-
According to the report of the International Diabetes Federation, there were 382 million people affected with diabetes in 2013 and it is expected that this number will increase to 592 million by the year 2035[1]. Diabetes is caused due to the interaction between environmental and genetic factors[2]. Residues of organophosphate pesticides (OPs), which constitute 40% of the pesticide market, have been detected in various environments such as air, soil, water bodies, vegetables, and tissues of humans and animals[3]. Moreover, several animal and human studies have demonstrated the association between exposure to OPs and the prevalence of diabetes[3]. Velmurugan et al. identified glucose intolerance through gluconeogenesis induced by gut microbial degradation of organophosphate insecticides as one of the mechanisms underlying OP-induced hyperglycemia[3]. Similarly, Peris-Sampedro et al. showed that APOE3 mice exposed to chlorpyrifos were most likely to develop obesity and related disturbances[4]. Our previous research has indicated that organophosphates could cause oxidative stress and ER stress, which could compromise insulin signaling in muscles[5]. Other studies have also suggested that hepatic insulin resistance may precede skeletal muscle insulin resistance and that hepatic insulin resistance is a major contributing factor to the development of T2D[6]. However, the mechanism by which organophosphates affect liver insulin signaling has not yet been completely elucidated. Therefore, this study was conducted to investigate the effects of organophosphates on liver insulin signaling by analyzing the changes in protein expression of major elements in insulin signaling, such as insulin receptor substrate (IRS), phosphatidylinositol 3-kinase (PI-3K), Akt, glucose transporter (GLU2, GLUT4), and two key players involved in the regulation of glucose and lipid metabolism, PPAR-r and PGC-1α.
The animal experimental procedures were approved by the Institutional Review Board of School of Public Health, Jilin University (Changchun, Jilin Province). A total of 60 ICR male mice weighing 18-22 g were purchased from Beijing Huafukang Bioscience CO.INC. The mice were allowed to acclimatize for 7 days, numbered, kept in light/darkness conditions (12 h each), and allowed free access to water and food. Mice in the control group received gastric injection of phosphate buffer saline (PBS), mice in the vehicle group were administered cotton oil, and mice in the treatment group were injected with omethoate via the gastric route at dosages of 0.5, 1, and 2 mg/kg bw for the low-dosage, medium-dosage, and high-dosage groups, respectively (each group consisted of 12 mice, and the gastric injection was performed once a day for 60 consecutive days).
After treating with omethoate for 60 days, the mice were fasted for 12 hours and then anesthetized using pentobarbital sodium at the dose of 30 mg/kg body weight. The serum glucose level, the insulin level, and the homeostasis model assessment of insulin resistance were determined according to the methods described by Zhang et al.[5]. The plasma acetylcholinesterase activity (AChE) was determined spectrophotometrically using a commercial kit manufactured by Nanjing Jiancheng Bioengineering Institute. The protein expression was determined by western blotting using β-actin as the reference. The levels of total cholesterol (TC) and triglycerides (TG) were determined using an automatic biochemistry analyzer. The levels of glucose and lipids, the body weight of the mice, and the protein expression levels were compared using one-way ANOVA, and comparison between two groups was performed by Student-Newman-Keuls (SNK) or least significant difference (LSD) test using SPSS 21.0 software (SPSS Inc., Chicago Illinois, USA).
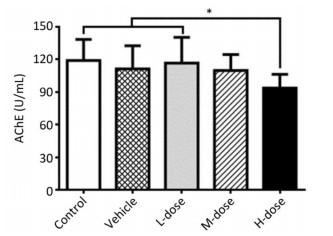
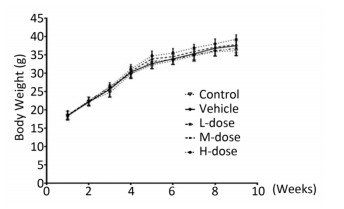
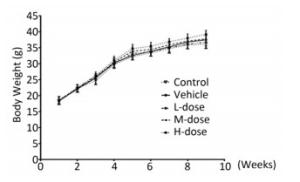
Results showed that the body weight of the mice increased with increase in the duration of the experiment; however, there was no significant difference in body weight among the different groups at the end of the experiment (Supplement Figure S1 available in www.besjournal.com). Omethoate exposure at the selected dosages had no influence on the ratio of organ to body weight (Supplement Table S1 available in www.besjournal.com). In addition, inhibition of the acetylcholinesterase activity was detected only in the high-dosage group (Supplement Figure S2 available in www.besjournal.com).
Organ Ratio of Organ to Body Weight F P Control Vehicle L-dose M-dose H-dose Liver 0.0503 ± 0.0056 0.0429 ± 0.0060 0.0412 ± 0.0074 0.0432 ± 0.0053 0.0404 ± 0.0096 2.596 0.052 Pancreas 0.0052 ± 0.0014 0.0058 ± 0.0009 0.0063 ± 0.0009 0.0062 ± 0.0008 0.0062 ± 0.0007 1.899 0.131 Spleen 0.0047 ± 0.0013 0.0050 ± 0.0023 0.0040 ± 0.0013 0.0049 ± 0.0024 0.0047 ± 0.0016 0.413 0.798 Kidney 0.0149 ± 0.0015 0.0147 ± 0.0014 0.0142 ± 0.0010 0.01437 ±0.0012 0.0136 ± 0.0015 1.207 0.324 Table Supplemental Table S1. Comparison of Ratio of Organ to Body Weight in Mice Among Different Groups (n = 10)
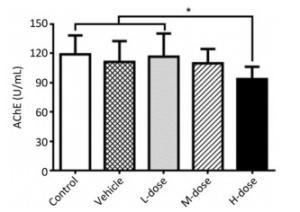
Figure Supplemental Figure S2. Acetylcholinesterase activity inhibition by omethoate. n = 10, *P < 0.05.
As shown in Table 1, exposure to omethoate for 60 days at the selected dosages had no significant effect on the levels of glucose and fasting insulin. There were also no significant changes in HOMA-IR among the groups treated with different dosages of omethoate, which indicated that omethoate exposure at the selected dosages for 60 days did not cause systemic insulin resistance.
Items Control Vehicle L-dose M-dose H-dose F P Glucose (mmol/L) 4.48 ± 0.35 4.21 ± 0.53 4.56 ± 0.69 4.34 ± 0.49 4.60 ± 0.42 0.755 0.561 FINS (mIU/L) 12.99 ± 1.17 13.93 ± 1.05 13.47 ± 1.35 12.89 ± 1.84 13.41 ± 1.53 0.657 0.625 HOMA-IR 2.59 ± 0.35 2.62 ± 0.43 2.75 ± 0.38 2.50 ± 0.35 2.78 ± 0.51 0.646 0.633 TC (mmol/L) 2.78 ± 0.67 3.01 ± 0.38 2.78 ± 0.40 3.42 ± 0.41ac 4.33 ± 0.66abcd 13.508 0.000 TG (mmol/L) 1.42 ± 0.30 1.46 ± 0.29 1.52 ± 0.34 1.71 ± 0.36 2.28 ± 0.41abcd 5.034 0.002 HDL (mmol/L) 3.46 ± 0.31 3.12 ± 0.25 2.78 ± 0.29ab 2.67 ± 0.20ab 2.47 ± 0.45abc 12.909 0.000 LDL (mmol/L) 0.97 ± 0.12 1.09 ± 0.21 1.04 ± 0.16 1.33 ± 0.24abc 1.42 ± 0.28abc 5.847 0.001 Note. aP < 0.05 vs. Control, bP < 0.05 vs. Vehicle, cP < 0.05 vs. L-dose, dP < 0.05 vs. M-dose. Table 1. Comparison of Systemic Insulin Resistance and Serum Lipid Levels among Different Groups (x ± s, n = 10)
However, mice from the medium- and high-dosage groups had significantly higher serum TC levels after omethoate exposure for 60 days than those of mice from the control, vehicle, and low-dosage groups (P < 0.05). Mice from the high-dosage group had significantly higher serum TC levels than those of mice from the medium-dosage group (P < 0.05). These results indicated exposure to omethoate even at the medium dosage could increase the TC levels. Similarly, the serum TG levels of mice from the high-dosage group were significantly higher than those of mice from the remaining groups (P < 0.05).
Regarding serum high density lipoprotein (HDL) levels of the mice, there was no significant difference between the low- and medium-dosage groups; however, these two groups showed significantly lower serum HDL levels than those of mice from the control and vehicle groups (P < 0.05). In addition, the serum HDL levels of mice from the control, vehicle, and low-dosage groups were significantly higher than those of mice from the high-dosage group (P < 0.05).
Analysis of serum low density lipoprotein (LDL) levels revealed that mice from the control, vehicle, and low-dosage groups had significantly lower levels than those of mice from the medium- and high-dosage groups (P < 0.05). Furthermore, there was no significant difference in serum LDL levels of mice between the medium- and high-dosage groups, which indicated that LDL levels are sensitive to omethoate.
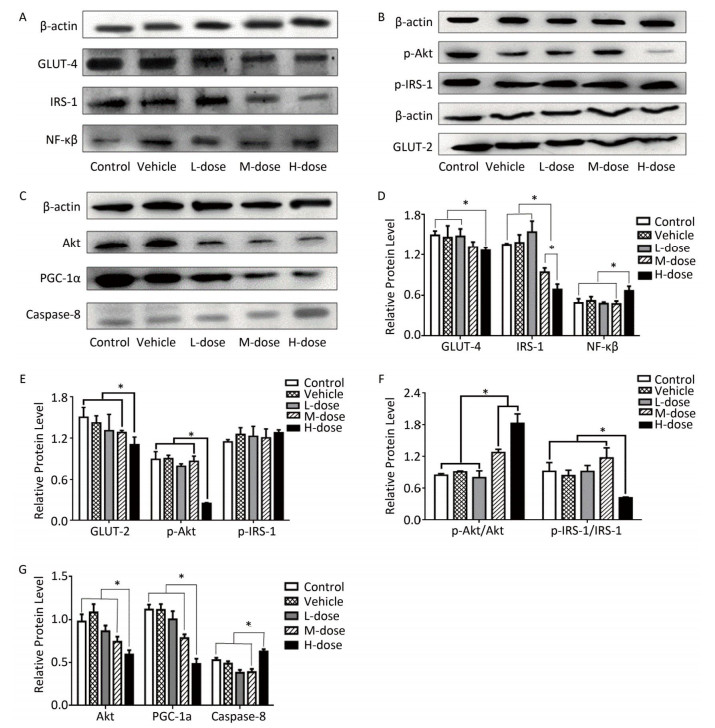
Regarding the effects of omethoate on liver insulin signaling after 60 days of exposure, we observed a decrease in the expression of GLUT-4, GLUT-2, IRS-1, p-IRS-1, Akt, p-Akt, and PI3K in the liver of mice from the high-dosage group (Figures 1 and 2A). Even at the medium dosage, the expression levels of IRS-1 and PI3K were decreased, which indicated that insulin signaling in the liver was compromised at these dosages.
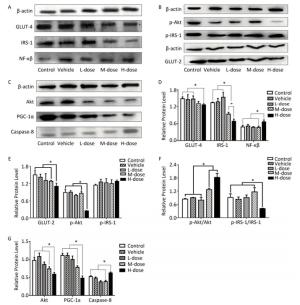
Figure 1. Comparison of the expression of IRS-1, GLUT-4, GLUT-2, Akt, NF-кB, and PGC-1α. (A-C) Western blot analysis of GLUT-4, IRS-1, NF-кB, p-IRS-1, p-Akt, GLUT-2, Akt, PGC-1α, and caspase-8. (D-G) The relative expression levels of GLUT-4, IRS-1, NF-кB, p-IRS-1, p-Akt, GLUT-2, Akt, PGC-1α, and caspase-8 were quantitatively analyzed by the image J software. *P < 0.05.

Figure 2. Comparison of the expression of PI3K, PPAR-γ, P38-MAPK, Bax, Bcl-2, and FADD. (A, C) Western blot analysis of P38-MAPK, PI3K, PPAR-γ, Bax, Bcl-2, and FADD. (B, D) The relative expression levels of P38-MAPK, PI3K, PPAR-γ, Bax, Bcl-2, and FADD were quantitatively analyzed using the image J software. *P < 0.05.
As shown in Figures 1A, 1D, 2A, and 2B, the expression levels of both NF-кB and P38-MAPK were significantly increased in the high-dosage group, and the expression of P38-MAPK was increased even in the medium-dosage group. Furthermore, as depicted in Figures 1C, 1G, 2A, and 2B, the expression levels of PPAR-γ and PGC-1α were significantly decreased in the liver of mice exposed to high dosage of omethoate for 60 days, and in addition, the expression of PPAR-γ was decreased even in the medium-dosage group.
After the 60-day exposure to omethoate, the expression levels of Bax, caspase-8, and Fas-associated death domain protein (FADD) in the liver of mice were significantly increased, whereas Bcl-2 expression was decreased, in the high-dosage group, which indicated the induction of apoptosis in the liver at the high dosage.
The major findings of this study are that exposure to omethoate for 60 days at the dosages of 1 and 2 mg/kg body weight resulted in a reduced expression of critical elements in the insulin signaling pathway of the liver. Moreover, these exposure dosages resulted in abnormal lipid levels. The changes and abnormalities in the insulin signaling pathway, such as the insulin receptor substrate (IRS), phosphatidylinositol 3-kinase (PI-3K), Akt, and the glucose transporter (GLUT), are known to cause insulin resistance and result in diabetes[6]. However, these changes are unable to induce systemic insulin resistance.
Previous studies have shown that insulin resistance will develop due to changes in the expression of the insulin receptor[7]. Our findings showed that exposure to omethoate for 60 days could reduce the expression levels of IRS-1, PI3K, Akt, and GLUT-4 in the liver, which are critical elements in the insulin signaling pathway. Velmurugan et al. reported that the fasting blood glucose level was elevated in mice that were treated with monocrotophos for 80 days[3]. Therefore, it is likely that the expression level will be further decreased with prolonged exposure. Thus, it is reasonable to predict that insulin resistance will develop in the liver at a certain time point, even though the intake of omethoate is at a low dosage each day. Moreover, it is also possible that omethoate treatment will predispose to hyperglycemia or insulin resistance since omethoate can induce oxidative stress, which is a contributing factor to insulin resistance[5]. As demonstrated in the present study, the expression levels of NF-кB and P38-MAPK were significantly increased in the high-dosage group, and the expression of P38-MAPK was increased even in the medium-dosage group.
Alterations in serum lipid levels observed in our study are highly similar to those observed in rats with streptozotocin-induced type 2 diabetes that were fed on a high-fat diet, which were characterized by higher levels of TC and TG[8]. Although we did not detect systemic insulin resistance, we did find changes in serum lipid levels. Both serum TC and TG levels in mice from the high-dosage group were significantly increased in our study, which indicated that omethoate treatment has the potential to induce type 2 diabetes.
It has been suggested that PGC-1α plays an important role in the pathogenesis of T2D by preventing lipotoxicity[6]. In the present study, the expression of PGC-1α was significantly decreased in the high-dosage group, and the serum levels of TC and TG were significantly increased in the high-dosage group, which indicated that omethoate could cause lipotoxicity and insulin resistance or that T2D would develop with prolonged time of exposure to omethoate.
PPAR-γ, which plays a key role in the regulation of glucose and lipid metabolism, is downregulated in tissues during insulin resistance conditions[9]. In our study, although we did not detect insulin resistance, we did find that the expression of PPAR-γ was reduced in the high-dosage group. It is possible that the decreased expression of PPAR-γ is not significant enough to cause insulin resistance.
The results of our study indicate that omethoate could contribute to the development of insulin resistance or T2D by affecting the expression of critical elements in the insulin signaling pathway in the liver and lead to a potential risk of developing insulin resistance.
No conflict of interest to declare.
Effects of Omethoate on Liver Insulin Signaling in Mice
doi: 10.3967/bes2018.086
the grant of research on management model of common chronic diseases 371182093427
Jipai Runda Environmental Inspection Technology Corporation Limited of Beijing 2016YX137
- Received Date: 2018-04-01
- Accepted Date: 2018-07-31
| Citation: | WANG Yuan, LI Yu Ling, MENG Fan Zhu, HOU Bao Lian, ZHUANG Chuan Ning, XIONG Shu Han, REN Shu Ping. Effects of Omethoate on Liver Insulin Signaling in Mice[J]. Biomedical and Environmental Sciences, 2018, 31(8): 627-631. doi: 10.3967/bes2018.086 |





 Quick Links
Quick Links
 DownLoad:
DownLoad: