-
Chronic obstructive pulmonary disease (COPD) is a major global health concern, and its burden is projected to increase as the population ages[1,2]. According to the 2019 Global Burden of Disease Study, approximately 212.3 million individuals worldwide were affected by COPD, which was associated with 3.3 million fatalities and a loss of 74.4 million disability-adjusted life years[3]. In China, COPD prevalence among individuals aged 40 years and over is 13.7%[4], with Gansu Province experiencing the highest burden (age-standardized incidence rate: 257.33 per 100,000)[5]. Although the incidence, prevalence, mortality, and disease burden of COPD have been decreasing and are projected to remain stable or decline in the Asia-Pacific Region until 2034, significant challenges remain in certain regions.[6] Projections suggest a 1.5-fold increase in COPD cases and deaths in China over the next 25 years[7,8], highlighting the urgent need for targeted interventions.
Sand-dust storms (SDS) are recognized as critical global public health issues. In the context of climate change, the world is projected to face more severe droughts, heightened wind erosion, and extreme weather events, all of which are expected to increase the intensity and frequency of SDS, leading to significant health impacts[9,10]. Numerous studies have shown a strong link between dust storms and higher rates of respiratory-related deaths and hospitalizations, particularly for conditions such as asthma, pneumonia, and COPD[11-13]. A multicenter time-series study conducted in China demonstrated that exposure to SDS events significantly increased COPD mortality, with an excess mortality risk of 11.55% (5.55%–17.89%)[14].
SDS is a prevalent meteorological hazard in arid and semi-arid regions, resulting in the release of substantial quantities of atmospheric mineral dust particles.[15] Inhalable particulate matter (PM10), which comprises both fine particulate matter (PM2.5) and coarse particulate matter (PM2.5–10), is well established as the principal constituent of SDS and poses significant risks to human health.[9,14] SDS events are frequently correlated with significant increases in the measured concentrations of both PM2.5 and PM10[16]. Particulate matter (PM) exposure is a leading environmental risk factor for public health, and the disease burden associated with it remains substantial in China[17,18]. Previous studies have demonstrated a strong association between ambient PM exposure and COPD development[19-21]. However, SDS events may increase the risk of exposure to environmentally persistent free radicals in atmospheric particles, boosting the oxidizing capacity of PM2.5 and leading to a higher prevalence of lung diseases and elevated health risks[22]. Although most studies have focused on the effects of air pollution in isolation, the implications of dramatic PM concentration spikes during SDS events on respiratory health and patients with COPD remain poorly understood, particularly within climatically diverse regions.
Given its arid and semiarid climatic conditions, Gansu Province frequently experiences severe air pollution stemming from both anthropogenic and natural factors[23]. The northwestern region exhibits a significantly higher SDS frequency than the southeastern region, primarily because of its proximity to desert areas and low vegetation coverage[24,25]. In China, more than 90% of SDS events that lasted over two hours occurred in the northwestern provinces, particularly Gansu[26]. Gansu Province, which is highly prone to SDS events, has experienced significant SDS-related respiratory hospitalizations[27]. The formation and propagation of SDS are strongly influenced by key meteorological factors, including temperature, precipitation, and soil moisture. With diverse climatic conditions and arid/semi-arid zones encompassing 75% of its land area, Gansu Province serves as an ideal natural laboratory for quantifying the respiratory health impacts of SDS-PM exposure.
To address these research gaps, this study employed an advanced space-time-stratified case-crossover design to systematically examine the differential impacts of PM exposure on COPD hospitalization during both SDS and non-SDS periods in Gansu Province. Concurrently, we assessed the burden of hospitalization for COPD associated with PM exposure on SDS days. This study sought to establish a critical scientific foundation for developing tailored prevention strategies and refined intervention protocols to improve COPD management in Gansu Province, with particular emphasis on addressing the health challenges associated with SDS events.
-
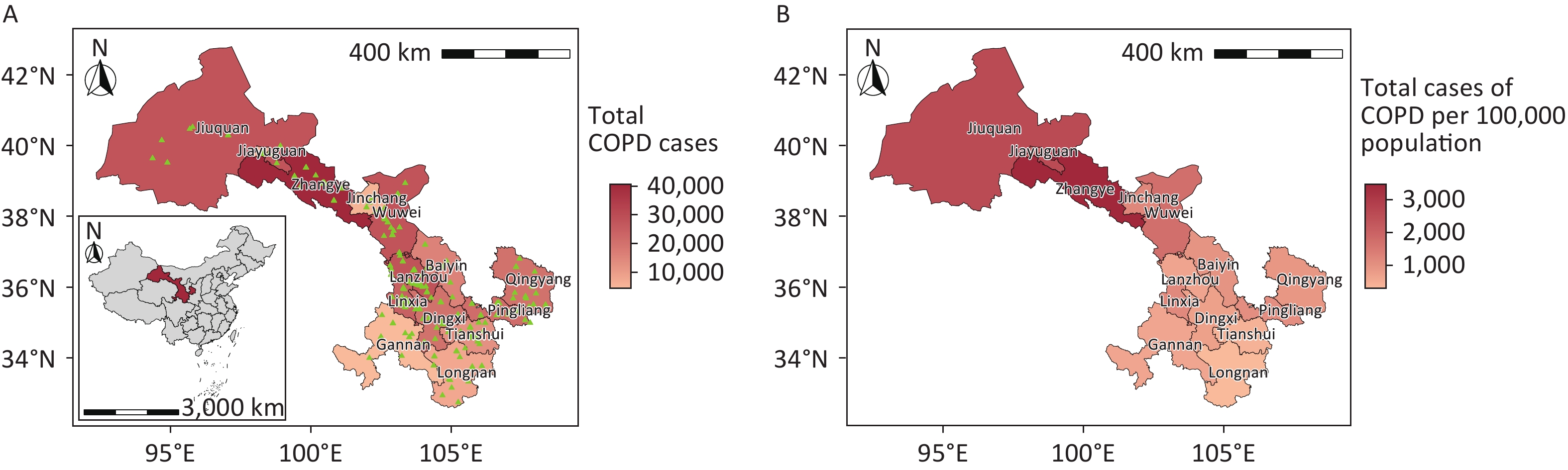
Gansu Province, situated in northwest China, comprises six distinct geographical regions, including the Loess Plateau in Longzhong, the Gannan Plateau, and the Hexi Corridor. The province exhibits diverse climatic conditions, ranging from arid to humid, spanning four wet and dry regions. Comprising 14 cities (prefectures), Gansu Province is categorized into three distinct climatic zones according to annual precipitation levels:[28] the arid region, with less than 200 mm of precipitation (Jinchang, Jiayuguan, and Jiuquan), the semi-arid region, with 200 mm to 400 mm of precipitation (Lanzhou, Zhangye, Wuwei, and Baiyin), and the humid and semi-humid region, with over 400 mm of precipitation (Dingxi, Linxia Hui Autonomous Prefecture, Tianshui, Longnan, Gannan Tibetan Autonomous Prefecture, Qingyang, and Pingliang) (Figure 1).
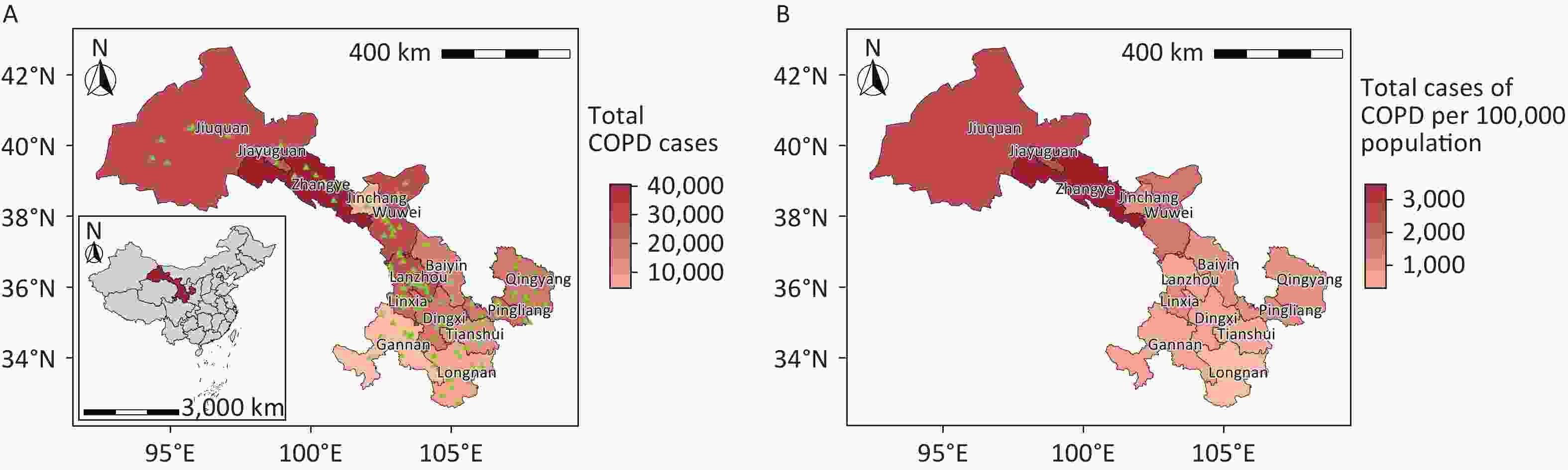
Figure 1. Spatial distribution of chronic obstructive pulmonary disease hospitalizations in Gansu Province, China, showing absolute case numbers (A) and incidence rates per 100,000 population (B). Hospital locations are indicated by green triangles. COPD, chronic obstructive pulmonary disease. Map approval number: GS(2024)1158.
This study analyzed data from 324 hospitals in Gansu Province collected from the Medical Information Management System in Gansu between January 1, 2018, and December 31, 2022. The dataset included information on sex, age, admission and discharge dates, total inpatient costs, disease diagnosis, and diagnosis codes for all COPD hospitalization cases. The International Classification of Diseases, Tenth Revision code relevant to this research was J44 (COPD).
-
We collected pollutant data for each hospital location from the China National Environmental Monitoring Centre (https://www.cnemc.cn/sssj/) for the study period January 1, 2018, to December 31, 2022. These data included the daily maximum 8-hour concentration of ozone and daily average concentrations of PM2.5, PM10, nitrogen dioxide, sulfur dioxide, and carbon monoxide. In addition, we obtained daily meteorological data from the Gansu Provincial Meteorological Bureau, including the daily average air temperature (Tmean) and relative humidity (RH) for each city in Gansu Province throughout the study period.
-
In the absence of official city-level records for SDS, we established SDS event criteria based on PM10 concentrations and the PM2.5/PM10 ratio, following methods from previous studies[12,14]. The regional background PM10 concentrations for the target dates were determined by calculating the median PM10 values within a 15-day moving window centered on each target date, encompassing 7 days before and 7 days after the measurement day. For each city, SDS events were defined as days when both of the following criteria were met: (1) the mean PM10 concentration difference from the regional background value was greater than 150 μg/m3 (aligned with the threshold set by the China National Air Environment Quality Grade II Standard); and (2) the daily PM2.5/PM10 concentration ratio was less than 0.4. The reduced PM2.5/PM10 concentration ratio demonstrates a significant correlation with long-range transported dust particles, serving as a critical diagnostic parameter for differentiating dust storm events from non-dust weather[14,29]. In China, the PM2.5/PM10 concentration ratio has typically been observed to be less than 0.4 during SDS events. Therefore, our definition of an SDS event is based on the PM2.5/PM10 concentration ratio, which is consistent with the methodology used by Zhang et al. and Zhou et al[12,14].
-
A space-time-stratified case-crossover analysis was performed to assess the associations between ambient air pollutants, meteorological factors, and daily COPD hospitalization. Unlike conventional case-control studies, the case-crossover design inherently controls for time-invariant confounders because each participant serves as their own control. Specifically, the case and control periods were matched through a stratified approach, selecting reference days on the same calendar day within the same month and year or from adjacent weeks within the study region. Each case period was matched with three to four control days selected from adjacent weeks within the same calendar month, a strategy that controlled for the day of the week, seasonality, long-term trends, and spatial variation bias. This methodological approach effectively controls for both time-varying and time-invariant individual characteristics[30]. Air pollution and meteorological data for each patient location were represented as city-level values[31].
Previous studies have shown that PM exposure can have a lag effect on COPD, with the largest effects typically occurring within seven days[21,32,33]. Based on an initial analysis (Supplementary Figure S1) and previous research, we quantified the lag-specific effects of PM exposure (lag 0–7 days) using multi-pollutant modeling frameworks that accounted for the temporal dynamics between pollutant concentrations and COPD hospitalization risks. The formula used is as follows:
$$ \begin{aligned} & Log\left[E\right({Y}_{t}\left)\right]=\alpha +{\beta }_{t}{PM}_{t}+ ns(Tmean,df=6)+\\ &ns(R{H}_{t}, df=3)+factor(stratum)+v({Holiday}_{t}) \end{aligned} $$ (1) t is the day of observation; E(Yt) represents the number of daily COPD hospitalizations on day t; α is the intercept; PMt is the air pollutant concentration on day t with its coefficient βt; ns is the natural cubic spline for nonlinear variables; Tmean and RH are the average temperature and average relative humidity on day t, with the degrees of freedom (df) of 6 and 3, respectively; stratum is time strata; Holiday is a binary variable representing public holidays, with its coefficient v; the choice of df for the variable terms is based on Akaike Information Criterion for the minimization model. The relationship between PM exposure and hospitalization for COPD in Gansu Province was measured using the relative risk (RR) and corresponding 95% confidence intervals (CI).
The PM-related burden of COPD hospitalization The attributable fraction (AF) and attributable number (AN) are epidemiological measures that quantify the disease burden attributable to specific exposures, including environmental pollutants or behavioral risk factors within a defined population[34]. Specifically, AN represents the number of increased COPD hospitalizations attributable to PM exposure, while AF quantifies both the proportion of COPD hospitalizations in Gansu Province attributable to PM exposure and the potential reduction achievable when PM levels remain below health-based thresholds[35]. The formula used is as follows:
$$ A{N}_{t}={N}_{t}\times ({RR}_{t}-1)/{RR}_{t} $$ (2) $$ {AF}_{t}=\frac{\sum _{t}{AN}_{t}}{\sum _{t}{N}_{t}} _{ } $$ (3) t is the day of observation; ANt is the number of attributable hospitalizations on day t; Nt is the number of COPD hospitalizations observed on day t; and RRt is the risk of hospitalization associated with PM exposure on day t.
-
To assess model robustness, we conducted sensitivity analyses using two approaches: (1) constructing two-pollutant models with PM2.5, PM2.5-10, and PM10 as primary predictors and (2) evaluating the stability of PM exposure effects on COPD hospitalizations by varying degrees of freedom (df = 3–6 per year) for temporal control.
We conducted several stratified analyses according to sex (male and female), age (0–49, 50–59, 60–69, 70–79, and ≥ 80 years), region (arid, semi-arid, humid, and semi-humid regions), and season (warm season from April to September and cold season from October to March of the following year). All analyses and plotting were performed using the R software (version 4.3.3; https://cran.r-project.org). A P value < 0.05 was considered statistically significant.
-
From January 1, 2018, to December 31, 2022, Gansu Province recorded 265,379 COPD hospitalizations, with men accounting for 153,527 cases (57.85%) and women accounting for 111,852 cases (42.15%). The majority of patients were over 60 years of age (84.01%). Hospitalizations were more frequent during the cold season and in the semiarid region of Gansu Province. Zhangye (3,462.45 per 100,000 people), Jiuquan (2,587.29 per 100,000 people), and Jiayuguan (2,118.66 per 100,000 people) reported the highest number of COPD cases per 100,000 people; these cities are primarily located in arid regions. The total expenses per hospitalization were higher for patients aged ≥ 80 years, in arid regions, and in Lanzhou and Jiayuguan (Table 1 and Figure 1).
Variable Cases (%) Total expenses per hospitalization (Mean ± SD)* Total 265 379 6 029.41±4 895.61 Gender Male 153 527 (57.85) 6 184.91±5 184.71 Female 111 852 (42.15) 5 814.34±4 456.08 Age (year) 0– 8 903 (3.35) 5 291.93±4257.34 50– 33 535 (12.64) 5 676.91±4 979.53 60– 79 710 (30.04) 5 828.90±4 615.70 70– 103 715 (39.08) 6 090.99±4 851.16 ≥ 80 39 516 (14.89) 6 747.94±5 503.44 Season Cold 143 999 (54.26) 5 986.62±4 778.77 Warm 121 380 (45.74) 6 080.19±5 030.27 Region Arid region 39797 (15.00) 5 927.93±4 258.96 Semi-arid region 112914 (42.55) 6 399.18±5 632.52 Humid and semi-humid region 112668 (42.45) 5 745.37±4 298.26 City (Prefecture) Lanzhou 28 315 (10.67) 8 853.81±8 251.58 Tianshui 13 000 (4.90) 5 971.46±4 396.31 Dingxi 20 328 (7.66) 5 560.75±2 951.48 Longnan 7 941 (2.99) 5 549.28±3 179.02 Qingyang 20 336 (7.66) 5 776.54±6 480.11 Linxia 24 965 (9.41) 6 272.48±3 652.98 Pingliang 21 502 (8.10) 5 517.85±4 038.58 Baiyin 16 127 (6.08) 6 102.87±4 797.78 Wuwei 27 996 (10.55) 5 780.47±4 319.56 Zhangye 40 476 (15.25) 5 228.05±3 593.20 Jiuquan 28 054 (10.57) 5 908.04±4 342.23 Gannan 4 596 (1.73) 4 324.60±1 881.32 Jinchang 5 601 (2.11) 4 812.50±2 856.81 Jiayuguan 6 142 (2.31) 7 035.93±4 648.62 Note. COPD, chronic obstructive pulmonary disease; SD, standard deviation; * unit, Chinese Yuan (CNY). Table 1. Demographic characteristics of COPD hospitalization cases in Gansu Province during 2018–2022
During the study period, Gansu Province experienced 791 sand and dust events, predominantly in winter and spring (Supplementary Figure S2). The arid region of northwestern Gansu Province exhibited a significantly higher SDS event frequency than the humid and semi-humid areas, with Jiuquan recording the highest incidence at 128 events (Supplementary Figure S3). Notably, PM concentrations during SDS days showed substantial increases, reaching levels three to six times higher than the 5-year average (Table 2). The increase in concentrations of PM10 (349.80 ± 295.94 μg/m3) and PM2.5–10 (265.73 ± 231.23 μg/m3) was more pronounced compared to that of PM2.5. Temporal variations in air pollutant concentrations, meteorological parameters, and COPD hospitalization records in Gansu Province (2018–2022) were systematically analyzed (Supplementary Figures S4–S5).
Air Pollutants Mean±SD Minimum P25 Median P75 Maximum PM2.5 (μg/m3) 29.33±19.55 8.43 17.41 25.29 36.29 369.71 PM2.5−10 (μg/m3) 47.74±64.40 3.00 24.73 33.79 48.64 1532.29 PM10 (μg/m3) 77.07±80.98 13.43 43.79 61.86 84.00 1902.00 SDS PM2.5 (μg/m3) 84.07±72.23 13.00 51.67 68.00 93.20 649.40 SDS PM2.5−10 (μg/m3) 265.73±231.23 10.33 163.00 206.33 310.50 2101.90 SDS PM10 (μg/m3) 349.80±295.94 35.00 220.50 271.67 400.67 2596.50 Note. SD, standard deviance; P25, 25th percentile; P75, 75th percentile. Table 2. Descriptive statistics of particulate matter pollutants over 2018−2022 in Gansu Province
-
Figure 2 demonstrates that each 10 μg/m3 increase in PM2.5, PM2.5-10 and PM10 significantly raised the risk of hospitalization for COPD in Gansu Province from lag 0 to 7 days. In single-day lag analyses, PM2.5 and PM2.5-10 exhibited peak associations with COPD hospitalization risk at lag 0, whereas PM10 demonstrated maximum significant effects at lag 4. The cumulative effect of PM exposure intensified with an increasing number of cumulative days, peaking at lag 07 with RRs of 1.015 (95% CI: 1.012–1.017) for PM2.5, 1.005 (95% CI: 1.004–1.006) for PM2.5-10 and 1.004 (95% CI: 1.003–1.004) for PM10.
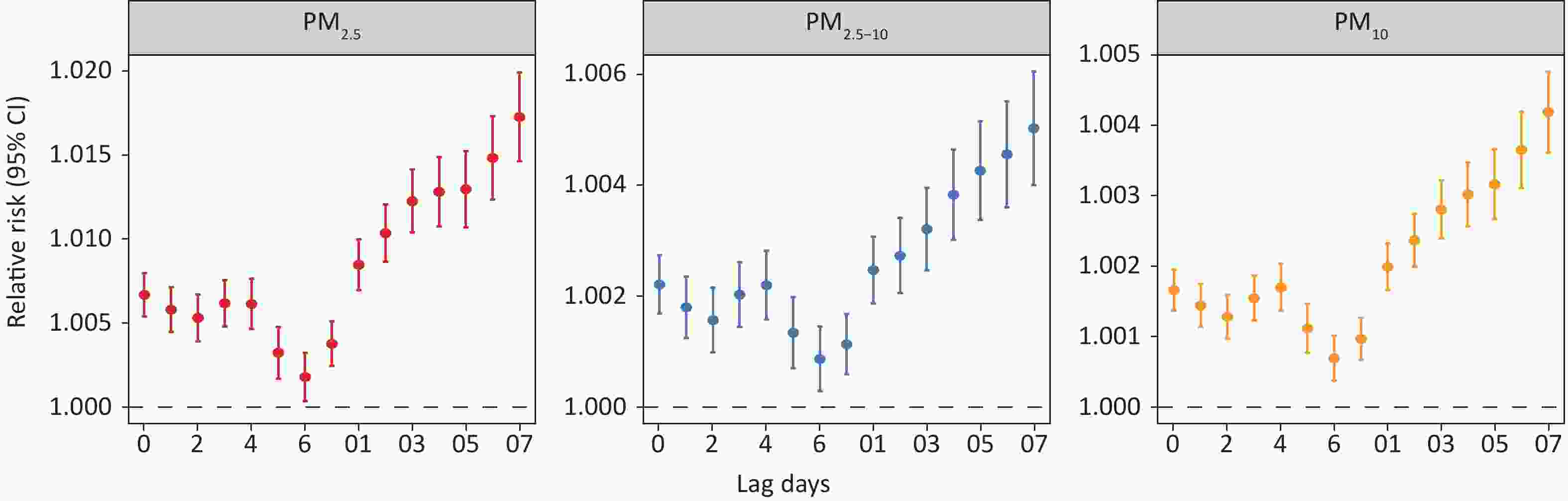
Figure 2. Relative risks of chronic obstructive pulmonary disease hospitalization associated with every 10 μg/m3 increase in PM2.5, PM2.5-10, and PM10 at different lag days. CI, confidence intervals.
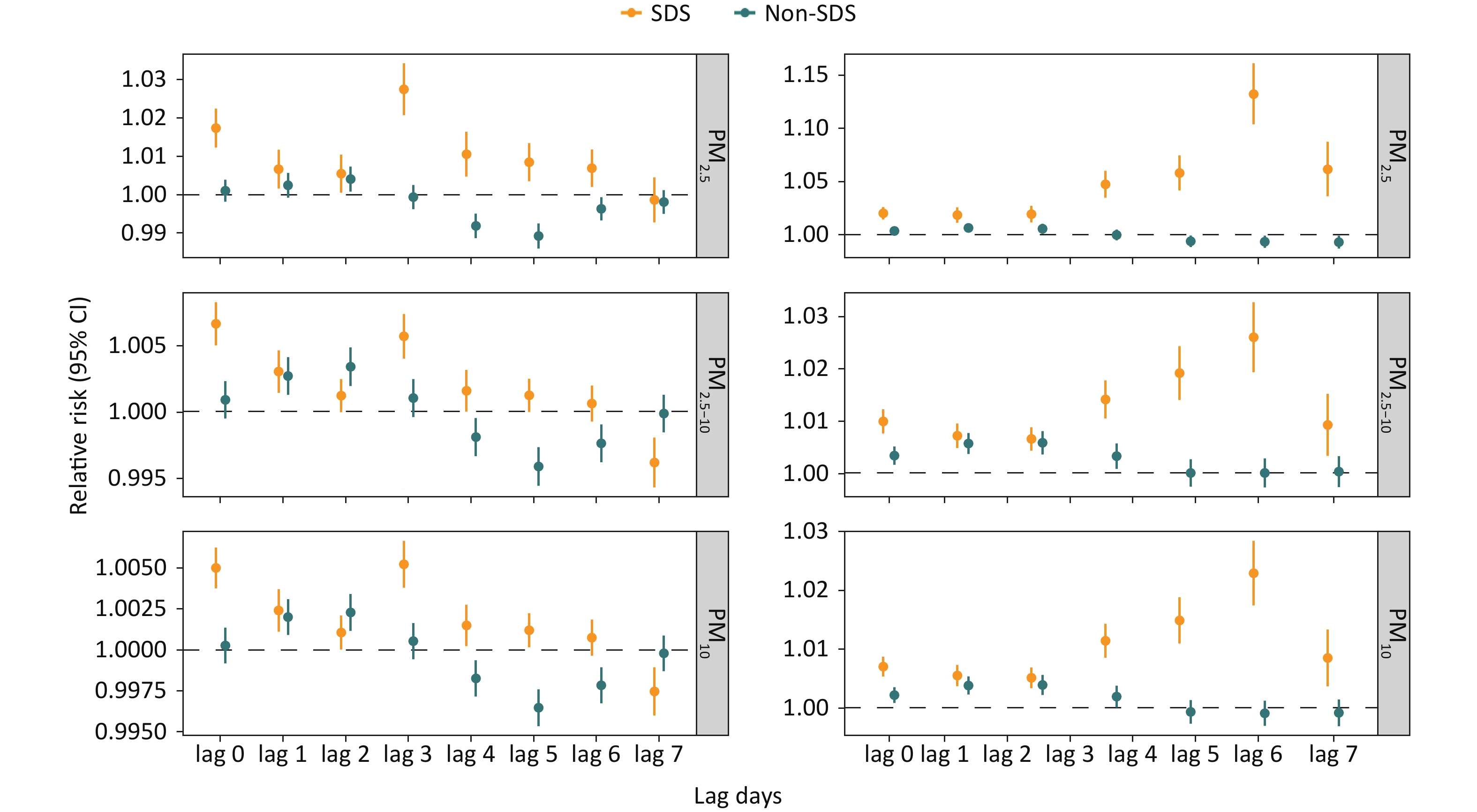
Figure 3 illustrates the differential impact of PM exposure on COPD hospitalization during the SDS and non-SDS periods. Our analysis revealed a markedly elevated COPD hospitalization risk associated with all three PM types during SDS days compared to non-SDS periods, with PM2.5 demonstrating the strongest association. In the single-day lag model, the risk of COPD associated with PM2.5 and PM10 on SDS event days was the highest at a lag of 3 days, with RRs of 1.028 (95% CI: 1.021–1.034) and 1.005 (95% CI: 1.004–1.007), respectively. In contrast, the lagged effect of PM2.5-10 was most pronounced at a lag of 0 days, with RR of 1.007 (95% CI: 1.005–1.008). On non-SDS days, the effects of PM exposure were primarily concentrated at lags of 1–2 days. During the SDS period, the cumulative effect of the three PM types increased with longer cumulative days, beginning with a significant increase at lag 03 and peaking at lag 06, after which the effect gradually diminished.
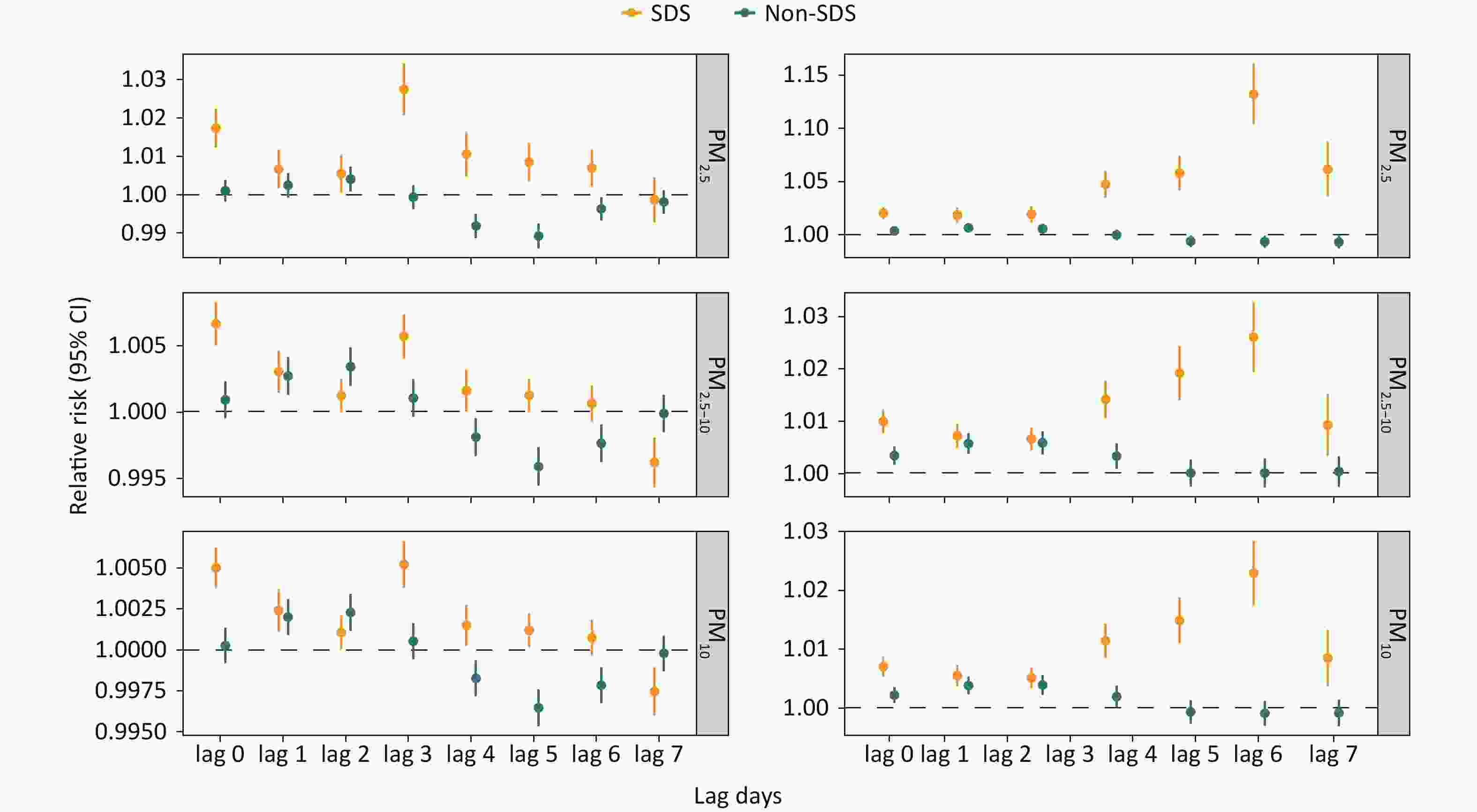
Figure 3. Relative risks of daily hospitalizations for chronic obstructive pulmonary disease associated with PM2.5, PM2.5-10, and PM10 during sand-dust storm (SDS) and non-SDS days on single-day lags (from current day to 7 days before: lag 0-lag 7) and multi-day moving average lags (from lag 0-1 to lag 0-7). SDS, sand-dust storm; CI, confidence intervals.
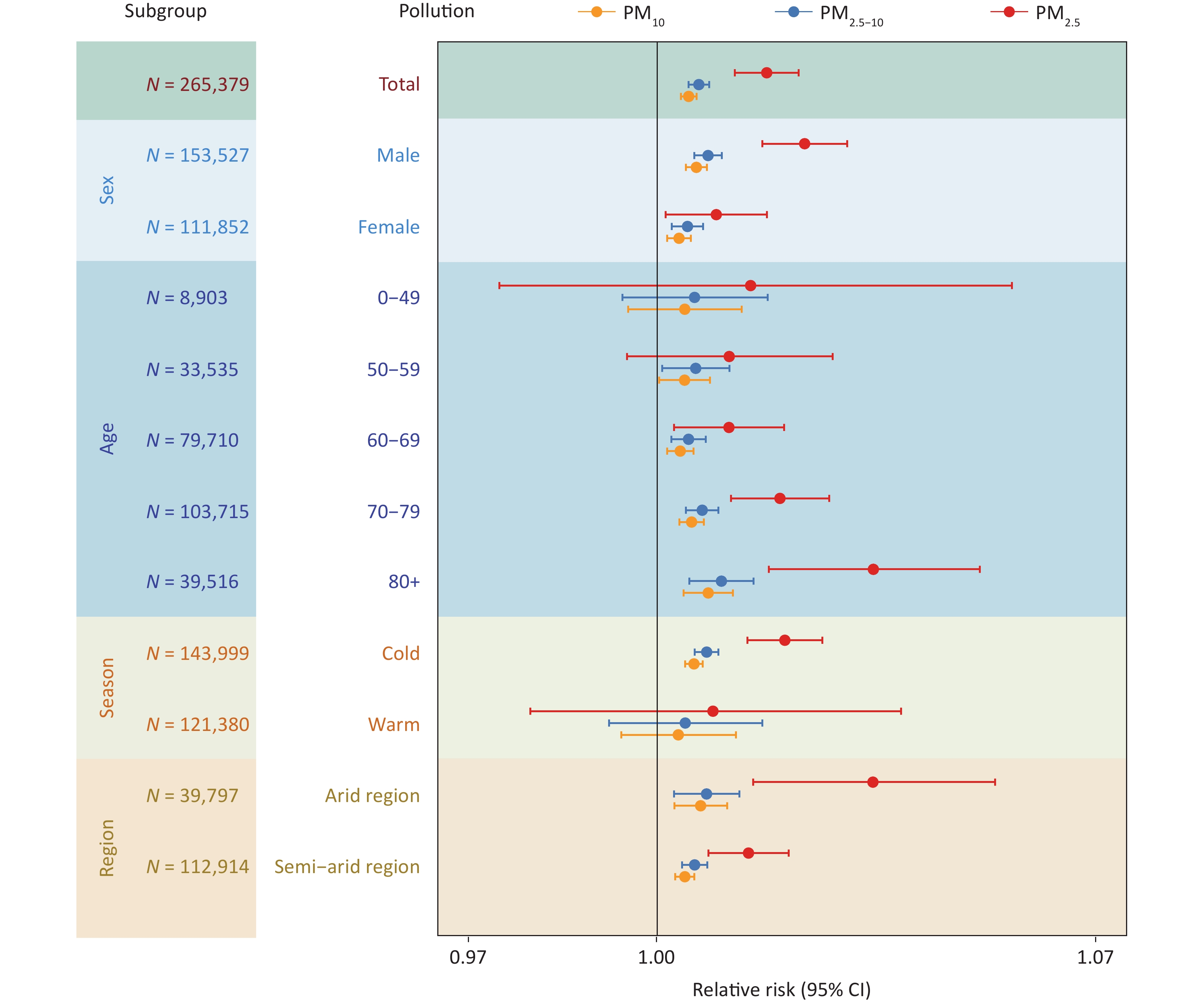
Figure 4 presents the results of subgroup analyses according to sex, age, season, and region. In analyzing of PM-related hospitalization risk on SDS event days stratified by sex, we found that the risk was more pronounced in men (RR = 1.024, 95% CI: 1.017–1.030 for PM2.5; RR = 1.008, 95% CI: 1.006–1.010 for PM2.5–10; RR = 1.006, 95% CI: 1.005–1.008 for PM10) than in women (RR = 1.009, 95% CI: 1.001–1.018 for PM2.5; RR = 1.005, 95% CI: 1.002–1.007 for PM2.5–10; RR = 1.004, 95% CI: 1.002–1.005 for PM10). Exposure to PM2.5, PM2.5–10 and PM10 increased the risk of hospitalization for COPD among individuals over 60 years of age, and this risk progressively increased with advancing age. We found that PM exposure during SDS events had a significantly greater impact on COPD hospitalization in the cold season than in the warm season. The strength of the association between PM exposure and hospitalization for COPD varied between regions. Overall, the association was more robust in arid regions, whereas it was not statistically significant in humid and semi-humid regions, likely because of the lower occurrence of SDS events.
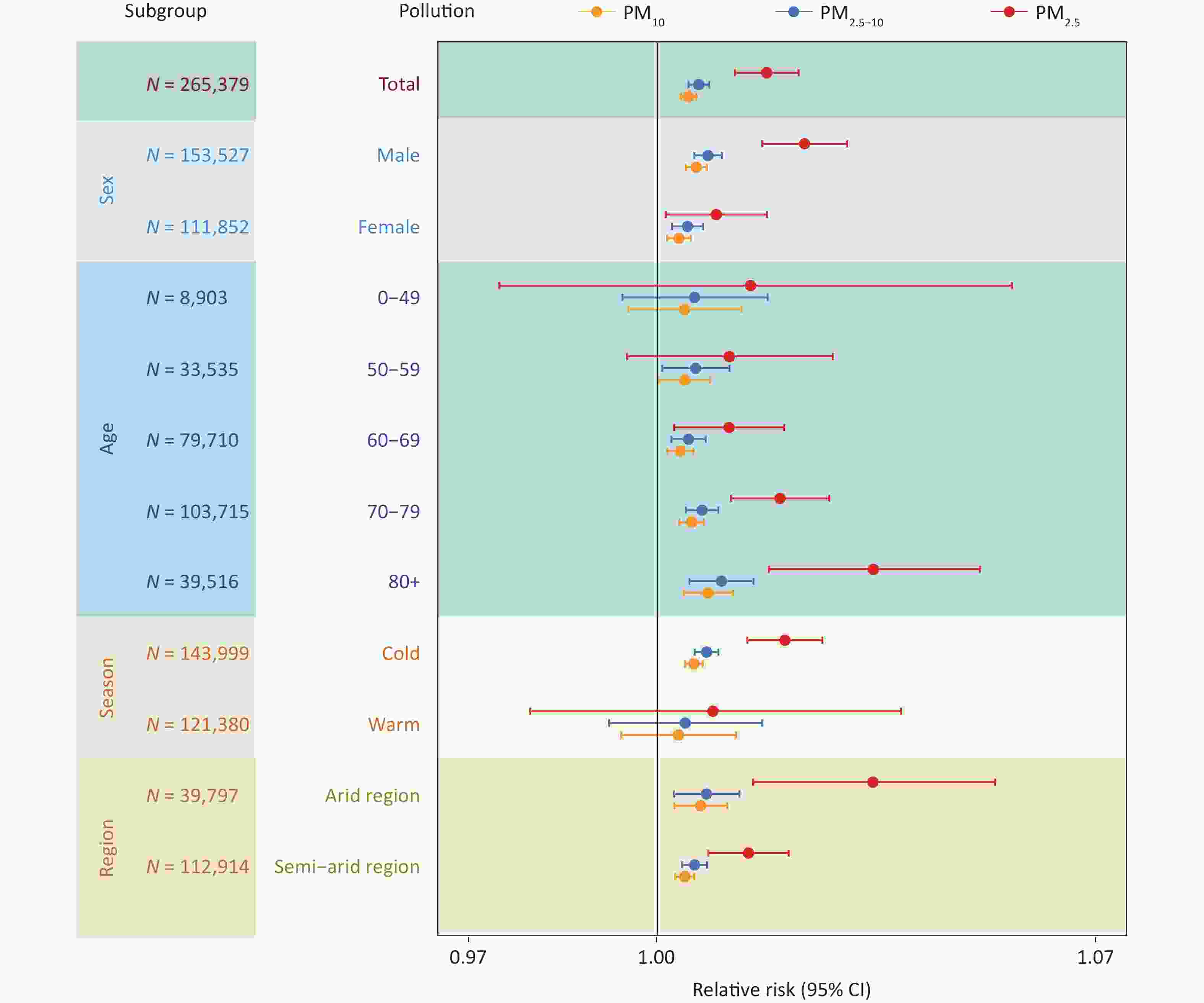
Figure 4. The effect (lag 0 day) of PM2.5, PM2.5-10, and PM10 during sand-dust storm (SDS) days on chronic obstructive pulmonary disease hospitalization across gender, age, season and region. Due to the limited number of SDS events in humid and semi-humid region during the study period, the relative risk (RR) value could not be reliably estimated in these areas. CI, confidence intervals.
-
The occurrence of SDS events significantly contributed to an increased burden of PM-related hospitalization, with 14.88% (95% CI: 13.67%–16.05%), 12.63% (95% CI: 11.43%–13.70%), and 13.74% (95% CI: 12.50%–14.94%) of COPD hospitalization attributed to PM2.5, PM2.5-10 and PM10 during SDS days. The corresponding attributable case numbers were 1,487 (95% CI: 1,366–1,604), 1,262 (95% CI: 1,143–1,378) and 1,373 (95% CI: 1,249–1,493), respectively (Table 3). Subgroup analyses revealed that the attributable fraction of COPD hospitalizations associated with SDS PM2.5 exposure was significantly higher among males, patients aged over 80 years, and during the cold season, particularly for inpatients with COPD in semi-arid regions. Similar patterns were observed for PM2.5–10 and PM10 exposures. At the city level, the burden of coarse particulate matter was predominantly concentrated in cities situated in arid and semiarid regions, such as Zhangye and Lanzhou, which displayed higher attributable numbers (Supplementary Tables S1–S2). Additionally, the Longnan and Tianshui regions exhibited significantly elevated attribution values for PM2.5 exposure. However, the comprehensive assessment of the PM-related disease burden during SDS events in these southern regions was constrained by the relatively low frequency of SDS occurrence, preventing definitive conclusions from being drawn.
Variables SDS PM2.5 SDS PM2.5−10 SDS PM10 Attributable fraction in %
(95% CI)Attributable number
(95% CI)Attributable fraction in %
(95% CI)Attributable number
(95% CI)Attributable fraction in %
(95% CI)Attributable number
(95% CI)Total 14.88
(13.67 to 16.05)1 487
(1 366 to 1 604)12.63
(11.43 to 13.7)1262
(1143 to 1378)13.74
(12.50 to 14.94)1373
(1249 to 1493)Sex Male 18.29
(16.74 to 19.78)1 040
(952 to 1 125)14.68
(13.25 to 16.05)835
(754 to 913)16.17
(14.66 to 17.61)919
(834 to 1002)Female 9.31
(8.61 to 10.00)401
(371 to 431)9.29
(8.46 to 10.11)400
(364 to 435)9.80
(8.98 to 10.61)422
(386 to 457)Age (years) 0−49 −1.57
(−1.68 to −1.47)−4.86
(−5.18 to −4.53)1.14
(1.05 to 1.22)3.51
(3.25 to 3.76)0.67
(0.62 to 0.71)2.06
(2.92 to 2.20)50−59 9.35
(8.66 to 10.04)107
(99 to 114)9.49
(8.65 to 10.32)108
(99 to 118)9.99
(9.16 to 10.81)114
(104 to 123)60−69 13.77
(12.68 to 14.83)425
(392 to 458)12.10
(10.98 to 13.18)374
(339 to 407)13.08
(11.94 to 14.19)404
(369 to 439)70−79 17.39
(15.90 to 18.83)701
(641 to 759)13.58
(12.24 to 14.88)548
(494 to 600)15.06
(13.64 to 16.43)607
(550 to 663)≥80 17.34
(15.83 to 18.79)253
(231 to 274)16.15
(14.44 to 17.79)236
(211 to 260)17.15
(15.44 to 18.79)250
(2254 to 274)Season Cold 16.64
(14.78 to 18.41)885
(786 to 979)10.76
(9.67 to 11.83)572
(514 to 629)12.31
(11.07 to 13.52)655
(589 to 719)Warm 4.15
(3.77 to 4.52)194
(176 to 211)2.83
(2.61 to 3.05)132
(122 to 143)3.59
(3.31 to 3.86)168
(155 to 181)Region Arid region 3.03
(2.69 to 3.37)93
(82 to 103)7.13
(6.35 to 7.90)218
(195 to 242)6.68
(5.98 to 7.37)205
(183 to 226)Semi-arid region 17.90
(15.44 to 20.22)917
(791 to 1 036)12.06
(9.93 to 14.09)618
(509 to 722)13.73
(11.44 to 15.90)704
(586 to 815)Humid and semi-humid region − − − − − − Note. COPD, chronic obstructive pulmonary disease; PM, particulate matter; SDS, sand-dust storm; CI, confidence intervals; Due to the limited number of SDS events in humid and semi-humid region during the study period, the attributable fraction and attributable number could not be reliably estimated in these areas. Table 3. The attributable fraction and attributable number of COPD hospitalization related to SDS-PM pollution in Gansu Province during 2018−2022
-
We selected the lag time corresponding to the maximum RR of the hazardous effect of each PM pollutant (lag 07) and adjusted the corresponding time variables (df = 3–6). After validating the two-pollution model and adjusting for df, no significant change was observed in the relative risk of PM's effect on COPD hospitalization. The results of the model chosen for this study remained relatively robust (Supplementary Figures S6–S7).
-
This space-time-stratified case-crossover analysis, which utilized daily COPD hospitalization records from Gansu Province, provided a comprehensive assessment of the attributable risk and associated burden on patients with COPD from ambient PM exposure, particularly during SDS days. PM exposure significantly increased the risk of COPD hospitalization during SDS days compared with non-SDS days, with PM2.5 specifically contributing to a larger attributable burden. Our findings offer new evidence relating to the importance of control and health protection during SDS weather.
Numerous studies have confirmed the significant health effects of PM exposure on SDS days, particularly the risks associated with respiratory diseases[9,11,36]. A study conducted in Greece found that the number of patients with COPD during SDS weather was 3.6 times higher than on non-SDS days[37]. A time-series analysis in southern Israel demonstrated that short-term exposure to elevated PM10 during dust storms was significantly associated with increased hospital admissions for COPD exacerbations (incidence rate ratio = 1.16; 95% CI: 1.08–1.24).[38] While accumulating evidence highlights the escalating health impacts associated with PM exposure during SDS events, achieving precise quantification of exposure-response relationships remains methodologically challenging. In this study, PM exposure during SDS days significantly increased the risk of COPD hospitalization compared to non-SDS days, with the cumulative lag effect intensifying as the lag time increased. These findings are consistent with previous related studies[37,39,40].
Our findings indicate that PM exposure during SDS events may have a more severe impact on COPD than during conventional PM pollution events. This is likely attributable to the unique characteristics of dust-storm particles, whose underlying mechanisms can be discussed at several levels. Particle size distribution is the primary factor that directly influences the respiratory deposition patterns[41,42]. During SDS weather, dust and particles inhaled by the body first enter the respiratory system. It is well-documented that larger particles are predominantly trapped in the nasal and pharyngeal regions and are efficiently cleared by the mucociliary system, whereas particles smaller than PM10 penetrate deeper into the fine bronchioles and alveoli[43]. Approximately 50% of PM2.5 is retained in the lung parenchyma, where it triggers apoptosis, autophagy, and oxidative stress, which can lead to airway damage[42,44,45]. The complex composition of the SDS particles provides a complementary mechanistic explanation supported by the established literature. Throughout long-range atmospheric transport, dust particles often adsorb a variety of chemical and biological substances, including sulfates, nitrates, polycyclic aromatic hydrocarbons, pollen, bacteria, and fungi[46-48]. Although our study did not assess changes at the cellular level, the results of previous experimental studies support our findings. In rat experiments, dust storm PM2.5 decreased superoxide dismutase activity and glutathione levels in lung tissues and increased thiobarbituric acid reactive substance levels, resulting in an imbalance between pro-oxidants and antioxidants, leading to oxidative damage[49]. In vitro studies have demonstrated that exposure of airway epithelial cells to sand and dust particles decreases cell viability and triggers a proinflammatory response[50]. Thus, it is plausible that the effect of SDS-PM exposure on COPD exacerbation is driven by a cascade of cellular processes, predominantly oxidative stress and the inflammatory response. Elucidating the specific contributions of these pathways warrants further mechanistic investigation.
Our study revealed a more pronounced impact of PM exposure during SDS days on hospitalization for COPD in men than in women. This finding aligns with that of previous research[27], possibly due to the higher smoking prevalence in men and sex differences in airway deposition patterns[51]. Furthermore, men spend more time working outdoors and are more likely to be exposed to air pollution, placing them at greater risk. Notably, our results demonstrated that exposure to air pollution substantially elevated the risk of COPD hospitalization in individuals aged 60 years and older, consistent with previous research findings[52]. Evidence has shown that older individuals are more susceptible to air pollution owing to reduced immune function, a higher likelihood of comorbidity, and a longer duration of illness[53,54].
The results demonstrate an elevated risk during cold seasons relative to warm periods, consistent with the findings of multiple studies[55]. Supplementary Figure S2 indicates that SDS weather in Gansu Province primarily occurred in winter and spring, leading to a significant increase in the health threat posed by COPD during the cold season. Numerous studies have confirmed that the impact of air pollution on hospitalization for COPD is greater during the cold season than during the warm season.[56-58] Our previous findings also demonstrated that combined cold stress and PM2.5 exposure significantly amplified inflammatory responses and oxidative stress in COPD rats, mediated through Ang-II/NF-κB pathway activation and Nrf2 signaling suppression[59,60]. However, further studies are needed to confirm the seasonal association between SDS events and hospitalization for COPD.
This study revealed the spatiotemporal heterogeneity of the risk and burden of PM-related COPD hospitalizations in the arid and semi-arid regions of China. In the northwestern and central parts of Gansu Province, the combination of dry climate, low precipitation, high wind speeds, and sparse vegetation has resulted in a particularly high frequency of SDS and subsequent COPD hospitalization. Dunhuang and Lanzhou are two of the cities most affected by air pollution in Northwest China, primarily because of spring dust storms that lead to exceedances in PM10 levels.[25,61] Moreover, the diverse atmospheric transport mechanisms of SDS result in significant spatial variations in both the chemical composition and particle size distribution of airborne dust. The regional heterogeneity in dust occurrence aligns with inherent climatological characteristics, where humid regions naturally experience fewer dust events. Such spatial variations reflect distinct patterns of dust storms across different climatic zones. Although the limited number of dust events in humid regions poses methodological challenges for risk assessment, these areas remain vulnerable to exposure to other forms of PM exposure[23]. The southern part of Gansu Province is predominantly mountainous and features plateaus, making it susceptible to localized pollutant accumulation because of weak winds and limited dispersion.[62] Simultaneously, biomass burning for heating worsens indoor air pollution and poses a significant threat to respiratory health. Differences in risk across various regions and cities in Gansu Province may be related to many unmeasured factors, including varying meteorological and medical conditions, socioeconomic status, industrial development, and living habits.
The main strength of our study is the comprehensive and detailed statistics of all COPD hospitalizations over 5 years. The substantial sample size significantly enhanced the reliability and representativeness of our research findings. Second, we utilized a space-time-stratified case-crossover design to quantify the acute association between PM exposure on SDS days and COPD hospitalization at the provincial and regional levels, which strengthened the validity of the study’s conclusions. Third, this study evaluated the attribution burden of PM exposure in SDS weather conditions, offering policymakers key insights for resource allocation in pollution control and a scientific basis for policymaking. The vast territory and diverse, predominantly arid climate of Gansu provide unique research opportunities. This study fills a crucial gap by examining arid northern regions. Moreover, the climatic diversity within Gansu offers unique supplementary evidence.
This study has several limitations. First, this study is ecological, and therefore, may be subject to ecological fallacies owing to the inherent limitations of the methodology. Second, we used city-level air pollution data, which may not accurately reflect individual exposure levels. Additionally, the lack of a precise definition of SDS events can lead to misclassification. Finally, despite conducting sensitivity analyses to ascertain the robustness of our findings and employing a case-crossover design to mitigate the impact of time-invariant confounders, it remains possible that certain time-varying factors such as influenza outbreaks and fluctuating pollen counts were not adequately addressed. Moreover, individual-level factors, such as comorbid conditions or treatment use, were not included, which could have influenced the observed associations.
The results of this study have significant implications for managing dusty weather and its associated health risks in the Gansu Province. First, as climate change intensifies, SDS weather is expected to increase, highlighting the urgent need for effective early warning systems for SDS events and the establishment of appropriate air quality standards. Second, this study found that SDS events significantly increased the COPD hospitalization burden associated with PM exposure. This underscores the need for the healthcare sector to optimize the allocation of healthcare resources in response to SDS weather warnings, particularly for sensitive and high-risk populations. Finally, public health education on SDS events should be enhanced to raise awareness of the health threats posed by SDS weather and to promote protective measures such as wearing masks and minimizing outdoor activities.
-
This province-specific study, performed in the arid and semi-arid regions of China, provides additional evidence linking short-term PM exposure on SDS days with an elevated risk of COPD hospitalization. This association was particularly pronounced among men and individuals over 60 years of age, during the cold season, and in arid and semiarid regions. Our study highlights the significant health impacts of SDS events in the Gansu Province and emphasizes the need to develop policies aimed at environmental improvement, particularly in arid and semiarid regions. Urgent action is needed to mitigate the growing burden of SDS events and develop effective preventive strategies to reduce their harmful effects.
Sandstorm-Driven Particulate Matter Exposure and Elevated COPD Hospitalization Risk in Arid Regions of China: A Spatiotemporal Epidemiological Analysis
doi: 10.3967/bes2025.134
- Received Date: 2025-06-28
- Accepted Date: 2025-09-11
-
Key words:
- Sand-dust storms /
- Particulate Matter /
- Chronic obstructive pulmonary disease /
- Hospitalization /
- Disease burden.
Abstract:
The authors declare that they have no competing interests.
All data used in this study were aggregated with no identifiable individual information and were processed by designated professionals from the Health Commission of Gansu Province before being authorized for use in the study. Ethical approval was obtained from the Medical Research Ethics Review Committee of the School of Public Health, Lanzhou University, China (No. IRB25011401).
&These authors contributed equally to this work.
| Citation: | Hao Zhao, Ce Liu, Erkai Zhou, Baofeng Zhou, Sheng Li, Li He, Zhaoru Yan, Jiabei Jian, Huan Chen, Huanhuan Wei, Rongrong Cao, Bin Luo. Sandstorm-Driven Particulate Matter Exposure and Elevated COPD Hospitalization Risk in Arid Regions of China: A Spatiotemporal Epidemiological Analysis[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2025.134 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: