-
Salmonella enterica serovar Enteritidis (S. Enteritidis
) is a major etiological agent of acute gastroenteritis in humans; the mode of infection being consumption of undercooked or improperly handled poultry meat or contaminated eggs or milk[1, 2]. Among more than 2,600 serotypes of Salmonella, S. Enteritidis frequently causes Salmonella infections in humans[3]. S. Enteritidis can invade host epithelial cells through the expression of virulence genes and causes diarrhea, endocarditis, meningitis, urinary tract infections, and even death in severe cases[4]. A better understanding of the pathogenesis of salmonellosis and development of new therapeutic strategies for S. Enteritidis infections will have important public health implications. A variety of animal models such as rhesus monkey, calf, and mouse models have been used to study the virulence of S. Enteritidis and host defense mechanisms. Among these, mouse models have been widely used due to their high degree of genetic homology with humans and similar tissue distribution of bacteria[5, 6]. In this study, S. Enteritidis isolate LN-248-0, which was identified as a causative agent for salmonellosis in humans in Liaoning Province, China in 2018, was isolated from a retail chicken product. This isolate had distinctive characteristics including high ability to invade intestinal epithelial cells and moderate replication capacity within macrophages. In addition, the isolate was sensitive to all 17 tested antibiotics. Furthermore, whole genomic analysis of LN-248-0 identified a 59-kbp plasmid carrying multiple virulence genes, including genes encoding type III and IV secretion system effectors, adhesins, fimbriae, and immune escape effectors. These distinctive features prompted us to further characterize its pathogenicity in mice. Therefore, we investigated the effect of oral infection caused by LN-248-0 in BALB/c mice (aged 6–8 weeks) to better understand the progression of salmonellosis induced by LN-248-0. All animal experiments and procedures related to animal use were approved by the Animal Ethical and Experimental Committee of Ludong University (license number LDU-IACUC2018007) and were based on the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
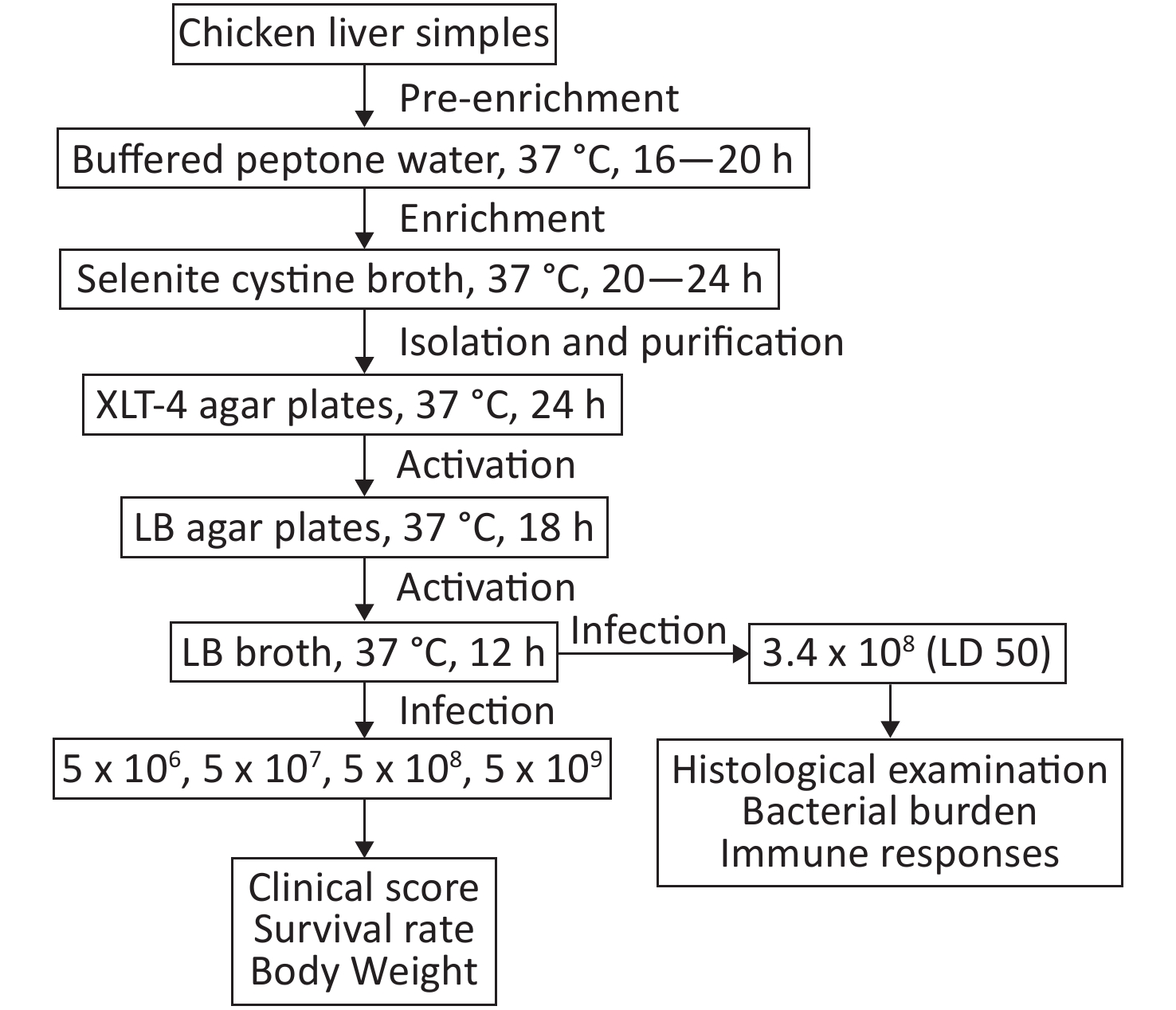
Twenty-five samples of frozen chicken liver were collected from retail stores located in Liaoning Province. Samples were transported to the laboratory immediately after collection and were kept frozen until analysis. The samples were thawed by overnight incubation in refrigerator. Twenty-five grams of each sample were placed into a stomacher bag containing 225 mL buffered peptone water and homogenized using a stomacher at 37 °C for 16 to 20 h. One milliliter of this homogenate was transferred to 10 mL of selenite cystine broth and incubated for 20–24 h at 37 °C. Plating was carried on xylose lysine tergitol-4 (XLT-4) agar and incubated at 37 °C for 24 h. The plates were examined for typical colonies of Salmonella (red with black center). Presumptive Salmonella colonies were confirmed using VITEK 2 system (bioMérieux). LN-248-0 was cultured in Luria–Bertani (LB) agar plates, and a single colony from the plate was inoculated for overnight culture in LB broth. The bacterial cultures that had reached saturation density were diluted (1/100) and cultured to mid-logarithmic growth phase (OD600, 0.4–0.6). Bacterial cultures were centrifuged, and the cell pellet was washed in phosphate-buffered saline (PBS) twice before use. Ten-fold dilutions (5 × 106 to 5 × 109 CFU) of LN-248-0 were administered to mice by oral gavage (Supplementary Figure S1, available in www.besjournal.com). The clinical scores were calculated as described previously[7].
Liver, spleen, lung, kidney, cecum, and feces of the mice were homogenized with 200 μL of sterile 0.1% Triton-100, plated onto XLT-4 agar plates, and incubated at 37 °C for 24 h to determine bacterial burden.
For isolation of mouse hepatic mononuclear cells (MNCs), the isolated livers were pressed through a 200-gauge stainless steel mesh and suspended in RPMI 1,640 medium, followed by centrifugation at 50 × g for 3 min. Supernatants containing hepatic MNCs were collected, resuspended in 40% Percoll (Sigma-Aldrich, St. Louis, MO), and centrifuged at 750 × g for 30 min. The cell pellet was collected and incubated in red blood cell lysis buffer at 4 °C for 10 min and washed twice with PBS.
For molecular staining of cell surfaces, the isolated mouse hepatic MNCs were blocked and incubated with the indicated fluorescent mAbs. The antibodies utilized were as follows: FITC-conjugated anti-CD3 mAb, PE-conjugated anti-CD4 mAb, PerCP-Cy5.5-conjugated anti-CD8 mAb, PE-conjugated anti-CD19 mAb, and APC-conjugated anti-CD69 mAb. All fluorescent antibodies were obtained from eBioscience, CA, U.S.A. The stained cells were analyzed using a flow cytometer (FACSCalibur, BD Biosciences, San Jose, CA, USA), and FlowJo software was used for data analysis (FLOWJO, Ashland, OR, USA).
All values are presented as mean ± SEM of three independent experiments. All analyses were performed using GraphPad Prism (San Diego, CA, USA). Survival data were analyzed using the log-rank test. Clinical scores were analyzed for statistical significance using two-way analysis of variance (ANOVA). Statistical analysis was performed using a paired Student's t-test. Results with P value < 0.05 were considered statistically significant.
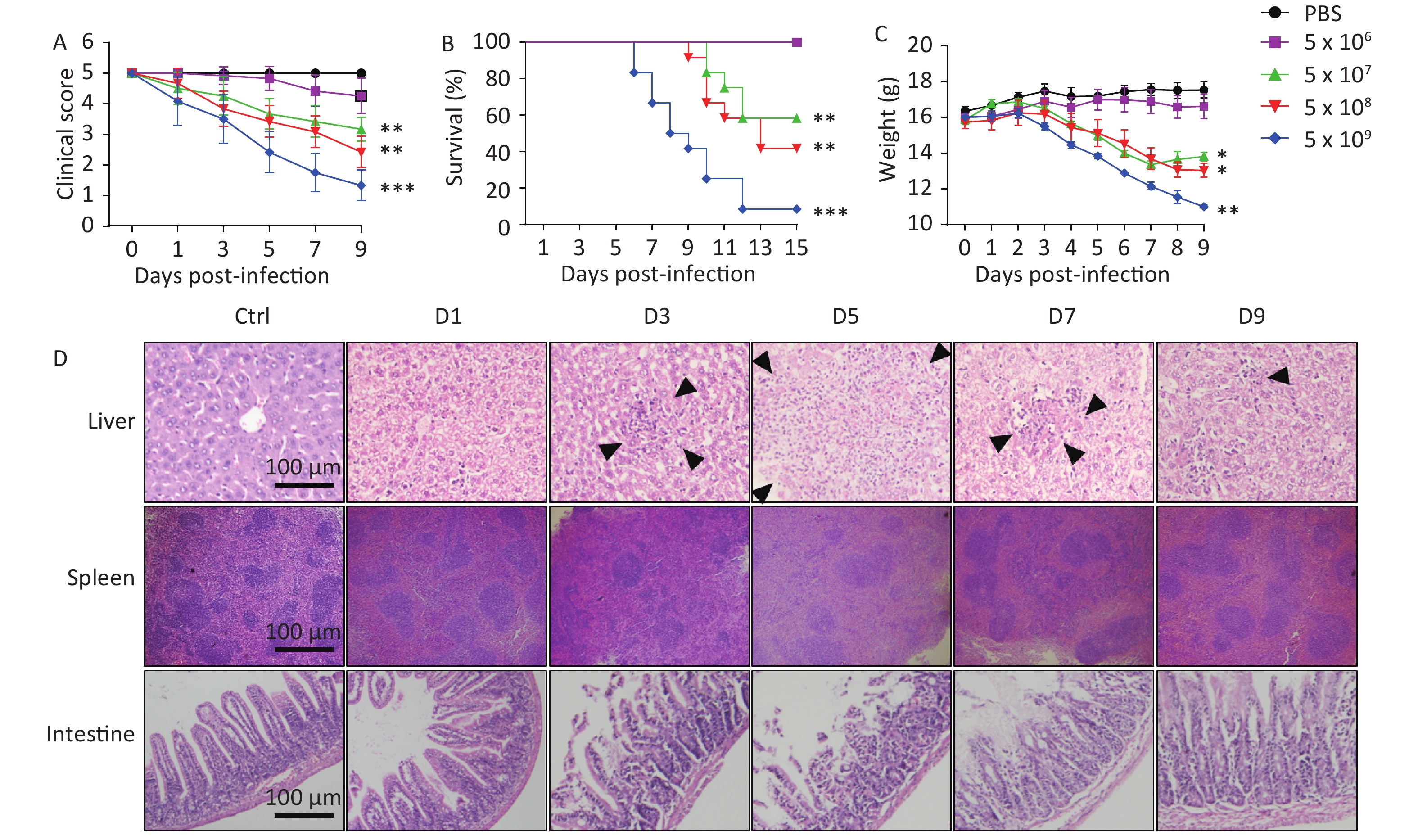
To evaluate the virulence of the isolate, BALB/c mice were infected with 5 × 106 to 5 × 109 CFU of LN-248-0. We found that the clinical score gradually decreased when the infectious dose was greater than or equal to 5 × 107; the most significant decrease was observed when the infectious dose was 5 × 109 (Figure 1A). Survival times were recorded to the nearest half day, over an observation period of 15 days. The highest mortality (91.7%) was observed for mice challenged orally with the highest infectious dose (5 × 109 CFU), followed by mice challenged with doses of 5 x 108 (58.3%) and 5 × 107 CFU (41.7%) (Figure 1B). The body weight of mice infected with 5 × 107, 5 × 108, and 5 × 109 CFU of S. Enteritidis significantly decreased as compared to that of control mice (Figure 1C). In contrast, all mice challenged orally with S. Enteritidis at the minimum infectious dose (5 × 106 CFU) survived, with no reduction in body weight (Figure 1B–C). The LD50 of LN-248-0, calculated by the method of Reed and Muench, was 3.4 × 108 CFU for BALB/c mice. These results suggest that oral administration of LN-248-0, at certain doses, can cause lethal infection in BALB/c mice.

Figure 1. Virulence of S. Enteritidis isolate LN-248-0 in BALB/c mice after oral infection. BALB/c mice were orally challenged with 5 × 106, 5 × 107, 5 × 108, or 5 × 109 CFU of LN-248-0 or PBS (n = 10 for PBS group and n = 12 for all other groups) on day 0. The clinical score (A), survival rate (B), and weight (C) of the mice were monitored at different days. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, compared to the PBS group. These data represent one of three independent experiments. (D) Histological examination of hepatic, splenic, and intestinal tissues. BALB/c mice were orally challenged with 3.4 × 108 CFU of LN-248-0 on day 0. Hematoxylin and eosin staining was performed on hepatic, splenic, and intestinal sections from control mice and mice infected with LN-248-0 at days 1, 3, 5, 7, and 9 post infection. Black arrows indicate the histological changes in the liver. Data are representative of three independent experiments.
Next, mice were infected orally with the LD50 of LN-248-0 and sacrificed at the indicated time shown in Figure 1D. Detailed histopathological changes in the liver, spleen, and intestine of the mice were explored. As shown in Figure 1D, on the day 3 post infection, mild swelling of the hepatocytes was observed, and small areas of parenchymal necrosis were randomly scattered throughout the liver. By the 5th day, the hepatic lesions were enlarged and more numerous. The early stages of transformation of smaller microabscesses into granulomas were obvious by day 7. The granulomas persisted until day 9, however, the size of the granulomas decreased and many of them showed central necrosis. Similarly, the spleen was also congested and showed minute foci of necrosis disseminated throughout both white and red pulps on the 3rd day post infection, and the lesions were enlarged on day 5. The white pulps appeared enlarged due to cellular proliferation, and the border between the white pulp and the red pulp began to disappear on the day 3 post infection, which exacerbated on day 5. On day 7 post infection, the splenic architecture started to improve. Histopathological observation of the small intestine revealed gradually increasing inflammatory cell infiltration in the mucosa and lamina propria of the lower small intestine in mice during the first 5 days. Moreover, the integrity of the epithelial barrier and the intestinal villi were severely damaged at day 5 post infection. Gradual recovery of the basic structure of the small intestine was observed on day 7. Collectively, these results suggest that LN-248-0 infection causes pathological changes in different organs and induces systemic infection in BALB/c mice.
After infection with LN-248-0, a general trend of increasing bacterial numbers was found in the liver, spleen, lung, and kidney during the first 5 or 7 days post infection, indicating successful bacterial infection. Subsequently, some mice died due to a more severe infection, whereas other mice showed a gradual decrease in bacterial load and an improvement in their physiological state from day 5 or 7 to day 9 post infection. In contrast, the bacterial load of cecum and feces decreased sharply on day 3 post infection as compared to day 1, and thereafter increased gradually until day 7 post infection, when the number of bacteria tended to decrease, thus indicating slight recovery from the infection (Table 1). We speculated that the high number of bacteria on day 1 post infection was due to the oral gavage and partial bacteria were excreted in feces, whereas others colonized the intestine and reproduced in the next few days. The number of bacteria peaked on day 7 post infection and decreased subsequently due to host immune response.
Organs 1 dpi 3 dpi 5 dpi 7 dpi 9 dpi Liver 2.70 ± 0.27 3.08 ± 0.11 3.86 ± 0.28 3.60 ± 0.42 3.39 ± 0.39 Spleen 4.12 ± 0.35 4.55 ± 0.32 5.65 ± 0.21 5.65 ± 0.31 3.98 ± 0.44 Lung 4.11 ± 0.67 4.29 ± 0.51 4.98 ± 0.53 5.55 ± 0.76 4.09 ± 0.65 Kidney 3.15 ± 0.55 3.74 ± 0.32 4.52 ± 0.51 3.67 ± 0.61 3.07 ± 0.50 Cecum 4.33 ± 0.50 2.31 ± 0.15 4.89 ± 0.50 6.06 ± 0.13 4.92 ± 1.09 Feces 5.38 ± 0.40 4.42 ± 0.17 4.36 ± 0.14 5.63 ± 0.32 5.32 ± 0.24 Note. dpi, days post infection. Table 1. The numbers of bacteria per gram of tissues on the 7th day post LN-248-0 infection (Lg10) (CFU/g)
To explore the involvement of hepatic T/B cell responses in mediating protective immunity against LN-248-0, we examined the percentage and activation of CD4+ T cells, CD8+ T cells, and B cells in the liver of mice infected with LN-248-0 using flow cytometry. As shown in Figure 2A, the isolated hepatic MNCs were first gated (FSC-H vs. SSC-H) to select lymphocytes and further gated on CD3+CD4+ for CD4+ T cells, CD3+CD8+ for CD8+ T cells, and CD3−CD19+ for B cells. Next, expression of CD69, an early activation marker of T/B cells, was analyzed to evaluate host immune response. The percentage of B cells decreased with an increase in the number of days post infection, possibly due to the recruitment of T cells and monocytes. The activation of B cells did not change significantly during the first 5 days but notably decreased after day 7 post infection (Figure 2B). In contrast, the percentage and activation of CD8+ T cells in the liver increased significantly on the day 7 and day 5 post infection respectively (Figure 2C). Similarly, the proportion of CD4+ T cells in the liver increased on day 7 post infection (Figure 2D), but the activation of CD4+ T cells did not change significantly (data not show). These results strongly suggest that S. Enteritidis isolate, LN-248-0, was able to activate adaptive immune responses in BALB/c mice.

Figure 2. Immune responses of BALB/c mice infected with LN-248-0. BALB/c mice were orally challenged with 3.4 × 108 CFU of LN-248-0 on day 0. (A) Gating strategy was used to identify CD4+ T cells, CD8+ T cells, and B cells in the liver of BALB/c mice. (B–D) Percentage and activation of B cells (B) and CD8+ T cells (C) and percentage of CD4+ T cells (D) in the liver were determined using flow cytometry. (E, F) BALB/c mice were orally challenged with 3.4 × 108 CFU of LN-248-0 on day 0. On days 0, 1, 3, 5, 7, and 9 post infection, serum levels of IL-6 (E) and IFN-γ (F) were measured using ELISA. Data are represented as mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, versus mice infected at day 0.
Several in vitro and in vivo studies have reported an inflammatory immune response to Salmonella infections by demonstrating an upregulation in the expression of the inflammatory cytokines, IL-1β and IL-6, chemokine IL-8, and the Th1 cytokines, IL-2, IFN-γ, and IL-12 in immune cells isolated from peripheral blood or lymphoid tissues[8, 9]. In our study, IL-6 and IFN-γ expression levels in the serum were measured using ELISA on different days post infection. As shown in Figure 2E and 2F, serum IL-6 levels gradually increased during the first 5 days post infection and subsequently decreased thereafter, indicating that the inflammatory response and inflammation-mediated pathological injury were probably most severe on day 5 post infection. In contrast, IFN-γ expression level was significantly enhanced since day 5 post infection and was maintained at a high level on days 7 and 9 post infection.
It is well known that Salmonella is a zoonotic pathogen that can spread to other animals via the food chain. Once introduced into the population, it can have widespread and serious consequences[10]. S. Enteritidis isolate, LN-248-0, responsible for causing salmonellosis in humans in Liaoning province in 2018, was studied for the first time in this study after obtaining it from the liver of a diseased retail chicken. Contamination of the chicken with LN-248-0 could have occurred during breeding, slaughter, or circulation. It is believed that this facilitated the dissemination of Salmonella into the food chain and its eventual transmission to humans. Furthermore, our study proved that LN-248-0 could induce pathological damages, activate immune system, and induce inflammatory cytokine secretion in BALB/c mice. These results indicate the intrusive and damaging effects of LN-248-0 infection on the host. Herein, we isolated LN-248-0 and evaluated its virulence in BALB/c mice, laying the basis for further studies on its virulence in humans.
Collectively, we established a BALB/c mouse model for studying infection by S. Enteritidis isolate, LN-248-0, and explored its bacterial virulence and host immune responses. The findings of the present study demonstrated that LN-248-0 isolated from chicken liver could successfully infect BALB/c mice, leading to obvious clinical symptoms at high infectious doses. At the LD50, LN-248-0 induced pathological damages to different organs and immune activation in BALB/c mice. The BALB/c mouse model of S. Enteritidis infection established in this study provides an experimental animal model for the study of S. Enteritidis infection and pathogenesis and lays a foundation for further research.
Virulence of Salmonella enterica Serovar Enteritidis Isolate LN-248-0 and Immune Responses in BALB/c Mice
doi: 10.3967/bes2020.083
- Received Date: 2019-11-24
- Accepted Date: 2020-05-21
| Citation: | LIU Yang, LI You Zhi, FENG Tao, GONG Pi Qian, JIN Ying Cai, ZHU Hong Wei, JIANG Lin Lin, ZHANG Jian Long, CHEN Guo Zhong, YU Xin, ZHANG Xing Xiao. Virulence of Salmonella enterica Serovar Enteritidis Isolate LN-248-0 and Immune Responses in BALB/c Mice[J]. Biomedical and Environmental Sciences, 2020, 33(8): 628-632. doi: 10.3967/bes2020.083 |








 Quick Links
Quick Links
 DownLoad:
DownLoad: