-
Diseases caused by nontuberculous mycobacteria (NTM) in humans have been recognized as an emerging public health problem[1,2]. Drug therapy of diseases caused by NTM is long, costly, and often associated with drug-related toxicities. Clinical improvement and prolonged culture conversion are not achievable for all patients[3]. There are two groups of NTM: rapidly growing mycobacteria (RGM) and slowly growing mycobacteria. Mycobacterium abscessus is the most common etiological agent of diseases caused by RGM[4-7]. Moreover, it is the etiological agent of a wide spectrum of infections in humans causing severe chronic pulmonary and disseminated infections[8] and is the causative agent in most patients with serious complications. At present, there is no reliable therapeutic antibiotic therapy, such as parenteral agents, based on in vitro drug susceptibility testing (DST) to cure M. abscessus infection[4]. M. abscessus infections may lead to an epidemic[9,10]. Previously, M. abscessus was thought to be independently acquired by susceptible individuals from the environment. However, whole-genome analysis of a global collection of clinical isolates indicates that most M. abscessus infections are acquired through transmission, potentially via fomites and aerosols, of recently emerged dominant circulating clones that have spread globally. This represents an urgent, international infection challenge[11].
The major issue with M. abscessus is its intrinsic resistance to the most available antibiotics. The American Thoracic Society has recommended different groups of agents, namely, macrolides (clarithromycin), aminoglycosides (amikacin), cephamycins (cefoxitin), and carbapenems (imipenem) to treat M. abscessus infections[4]. Moxifloxacin (MFX) emerged as a promising candidate for the treatment of RGM infections[4,12,13]. It showed good activity in vitro against M. abscessus[14] and was suggested as one of the antibiotics to treat adults with M. abscessus disease[12]. However, several other studies showed MFX to have lower or no activity against M. abscessus in vitro[15-18]. DST in vitro might be an option but it is not fully standardized[4]. More significantly, the clinical response to drugs does not correlate well with in vitro DST. It was recognized that future work should address MFX efficacy in vivo[19], and that there is a need for suitable animal models[20,21]. Recently, the M. abscessus-zebrafish (ZF) model provided important insights into the pathogenesis of infectious diseases. It is rapidly being recognized as a useful model to study bacterial interactions[22-25]. Because of its genetic tractability and optical transparency, ZF represent an exquisite model to study many aspects of M. abscessus. Such a simple and innovative system may be particularly suited for assessing potential antibacterial activities in the process of discovering new, urgently needed drugs to fight M. abscessus[19].
In this study, we report experimental conditions for in vivo imaging of M. abscessus, and their use to test the efficacy of drug treatments. The ZF model is of interest as it could be applied to high-throughput testing of drug efficacy against the most drug-resistant mycobacterial species in vivo; it can be applied to clarify the currently uncertain suitability of MFX to treat M. abscessus infections.
-
The reference strain ATCC19977 was used for culture. Isolates were sub-cultured on Lowenstein-Jensen medium at 37 ℃ for 4–6 d to observe colony morphology. Solutions of these drugs were prepared according to the Clinical and Laboratory Standards Institute (CLSI) recommendations[14]. The final concentrations of MFX and azithromycin (AZM) were in the range of 0.0625 to 32 μg/mL and 0.5 to 256 μg/mL, respectively. MICs of each drug were determined by broth-microdilution method as recommended by CLSI using 96-well plates. The MICs were determined 3 d after incubation in the following manner. To each well, 70 μL of Alamar blue dye (Serotec, 20-μL Alamar blue + 50-μL 5% Tween 80) was added, and the plates were re-incubated for 24 h[14]. A color change from blue to pink indicated bacterial growth. MIC was defined as the lowest concentration of the drug that resulted in no color change, i.e., the lowest concentration capable of inhibiting the visible growth of tested isolates. DST results were evaluated according to CLSI break-points recommendations.
-
ZF experiment was approved by the Ethics Committee of the Beijing Chest Hospital affiliated to Capital Medical University. A previously reported protocol[19] was used to assess the activity of MFX and AZM against M. abscessus in ZF. M. abscessus ATCC19977 with a smooth (S) morphotype were cultured in Middlebrook 7H9 broth (Becton Dickinson) supplemented with 10% OADC (Becton Dickinson) and 0.05% Tween 80 (Sigma-Aldrich) at 30 °C for 5 to 7 d. Mid-log-phase cultures of M. abscessus were centrifuged, washed, and resuspended in phosphate-buffered saline (PBS) supplemented with 0.05% Tween 80. Bacterial suspensions were then homogenized and sonicated, and the remaining clumps were allowed to settle for 5 to 10 min as previously described[26]. Bacteria were concentrated in PBS and administered intravenously. M. abscessus labeled with red-fluorescent CM-DiI was micro-injected 3 d ost-fertilization (dpf) into caudal vein of wild-type ZF. Different drug concentrations and amounts of bacteria over different observation periods were tested to establish the infected ZF model.
-
Ten 3-dpf ZFs without M. abscessus infection were randomly selected and placed into one well of 24-well plates, each well containing 1 mL of water. MFX or AZM were then added to the water. MFX concentrations of 10, 100, 250, 500, 1,000, and 2,000 μg/mL and AZM concentrations of 1, 10, 100, 250, 500, and 1,000 μg/mL were tested separately. Drug-containing water was renewed daily for 5 d. A group with no drug treatment was used as control. ZF-containing plates were maintained at 35 ℃. The MTC of each drug was defined as the highest concentration that caused no ZF death.
-
Ten 3-dpf ZFs with homogeneous distribution of M. abscessus were selected and placed randomly into 24-well plates containing 1 mL of water in each well. In preliminary experiments, noninfected embryos were exposed to increasing concentrations of MFX and AZM and observed under a microscope to establish the drugs’ concentrations that did not cause toxicity-induced killing or developmental abnormalities. Doses corresponding to 31.25×, 62.5×, 125×, 250×, and 500× MIC of MFX and 3.9×, 7.8×, 15.625×, 31.25×, and 62.5× MIC of AZM were tested according to the values determined using the microdilution method. MFX concentrations of 62.5, 125, 250, 500, and 1,000 µg/mL and AZM concentrations of 15.625, 31.25, 62.5, 125, and 250 μg/mL tested separately in individual wells caused no ZF death. The maximum concentrations tested in the next process were chosen to be below the drugs’ MTCs.
Twenty 3-dpf ZFs were tested for each of the aforementioned concentrations. Drug-containing water was renewed daily for 5 days of infection. A control group without a drug was maintained. ZFs were cultured at 35 ℃. Survival curves were determined by recording the number of ZFs that died each day.
Three days after infection, 10 ZFs from each concentration group were collected and pictured. Fluorescence microscopy of infected ZFs was performed using Nikon NIS-Elements D 3.10 fluorescence microscope. Final image analysis and visualization were performed using GIMP 2.6 freeware to merge fluorescent and differential-inference-contrast images, to adjust brightness level, and to remove out-of-focus background fluorescence. Images of fluorescence intensity at each concentration were evaluated by counting fluorescent pixels.
Three days after infection, 5 ZFs in each concentration group were imaged, and the inhibition rate (%) at each concentration was calculated using the following formula: inhibition rate (%) = (Scontrol group - Sdrug group)/Scontrol group × 100% (where S is fluorescence intensity as determined by pixel count).
M. abscessus may be disseminated in the heart, brain, veins, liver, and eyes. Thus, to analyze the efficacy of the drugs against M. abscessus dissemination, fluorescence in ZF at various drug concentrations was observed, pictured, and analyzed.
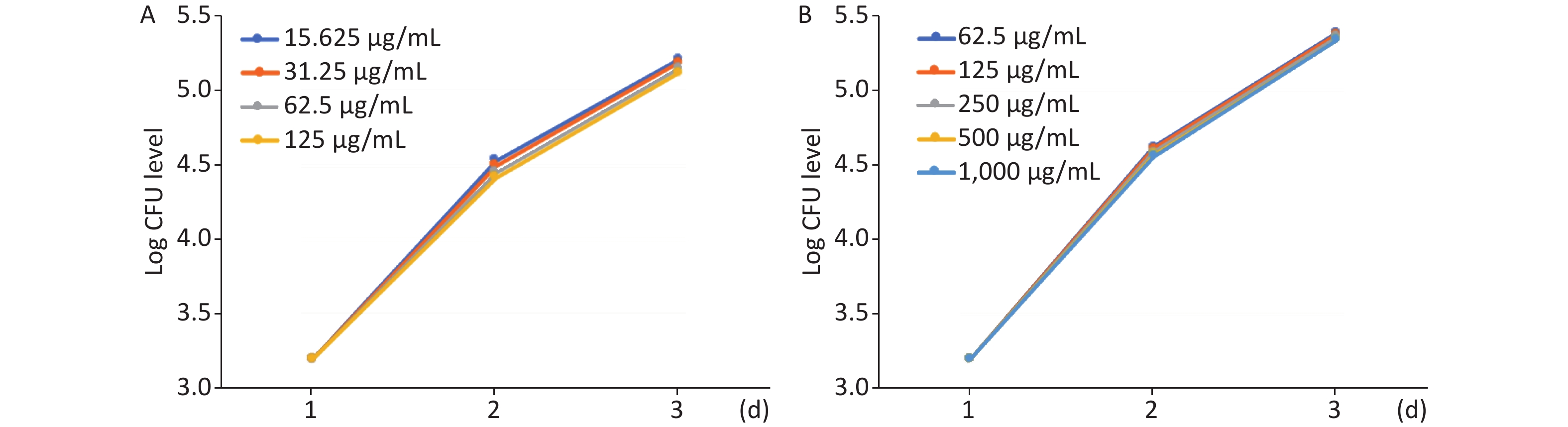
From day 1 to day 3, 5 ZFs at each tested-drug concentration were collected, lysed individually in 2% Triton X-100-PBS, and resuspended in PBS with Tween 80. Several 10-fold dilutions of homogenates were plated on 7H10 containing 500 mg/L hygromycin and BBL MGIT PANTA (Becton Dickinson) and used as recommended by the supplier. Colony-forming units (CFUs) were enumerated after 4 days of incubation at 35 °C. Results are expressed as mean log10 CFU per ZF.
-
Statistical analyses of comparisons between Kaplan-Meier survival curves were performed using the log-rank test using the SPSS software (IBM SPSS Statistics version 24). CFU counts and quantification experiments were analyzed using one-way analysis of variance and Fisher’s exact tests, respectively. Statistical significance was assumed at P values < 0.05.
-
MIC values of MFX and AZM against M. abscessus reference strain were determined as 2 μg/mL and 8 μg/mL, respectively; this suggests a moderate susceptibility to MFX and susceptibility to AZM (Supplementary Table S1 available in www.besjournal.com).
Drug Culture time (d) MIC value (μg/mL) Susceptible breakpoint (μg/mL) Moderately susceptible breakpoint (μg/mL) Resistant breakpoint (μg/mL) Azithromycin 3 8 ≤ 1 2 ≥ 4 Moxifloxacin 3 2 ≤ 16 32 ≥ 64 Table S1. Results of DST by Alamar blue 2-fold dilution method
-
Different bacteria concentrations and durations of observation were tested for establishing infected ZF model. High concentration would be expected to cause a rapid death. Low concentration would not generate enough fluorescence to generate permanent records of images when observed under the microscope. Three M. abscessus concentrations – 1.6 × 109 µg/mL, 2 × 109 µg/mL, and 5 × 109 µg/mL were chosen for testing. The following amounts of bacteria (in units, 1 unit means 1 M. abscessus) – 12,800, 6,400, 3,200, 1,600, and 800 were tested by injection and followed over the observation period from 3 to 7 d. For the final testing, M. abscessus concentration of 5 × 109 µg/mL containing 1,600 units of M. abscessus was injected into ZFs and observed over a period of 5 d.
-
Several MTCs of the drugs were tested (Table 1), with the purpose that concentrations that did not affect ZF survivability would be selected for a follow up process. We established that MFX at ≤ 1,000 μg/mL and AZM at ≤ 250 μg/mL would not impact ZF survivability. Hence, MFX concentrations of 62.5 μg/mL, 125 μg/mL, 250 μg/mL, 500 μg/mL, 1,000 μg/mL, and AZM concentrations of 15.625 μg/mL, 31.25 L, 62.5 μg/mL, 125 μg/mL, and 250 μg/mL were chosen for the subsequent process.
Group Concentration (μg/mL) Death number Mortality (%) Control group (Healthy ZF) − 0 0 Moxifloxacin 0 0 0 62.5 0 0 125 0 0 250 0 0 500 0 0 1,000 0 0 2,000 10 100 Azithromycin 0 0 0 15.625 0 0 31.25 0 0 62.5 0 0 125 0 0 250 0 0 500 7 70 Table 1. ZF survivability at different concentrations of MFX and AZM (n = 10 of in each group)
-
We tested AZM at a wide range of concentrations from 15.625 μg/mL to 250 μg/mL, and MFX at concentrations ranging from 62.5 μg/mL to 1,000 μg/mL. Exposing ZF to aqueous solutions these drug concentrations did not show any indication of toxicity in our preliminary experiments.
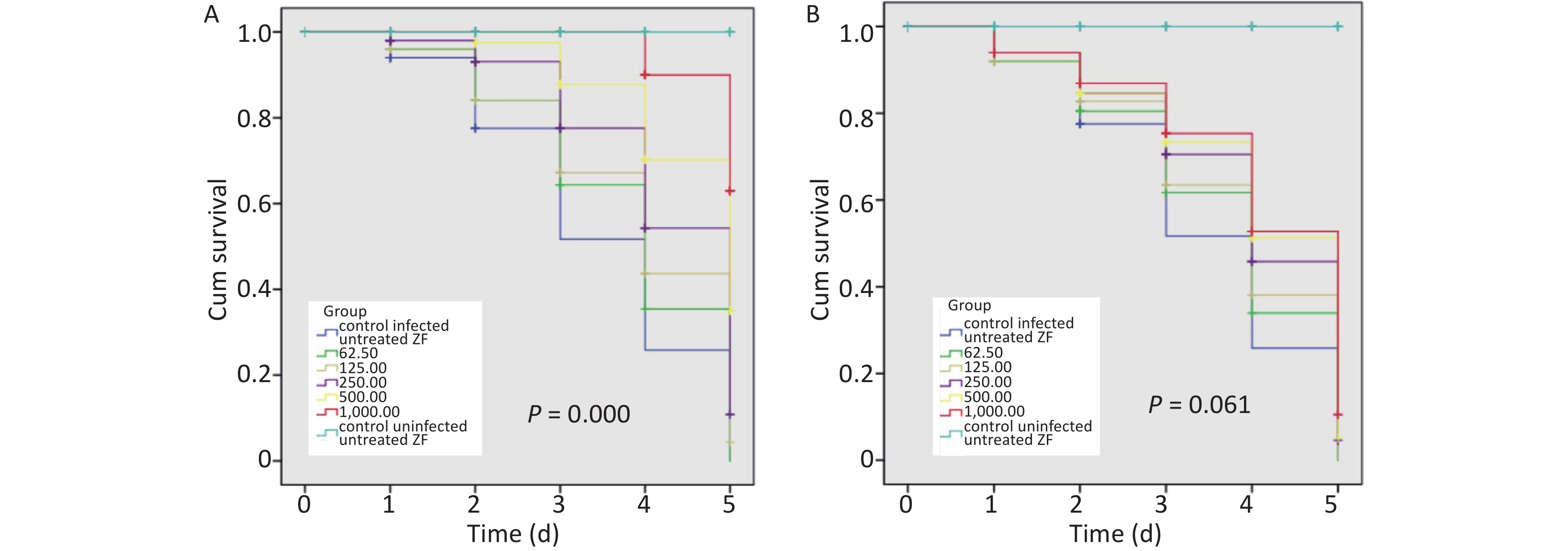
When infected ZFs were exposed for more than 2 d to the above AZM concentrations, a significant increase in survival rate (P = 0.000) was observed depending on AZM concentration (Figure 1A); higher doses of AZM increased ZF survival. The treatment with low AZM doses failed to restrict mycobacterial growth. This result shows that AZM has a significant activity against M. abscessus in vivo in the M. abscessus-infected ZF test system. However, although some restriction to mycobacterial growth by MFX was observed, the association between the increased survival and the high dose of MFX was not found to be significant (Figure 1B). With the increasing MFX concentration, the survival curve did not show a corresponding significant increase in ZF survival (P = 0.061).
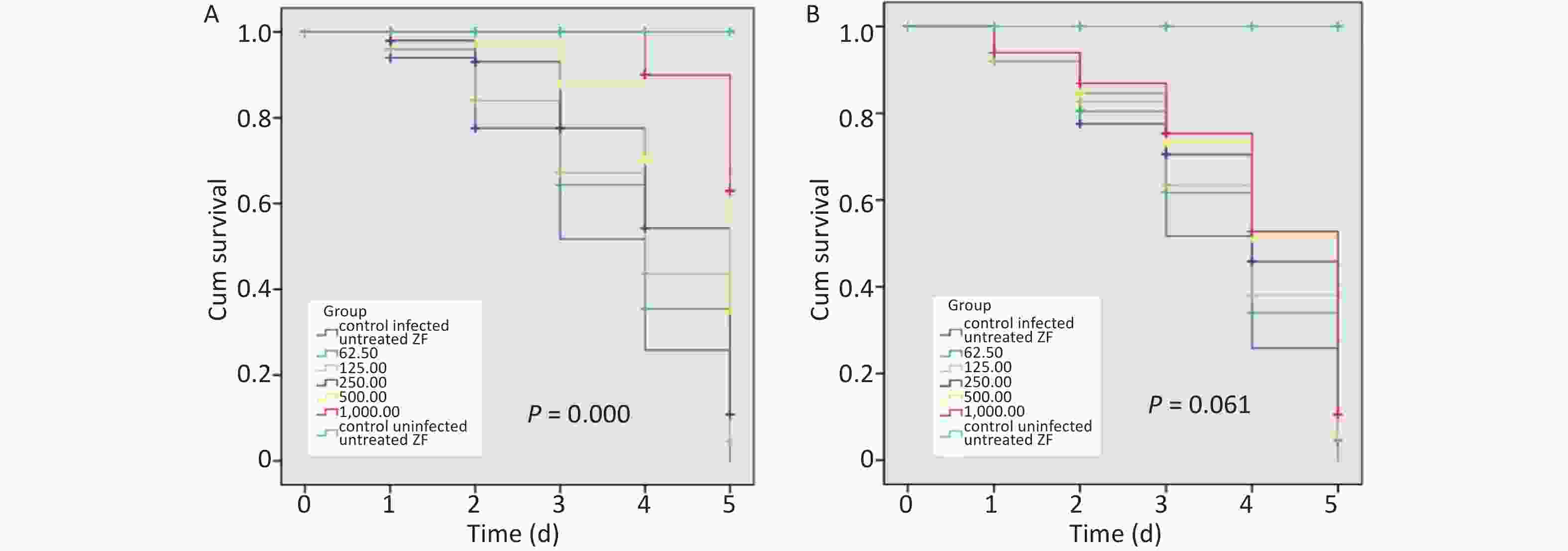
Figure 1. The survival analysis of AZM and MFX against M. abscessus infected ZF. (A) Increased survival was associated with a high dose of AZM. The treatment with low AZM doses failed to restrict mycobacterial growth. The survival curve showed significant difference between different AZM concentration group (P = 0.000). (B) Although some restriction to mycobacterial growth by MFX was observed, the association between increased survival and high dose of MFX is not significant (P = 0.061). Statistical comparison was tested between different drug concentration but without uninfected/untreated ZF control.
-
The effect of MFX and AZM on bacterial burden was analyzed by quantifying CFU loads. Increased AZM concentration was associated with lower bacterial burdens as determined quantitatively by CFU plating (Figure 2A). Treatment with lower doses was correspondingly less effective in restricting mycobacterial growth. The same trend was observed with MFX. MFX concentration correlated with CFU loads (Figure 2B). In both AZM and MFX groups, no significant differences were observed between CFU loads at different concentrations (P > 0.05).
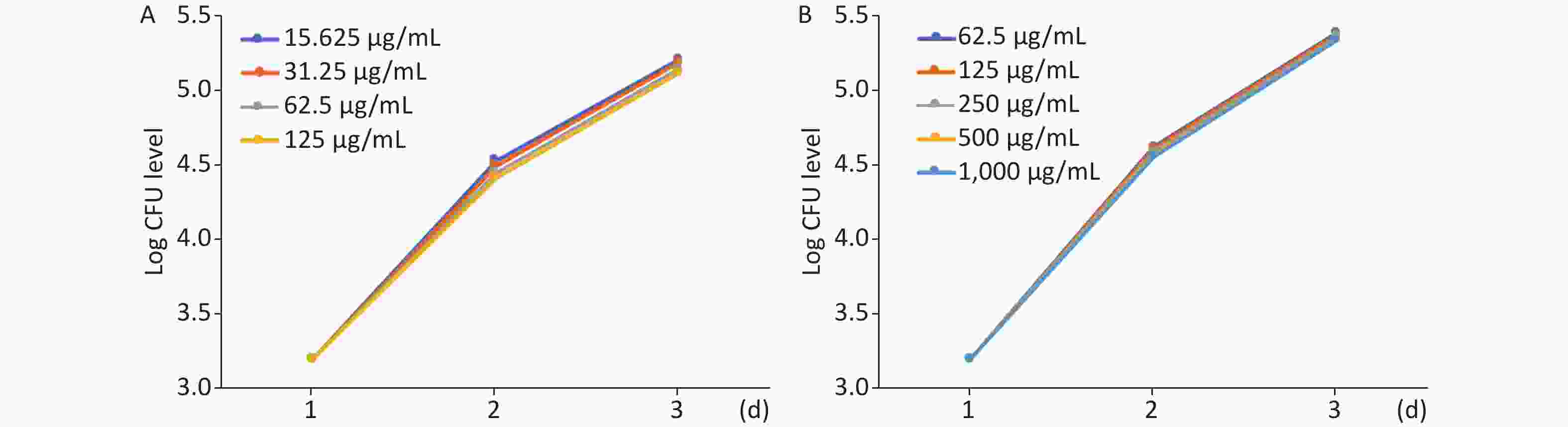
Figure 2. The analysis of AZM and MFX efficacy against M. abscessus infected ZF by CFU loads. (A) From day 1 to day 3, 5 ZF from each tested concentration were collected and were lysed and plated on 7H10. Increased AZM concentration was associated with lower bacterial burdens as determined quantitatively by CFU plating. Treatment with lower doses had less effect on mycobacterial growth. No significant difference was observed between different AZM concentrations (P > 0.05). (B) The same trend was observed with MFX. MFX concentrations correlated with CFU loads. No significant difference was observed among different MFX concentrations (P > 0.05).
-
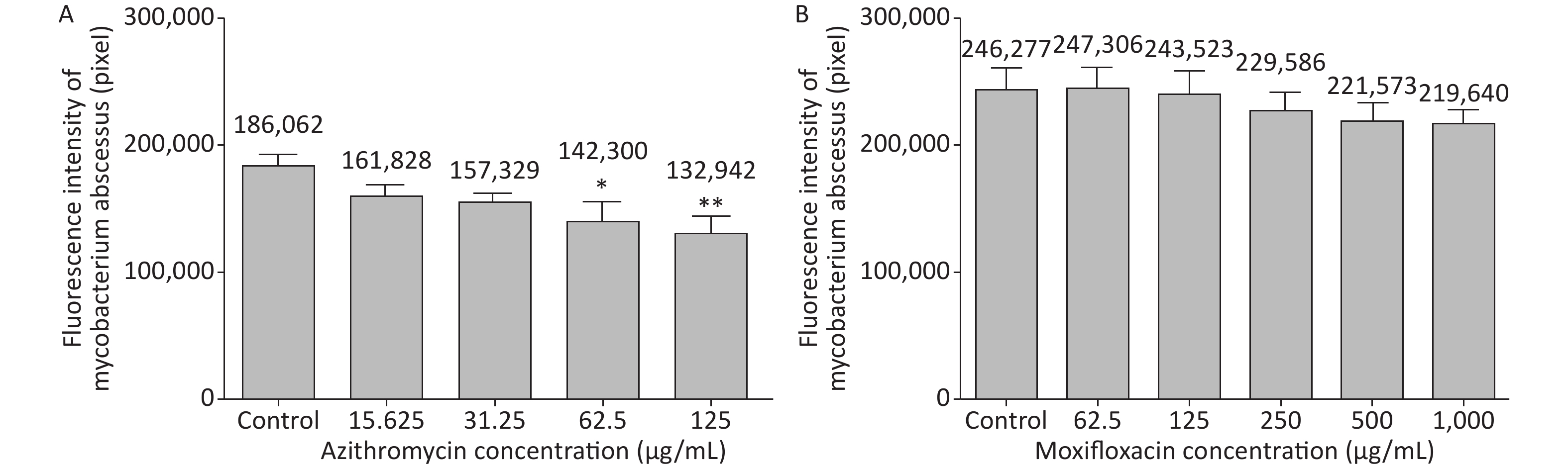
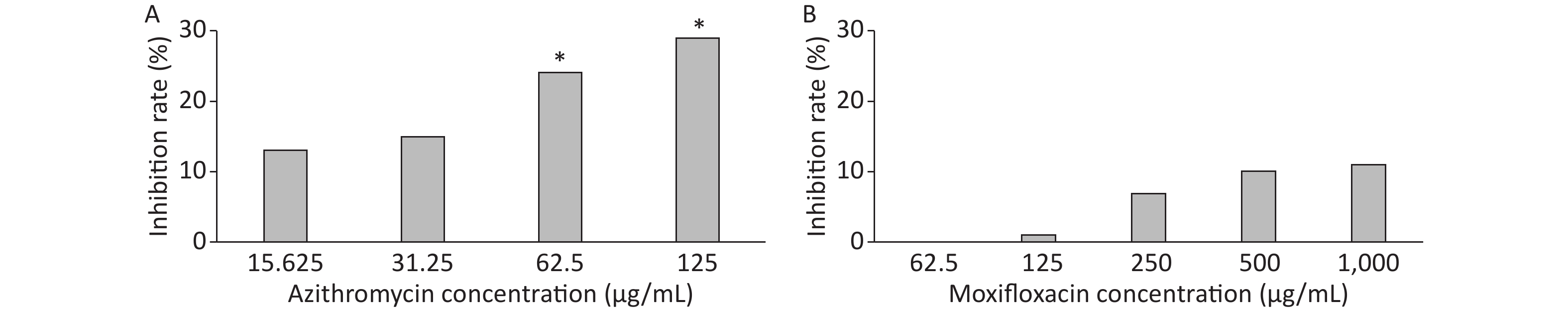
3 days after infection, 5 ZFs in each concentration group that generated images of adequate quality were collected and analyzed. Exposure to AZM was associated with a significant reduction in the number of abscesses (Figure 3A). With increasing AZM concentration (15.625 μg/mL, 31.25 μg/mL, 62.5 μg/mL, 125 μg/mL), bacterial fluorescence intensity in ZF showed significant decrease (161,828 ± 6,605, 157,329 ± 5,356, 142,300 ± 13,715, 132,942 ± 11,243) (Figure 3A). This decrease in fluorescence intensity was consistent with the inhibition rate. AZM inhibition rates at 15.625 μg/mL, 31.25 μg/mL, 62.5 μg/mL, and 125 μg/mL concentration were 13%, 15%, 24%, and 29%. The inhibition rate also showed significant difference when compared with no-drug group (P < 0.05) indicating that AZM possesses good inhibition efficacy (Figure 4A). However, exposure of infected ZF to MFX showed no significant decrease in the frequency of abscesses (Figure 3B), although increased MFX concentrations did decrease fluorescence intensity slightly when observed under fluorescence microscope. At MFX concentrations of 62.5 μg/mL, 125 μg/mL, 250 μg/mL, 500 μg/mL, and 1,000 μg/mL, fluorescence intensities in ZF were 247,306, 243,523, 229,586, 221,573, and 219,640 pixels (Figure 3B), and the inhibition rates were 0%, 1%, 7%, 10%, and 11%, respectively, with all P value > 0.05 indicating statistical insignificance when comparing with the control group (Figure 4B).
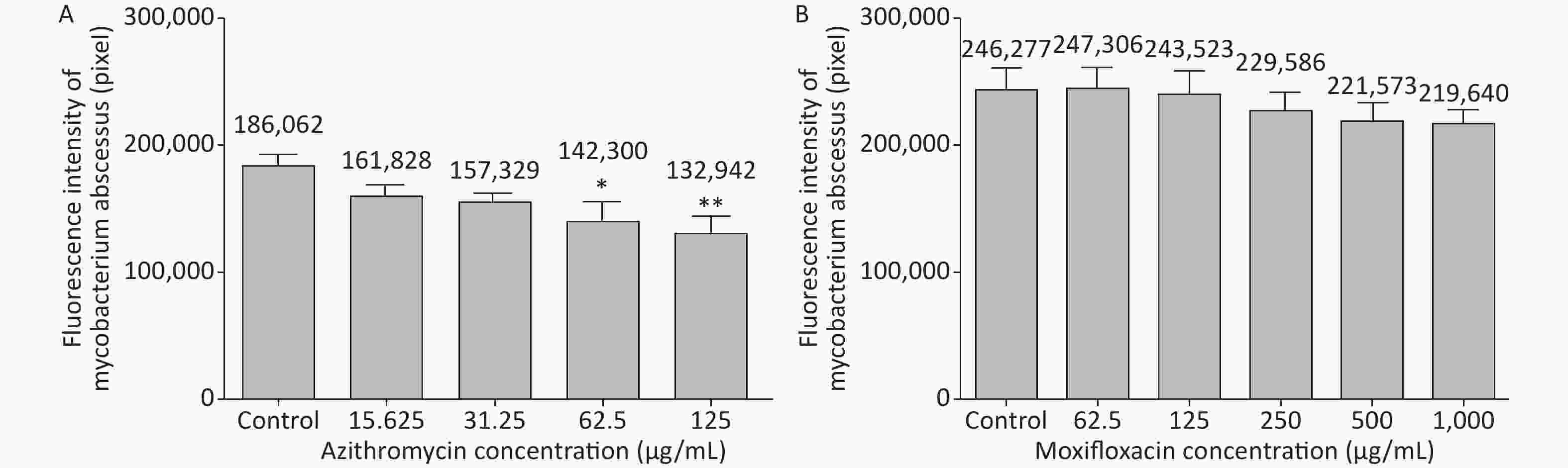
Figure 3. The analysis of AZM and MFX efficacy against M. abscessus infected ZF by fluorescence intensity (by pixel). Three days after infection, 5 ZFs from each concentration were collected and imaged. The fluorescence intensity (by pixel) of M. abscessus treated with different concentrations of AZM (A) and MFX (B) were compared with that each of M. abscessus treated without drug. n = 20 for each group. *P < 0.05; **P < 0.01.
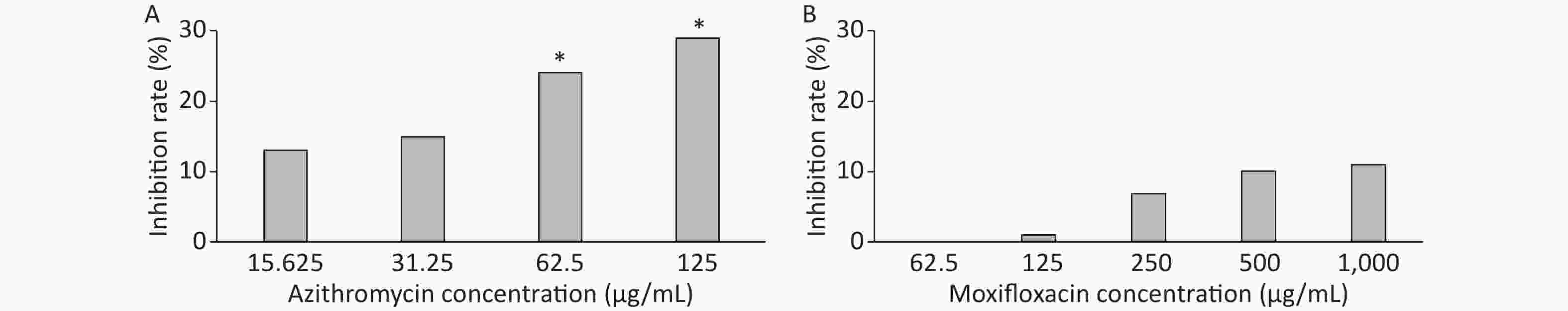
Figure 4. The analysis of AZM and MFX efficacy against M. abscessus infected ZF by inhibition rate(%). Three days after infection, 5 ZFs at each concentration were imaged, and the inhibition rate for each concentration was calculated. Inhibition rates at different concentrations of AZM (A) and (B) were compared with that each of M. abscessus treated without drug. One-way analysis of variance and t test were performed. *P < 0.05.
-
The effect of AZM and MFX on bacterial fluorescence dissemination was examined. In the AZM control group (without drug), M. abscessus disseminated in the heart, brain, and veins. The transfer-occurrence rate was 50%. In 15.625 μg/mL AZM group, M. abscessus disseminated in the brain and veins, with transfer-occurrence rate of 30%. In 31.25 μg/mL, 62.5 μg/mL, and 125 μg/mL AZM, M. abscessus disseminated only in the vein, with transfer-occurrence rate of 20%. All the transfer rates at different concentrations were compared with those in the control group with P > 0.05. In the MFX control group (without drug), M. abscessus disseminated in the liver, heart, brain, and veins; the transfer-occurrence rate was 70%. In 62.5 μg/mL MFX, M. abscessus disseminated in the heart and veins, with transfer-occurrence rate of 60%. In 125 μg/mL MFX, M. abscessus disseminated in the brain and veins, with transfer-occurrence rate of 50%. In 250 μg/mL, 500 μg/mL, and 1,000 μg/mL MFX, M. abscessus disseminated in the brain and veins, with transfer-occurrence rate of 40%. All the transferring rates at different concentrations compared with those in the control group showed P > 0.05. Therefore, although both two groups showed some inhibition of M. abscessus dissemination, no significant differences were observed for AZM and MFX groups when compared with the control group.
Together, these results suggest that AZM exerts a therapeutic effect, whereas MFX exerts a limit therapeutic effect, by preventing the development of abscesses and protecting ZF by killing bacteria.
-
Animal models for examining pathogenesis are currently limited unless very large doses of microorganisms are given intravenously. There is little evidence regarding whether adequate infection is fully induced when small doses of microorganisms are administered. Thus, better models were needed for elucidating the pathogenesis of M. abscessus that would enable testing of new drugs to treat infection caused by this organism. This need stimulated the recent development of the ZF model that can be used to assess the suitability and sensitivity of clinically relevant drugs in M. abscessus-infected embryos[19,25,27,28]. Small bacterial doses can be used in this model to allow visualizing, in a dose- and time- dependent manner, the dynamics of infection and physiopathological markers, such as cords and abscesses, in the presence of an active test compound[19]. Injecting a small amount of inoculum allows administration of homogenous bacterial suspensions without obstructing the needle during the microinjection procedure. In this study, we evaluated in vivo drug activity using this ZF model.
Recognized as a cause of chronic pulmonary infections especially in individuals with altered host defenses or disrupted airway-clearance mechanisms, M. abscessus appears to be a major infectious threat to the airway in cystic fibrosis patients for which an increased prevalence has been reported in recent years[19,29]. This situation is worsened by the fact that antibiotic treatment of M. abscessus is often unsuccessful and/or poorly tolerated by patients. M. abscessus is notorious for being intrinsically resistant to most antibiotics[7], thus rendering these infections particularly complicated, difficult to treat, and associated with a high rate of therapeutic failure[30].
Some studies showed that MFX had a low or no activity against M. abscessus in vitro[15-18]. However, some other studies showed that MFX had good activity in vitro against M. abscessus and recommended it for a possible antibiotic regimen to treat adults with M. abscessus disease[12,14]. There is not enough data to support recommendation for a preferential therapeutic use of this compound, hence there is a need to examine MFX efficacy in vivo.
We have previously shown that MFX moderately but significantly inhibits M. abscessus in vitro[14]. In this study, we evaluated the in vivo activity of MFX against M. abscessus, which exists as two variants: rough (R) and S. Ex vivo and in vivo studies have described the hypervirulence phenotype of the R versus the S morphotype[31,32]; epidemiological studies have confirmed the persistence and acute respiratory syndromes caused by the R morphotype[33-35]. The major difference between the R and S variants is the loss of a surface-associated glycopeptidolipid in S variants[36]. In this study, we chose the S morphotype for testing as it is associated with 53% of all M. abscessus cases in China[37].
In agreement with a recent study addressing the activity of MFX against several NTM[14], we found that MFX exhibited low MIC values against standard M. abscessus isolate in vitro. Further, we examined the efficacy of MFX against M. abscessus by monitoring the survival and bacterial burden of infected ZF treated with MFX. AZM has an excellent activity against M. abscessus and was tested together with MFX for comparison. Fluorescence intensity of M. abscessus under various concentrations in ZF was analyzed. AZM showed good activity for decreasing bacteria amount in ZF thus further verifying our choice and experimental design. However, MFX showed no significant ability to inhibit bacteria compared with the control group. Although we could see a modest decrease of bacteria fluorescence intensity with increasing MFX concentration, this was not significantly different when compared with control group. AZM showed significant inhibiting effect on bacterial growth compared with the control group whereas no such effect was observed for MFX. In our experiment, AZM increased the survival of ZF as previously reported, with a statistically significant difference compared with no drug treatment. However, the efficacy of MFX is likely to be poor since the Kaplan-Meier survival curve did not show significant inhibition of infected ZF mortality. All these results demonstrate that MFX may have very limited activity against M. abscessus in vivo compared with AZM.
Analysis of bacterial dissemination and CFU loads showed no significant effect even for the AZM group. Although some inhibition of MFX and AZM on M. abscessus dissemination in ZF was observed, there was no significant difference when compared with the control group. Same outcome was observed when analyzing the drugs’ efficacy on CFU loads. Although some positive inhibiting effect on CFU was observed, it was not significant. Although measuring the CFU in ZF was recommended[19,24], our study did not find it of use for assessing drug activity in vivo.
In summary, we report here a robust and sustained effect of MFX on infected zebrafish. The use of this model for testing efficacy of MFX in vivo allows visualization in a dose- and time-dependent manner the dynamics of bacterial fluorescence and loads. MFX exerted a limited impact on ZF survival. This comports well with the failure of MFX-containing regimens in clinical practice[38]. However, such conclusion will need to be supported further by multivariant pharmacometric analyses of clinical data that is currently not available for MFX applied to treating M. abscessus pulmonary disease[39]. In fact, as far as we know, no such data exist for any of the drugs currently used in treating pulmonary M. abscessus; there is a lack of clinical trials and of large prospective clinical-cohort studies for this disease[38].
Furthermore, the present study reports the usefulness of ZF as a preclinical model for evaluating in real time the efficacy of MFX and AZM against M. abscessus infection. As ZF have been successfully used in the past to test the efficacy of three clinically relevant drugs, clarithromycin, imipenem, and bedaquiline[19,24,25], future studies should address the in vivo efficacies of other drugs intended to treat M. abscessus infection using the ZF model.
However, the following limitations of our study should be noted. 1) Small sample size may have limited the demonstration of drug efficacy. 2) We only tested one reference strain (ATCC19977). More studies should be conducted to compare the intrinsic activity of antibiotics in vivo in ZF infected with the three subspecies of the M. abscessus complex, M. abscessus sensu stricto, M. massiliense, and M. bolletii, which are known to respond differently to antibiotics in vitro. 3) Owing to these strain-to-strain variations, clinical strains should also be tested, which may help clinicians select optimal drug treatments. 4) The time course of death induced by M. abscessus is rapid, with up to 50% of ZF dying at 5 d post-infection and 100% within 10 d post-infection. Hence, the observation time of ZF survival is limited.
Efficacy of Moxifloxacin against Mycobacterium abscessus in Zebrafish Model in vivo
doi: 10.3967/bes2020.047
- Received Date: 2020-02-27
- Accepted Date: 2020-03-11
-
Key words:
- Mycobacterium abscessus /
- Moxifloxacin /
- Azithromycin /
- Zebrafish
Abstract:
| Citation: | NIE Wen Juan, XIE Zhong Yao, GAO Shan, TENG Tian Lu, ZHOU Wen Qiang, SHANG Yuan Yuan, JING Wei, SHI Wen Hui, WANG Qing Feng, HUANG Xue Rui, CAI Bao Yun, WANG Jun, WANG Jing, GUO Ru, GE Qi Ping, NIE Li Hui, HAN Xi Qin, DU Ya Dong, CHU Nai Hui. Efficacy of Moxifloxacin against Mycobacterium abscessus in Zebrafish Model in vivo[J]. Biomedical and Environmental Sciences, 2020, 33(5): 350-358. doi: 10.3967/bes2020.047 |





 Quick Links
Quick Links
 DownLoad:
DownLoad: