1 + PCB2 group versus the AFB1 group (P < 0.01 or P < 0.05). The oxidative extent between the control and PCB2 groups was comparable (all P > 0.05). Since 8-OHdG is the biomarker of DNA oxidative damage, the results indicate that PCB2 could prevent the oxidative injury of liver tissue and DNA oxidative damage caused by AFB1 (Figure 4A–E).
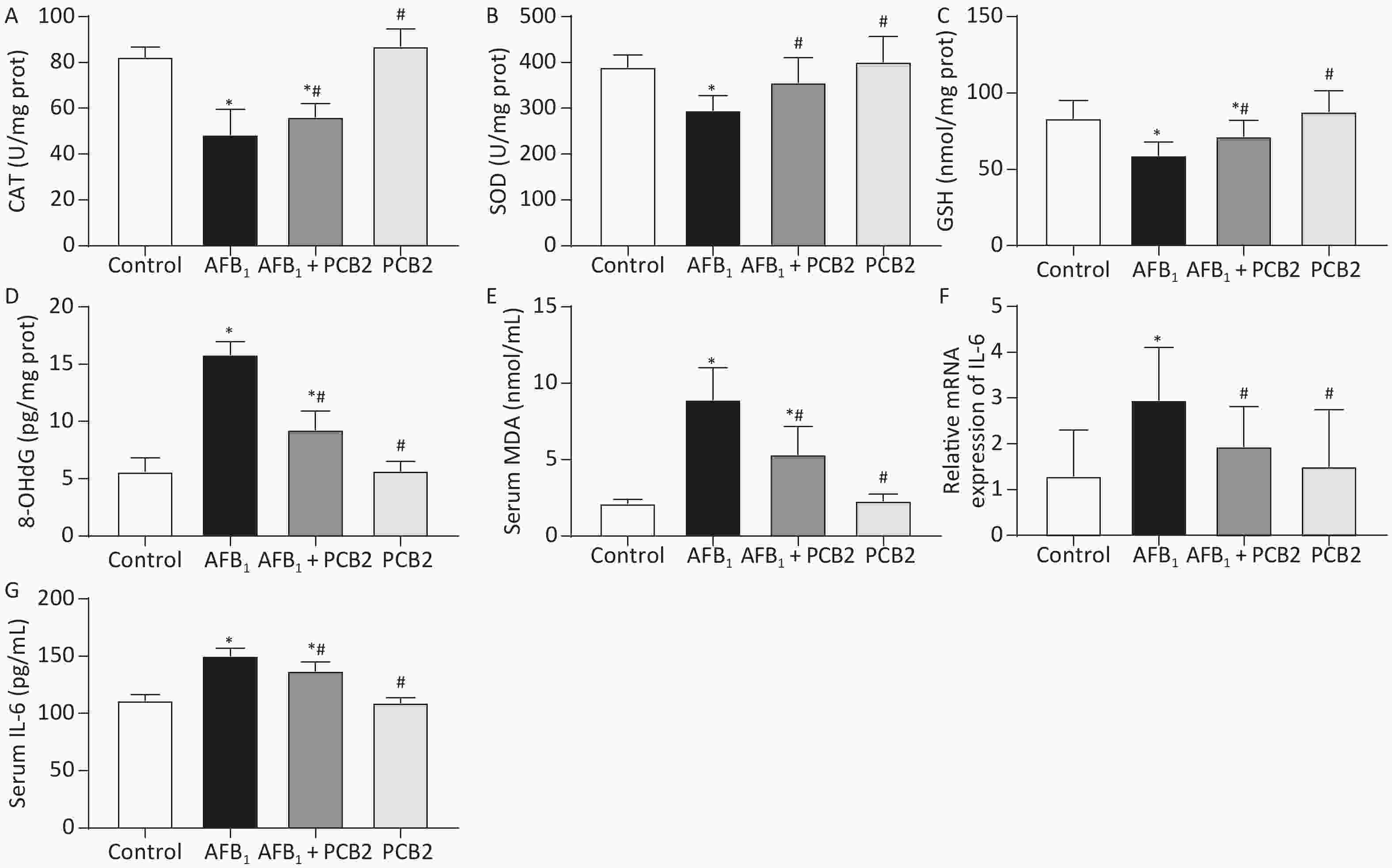
Figure 4. Procyanidin B2 (PCB2) suppressed aflatoxin B1 (AFB1)-induced oxidative injury and inflammatory response. (A) Hepatic catalase (CAT) activity, (B) hepatic superoxide dismutase (SOD) activity, (C) hepatic glutathione (GSH) content, (D) hepatic 8-hydroxy-2′-deoxyguanosine (8-OHdG) content, (E) serum malondialdehyde (MDA) content, (F) relative mRNA expression of IL-6, and (G) serum interleukin-6 (IL-6) concentration. The data are expressed as mean ± standard deviation. *P < 0.05 vs. the control group; #P < 0.05 vs. the AFB1 group.
-
AFB1 treatment significantly increased hepatic IL-6 mRNA expression and serum IL-6 compared with the control group (P < 0.05 or P < 0.01), whereas PCB2 treatment decreased the expression and levels of these two inflammatory response markers compared with the AFB1 group (P < 0.05 or P < 0.01). There was no significant difference between the control and PCB2 groups in IL-6 mRNA expression and serum levels of IL-6 (both P > 0.05). The results hinted that PCB2 could hinder inflammatory response induced by AFB1 (Figure 4F and G).
-
The mRNA and protein ratios of bcl-2/bax suggest apoptosis of hepatocytes. When the ratio decreases, it indicates that hepatocytes may undergo apoptosis. Compared with the control group, the mRNA and protein ratios of bcl-2/bax were significantly lower in the AFB1 group (both P < 0.01). However, the mRNA and protein bcl-2/bax ratios were higher in the AFB1 + PCB2 group than the AFB1 group (P < 0.01 or P < 0.05). The mRNA and protein bcl-2/bax ratios in the PCB2 group were comparable with those in the control group (both P > 0.05). The results revealed that PCB2 might decrease AFB1-induced apoptosis of hepatocytes (Figure 5A–G).
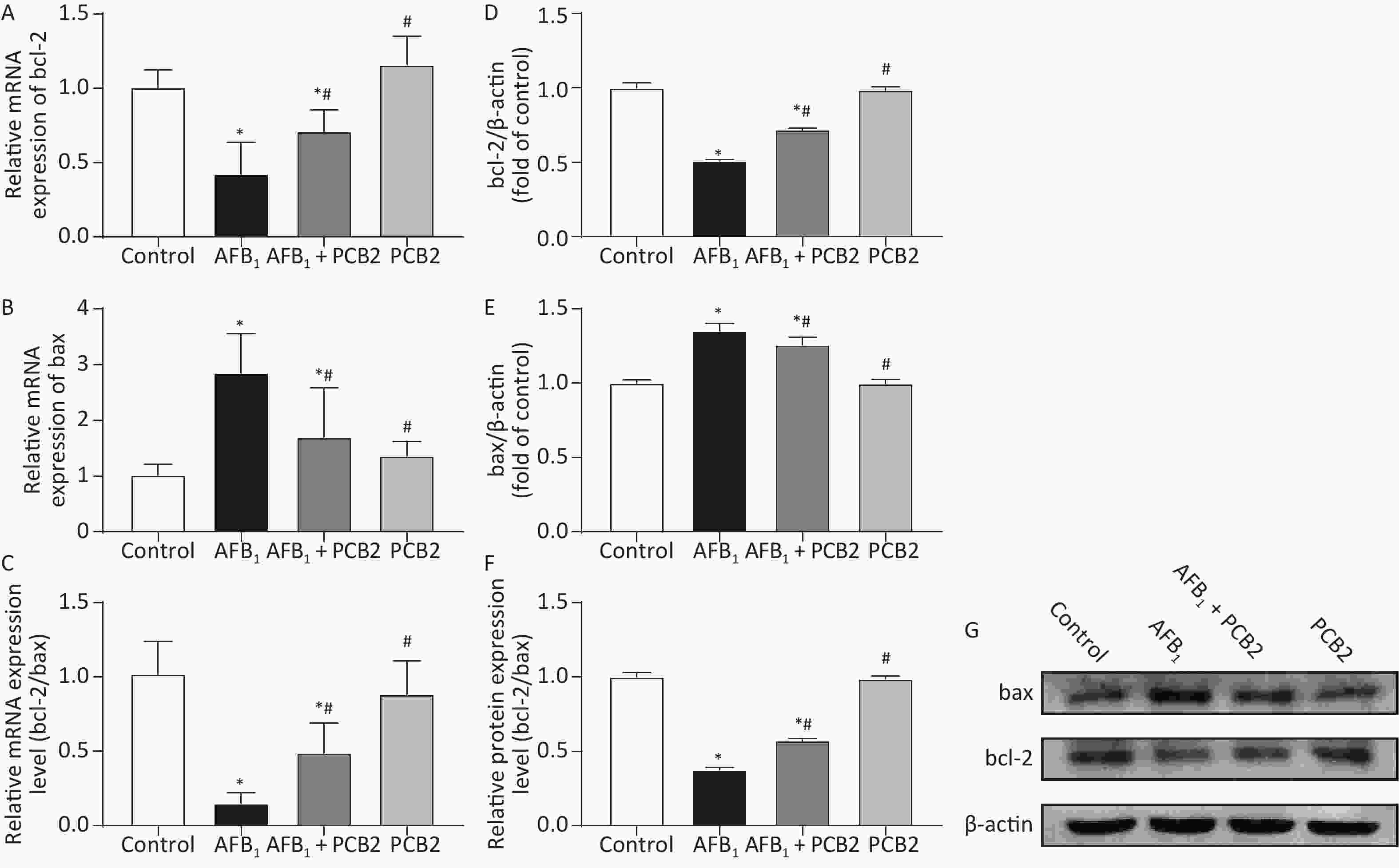
Figure 5. Procyanidin B2 (PCB2) decreased aflatoxin B1 (AFB1)-induced apoptosis of hepatocytes. Relative mRNA expression of (A) bcl-2, (B) bax, and (C) bcl-2/bax mRNA ratio were measured. Western blot analysis of (D) bcl-2, (E) bax, and (F) bcl-2/bax protein ratio were performed. (G) Protein strip of bax, bcl-2, and β-actin. The data are expressed as mean ± standard deviation. *P < 0.05 vs. the control group; #P < 0.05 vs. the AFB1 group.
-
AFB1 has been classified as a group 1 carcinogen, which is the top level of carcinogenicity categories, by the International Agency for Research on Cancer recently[24]. Previous studies have shown that AFB1 exerts carcinogenesis after being transformed into intermediate metabolites[3]. Meanwhile, the formation of AFBO and reactive oxygen species (ROS) deplete GSH, resulting in oxidative stress[5]. Histopathological change, liver dysfunction, and inflammation always exist in AFB1-induced hepatotoxicity[25-27]. In this study, PCB2 significantly ameliorated these adverse effects, which may partially explain its protective properties on AFB1-induced liver injury.
The organ coefficient is the ratio of organ mass to body weight, which reflects the influence of a substance on experimental animals. Changes in body weight and organ mass during the course of an experiment can affect the organ coefficient. In this study, the weight gain of the AFB1 group rats was lower than that of the control group, whereas the liver, spleen, and kidney coefficients were higher (Figure 2A–D), indicating that body weight change is one of the main factors affecting the organ coefficient of rats subjected to acute liver injury. Previous studies have also shown that AFB1 has a negative effect on weight gain[28, 29], probably due to the impaired liver function affecting appetite and biosynthesis. Compared with the AFB1 group, there was no significant change in the weight gain of the AFB1 + PCB2 group, but the liver and kidney coefficients decreased (Figure 2A–C), suggesting that PCB2 may reduce the abnormal weight gain of liver and kidney caused by AFB1.
Histopathological examination is the gold standard for determining hepatic damage. Once a liver injury occurs, hepatocytes may undergo edema, necrosis or apoptosis, and inflammatory cell infiltration[30]. AFB1 was reported to cause acute liver injury at an intraperitoneal injection dose of 1.5 mg/kg, characterized by the structure of hepatic lobule becoming abnormal, hepatocytes showing a balloon-like change, and bile duct hyperplasia in the portal area accompanied by inflammatory cell infiltration[31]. In our study, the pathological damage of liver tissue was observed 48 h after exposure to 2 mg/kg AFB1 in SD rats. The hepatocytes showed obvious balloon-like changes; the hepatic cord structure was destroyed; the texture of the tissue was disordered, and there were spotted necrosis and inflammatory cell infiltration (Figure 3B), which was similar to previous studies. While the clearer structure of liver tissue could be seen in the AFB1 + PCB2 group (Figure 3C), this finding indicates that PCB2 has a protective effect on AFB1-induced hepatic pathological damage.
Intracellular ALT, AST, and ALP are released into the peripheral blood, increasing the concentration of these enzymes in the serum when hepatocytes undergo damage. In addition, the ability of hepatocyte to ingest, transform, and excrete bilirubin is reduced, resulting in elevating levels of serum bilirubin[32, 33]. Therefore, serum ALT, AST, ALP, and bilirubin can serve as biomarkers of liver dysfunction. It was reported that when rats were exposed to AFB1, serum ALT, AST, and TBIL increased[34, 35]. The current study showed that the serum ALT, AST, ALP, TBIL, and DBIL of rats were elevated after the exposure to AFB1, whereas PCB2 administration could reduce these five indexes (Figure 3E–I), suggesting that PCB2 could ameliorate liver dysfunction.
GSH, CAT, and SOD are proteins or enzymes that scavenge oxygen free radicals and toxins in the body. GSH, composed of glutamate, cysteine, and glycine, is the main antioxidant of the human body exerting a detoxification effect[36]. SOD is an essential antioxidant enzyme widely found in living organisms, which catalyzes the disproportionation of superoxide anion to oxygen and hydrogen peroxide[37]. CAT is the enzyme that catalyzes the decomposition of hydrogen peroxide[38]. The concentration of these proteins or enzymes serves as a surrogate measure of the body’s ability to resist oxidative damage. MDA is the end product of lipid peroxidation, which reflects the degree of lipid peroxidation damage in the body[39]. 8-OHdG is the biomarker of DNA oxidative damage, mainly caused by ROS attacking the eighth carbon atom of the guanine base in DNA[40]. Oxidative damage induced by AFB1 can be monitored by detecting SOD, CAT, MDA, and 8-OHdG[41, 42]. Our study found that AFB1 leads to obvious oxidative damage with descending GSH, CAT, and SOD and elevating 8-OHdG and MDA, whereas PCB2 administration could inversely change the tendency (Figure 4A–E). Therefore, PCB2 may have a protective effect on AFB1-induced oxidative damage.
When inflammation occurs in the body, the immune system secretes a large number of cytokines into the blood, leading to an increasing concentration of these factors in the serum[43, 44]. Previous studies showed that AFB1 could promote inflammation via elevating the levels of interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α and involving the activated nuclear factor kappa B (NF-κB) signaling pathways by upregulation of toll-like receptor 2 (TLR2) and TLR4[45, 46]. IL-6 is an important pro-inflammatory factor mediating the acute inflammatory response and inducing B/T cell proliferation[47]. In our research, relative IL-6 mRNA expression and serum IL-6 levels increased in the AFB1 group compared with that of in the control group. Conversely, PCB2 treatment could decrease IL-6 gene expression and serum IL-6 level (Figure 4F and G). Long et al.[48] and Hinton et al.[49] observed that AFB1 treatment led to increased serum IL-6, whereas GSPE could lower the concentration of IL-6[48], which was similar to our findings. These results indicated that PCB2 could alleviate the inflammatory response caused by AFB1, but the underlying mechanism needs further research.
Bcl-2 and bax are proteins regulating the initiation of apoptosis. When the ratio of bcl-2/bax protein expression decreases, cells are more likely to undergo apoptosis[50]. Apoptosis is a biological process of programmed cell death[51]. According to previous studies, AFB1 invokes apoptosis in the process of exerting hepatotoxicity, which upregulation of p53, caspase-3, and bax and downregulation of bcl-2 can be measured[52]. Meanwhile, the apoptosis induced by AFB1 is related to the death receptor pathway[12] and the Nrf2 signal pathway[53]. In this study, the gene and protein expression of bcl-2 in single AFB1-treated rats decreased (Figure 5A–D), whereas the gene and protein expressions of bax increased (Figure 5B and E), resulting in a decreased bcl-2/bax ratio (Figure 5C and F). Compared with AFB1-treated rats, the gene and protein expressions of bcl-2 in PCB2 + AFB1 rats increased (Figure 5A and D), bax expression decreased (Figure 5B and E), and bcl-2/bax ratio increased (Figure 5C and F). It is speculated that AFB1 may cause apoptosis during the process of inducing acute liver injury; PCB2 could inhibit liver damage by reducing hepatocyte apoptosis. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay is a better method to detect tissue apoptosis[54, 55], thus, it would be better to apply it for further research.
GSPE has been proven to display a protective effect on AFB1-induced toxicity. Administration of GSPE was found to attenuate hepatotoxicity, immunotoxicity, and oxidative stress caused by AFB1 via modulating NF-κB and Nrf2 pathways, which restored liver dysfunction and contents of antioxidant enzymes and inhibited mRNA expression of inflammatory factors[13, 46, 48]. Although these findings indicate that GSPE may be a potent substance resistant to AFB1 toxicity, it remains unknown which components play the main role, for GSPE is a mixture of procyanidins. In this study, we observed that PBC2, one of the main components of GSPE, reduced serum AST, ALT, MDA, and IL-6 and elevated hepatic CAT, GSH, and SOD under the acute exposure of AFB1, exhibiting the similar characteristics of GSPE. Previous studies also showed that PCB2 exerted hepatoprotective effect through anti-oxidation and anti-inflammatory on liver injury induced by chemical substances like CCl4[17, 18]. In summary, PCB2 may protect against toxicant-induced liver damage mainly targeting oxidation and inflammation, while the mechanisms of which need further exploration.
In conclusion, this study reveals that PCB2 has an inhibitory effect on AFB1-induced acute liver injury, mainly in reducing histopathological damage, oxidative damage, inflammation, and probable apoptosis in hepatocytes. Therefore, PCB2 may serve as a potential phytochemical with anti-AFB1 toxicity. However, this study does not explore the dose-effect relationship between PCB2 and AFB1. Further research needs to be conducted to address this relationship.
-
The authors declared no conflict of interests.
-
DENG Zhi Ji and ZHAO Jing Fang conducted most experiments, DENG Zhi Jie wrote the paper; HUANG Feng assisted in the completion of animal experiments; SUN Gui Li and GAO Wei collected the experiment data; LU Li and XIAO De Qiang designed this study, LU Li revised this paper.
Protective Effect of Procyanidin B2 on Acute Liver Injury Induced by Aflatoxin B1 in Rats
doi: 10.3967/bes2020.033
- Received Date: 2019-10-24
- Accepted Date: 2020-02-05
-
Key words:
- Procyanidin B2 /
- Aflatoxin B1 /
- Acute liver injury /
- Oxidative stress /
- Inflammation
Abstract:
| Citation: | DENG Zhi Jie, ZHAO Jing Fang, HUANG Feng, SUN Gui Li, GAO Wei, LU Li, XIAO De Qiang. Protective Effect of Procyanidin B2 on Acute Liver Injury Induced by Aflatoxin B1 in Rats[J]. Biomedical and Environmental Sciences, 2020, 33(4): 238-247. doi: 10.3967/bes2020.033 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: