-
Gastroenteritis is an infectious diarrhea that has been considered as an important cause of hospitalizations and death in children aged < 5 years, particularly in developing countries. Unsanitary water, contaminated food, poor hygiene, and inadequate disposal of waste and feces are all risk factors for gastroenteritis, resulting in the higher incidence in developing countries. Gastroenteritis is generally caused by viral infections, among which rotavirus (RV) infections have been reported to be the most common, especially among young children aged < 5 years with acute gastroenteritis in Asia and Africa[1]. Other viruses associated with acute gastroenteritis include human Adenovirus (HAdV), Norovirus, Sapovirus (SaV), human Astrovirus (HAstV), and Aichi virus. Recent research has reported that adenovirus types 40 and 41, belonging to species F, cause gastroenteritis and were therefore termed as enteric adenoviruses. In addition, non-enteric HAdV species such as A, B, C, and D have been associated with diarrhea[2].
Acute gastroenteritis caused due to viral infection in children in Fujian has not yet been investigated in detail. Therefore, we attempted to explore the prevalence of RV of group A (RVA) and HAdVs among children with gastroenteritis and determine the predominant types of the two viruses circulating in Fujian from February 2009 to December 2017.
This study was conducted in Fujian, a province with a typical subtropical monsoon climate, with approximately 36.9 million inhabitants and an area of 124, 000 km2 located in southeastern China. All children hospitalized in Fuzhou Children's Hospital in the capital city and Pingtan County Hospital (a general hospital in Pingtan Island) with acute gastroenteritis aged < 5 years were enrolled. An acute gastroenteritis case was defined as defecation for three or more times a day with a change in the characteristic of stool or vomiting. Specimen and individual information were collected. Stool samples were collected monthly from children admitted to the designated hospitals with diarrhea from 2009 to 2017. Details regarding the patient's demographic characteristics, epidemiological and clinical data such as manifestations, and therapy were collected. Every specimen was assigned an 8-digit ID based on a naming convention, including the collection year, the institution code, and the serial number. The requirement for an informed consent was waived due to the regular long-term surveillance.
Fecal samples were screened for RVA antigen using Prospect Rotavirus Microplate Assay (Oxoid, UK), and nucleic acid was extracted using the EX-RNA/DNA reagents on an automatic NP968S nucleic acid extractor (Tianlong, China). For RVA genotyping, the viral genes of VP7 and VP4 were amplified using a semi-nested RT-PCR method with a cocktail of specific primers, respectively[3]. For HAdV serotyping, the hexon gene of HAdV was amplified[4] and purified by gel extraction and sequenced. Sequences of HAdV were aligned using the Clustal program, and phylogenetic relationships were determined by the maximum likelihood method using the Mega software (ver. 6.0, America). The SPSS Statistics software (ver. 17.0, America) was used for data analysis. Pearson's chi-square and Fisher's exact tests were used, and P values < 0.05 were considered as statistically significant.
A total of 2, 745 stool specimens were collected from February 2009 to December 2017. Among the study patients, 1, 808 were males and 937 were females with a sex ratio (male/female) of 1.93. Median age of the patients was 0.83 years, and mean age was 1.12 ± 0.93 years. Regarding age distribution, 747 children (27.21%) were aged ≤ 6 months, 940 (34.24%) were aged 6-12 months, 710 (25.87%) were aged 13-24 months, and 348 (12.68%) were aged 2-5 years. Diarrheal disease was predominant in the 6- to 12-month age group.
The results demonstrated that the total positive rate of the two viruses was 26.96%. The positive rate of RVA (23.5%) was significantly higher than that of HAdV (3.86%, χ2 = 342.9, P < 0.0001). The mean prevalence of RVA was lower than Tianjin (27.94%)[5] and HAdV was significantly different from Beijing (9.8%)[6]. The reason for this difference may be attributed to the different conditions of these studies, such as the target population, the period of sampling, and the distinct geographic location. Unexpectedly, the positive rate of these two viruses was significantly lower among male children (25.5%) than among female children (29.77%, χ2 = 5.736, P < 0.017). In addition, the prevalence of the viruses exhibited a significant age difference (χ2 = 84.87, P < 0.0001). The hospitalized children aged > 6 months had higher positive rates, especially the rate of RVA infection, than the children aged < 6 months. This finding might be attributed to the steady loss of maternal antibody among children. Considering each virus, the prevalence of RVA was significantly different across age groups (χ2 = 86.05, P < 0.0001), ranging from 12.18% (among children aged < 6 months) to 31.91% (among children aged between 1 and 2 years). Although the prevalence of HAdV ranged from 2.95% (among children aged < 6 months) to 6.03% (among children aged between 2 and 5 years), the difference was not statistically significant (χ2 = 6.125, P = 0.106).
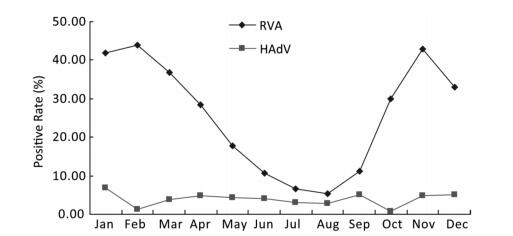
As shown in Figure 1, the prevalence of RVA in Fujian exhibited an obvious seasonality. RVA infection peaked during the autumn-winter season with low temperature, high atmospheric pressure, and relatively low humidity and rainfall, which was similar to that in other regions in Asia such as Hong Kong, China and Bangladesh[7]. In contrast to RVA, the prevalence of HAdV showed no clear seasonality, although the positive rate fluctuated from 0.78% to 6.87%. We also found that the incidence of RVA infections had decreased consecutively from 42.29% in 2010 to 9.71% in 2016 and reverted to 20.41% in 2017 (Figure 2). The reasons for these changes in RVA incidence still remain unknown.
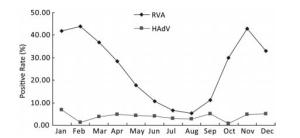
Figure 1. Seasonal distribution of RVA and HAdV in hospitalized children aged < 5 years with acute gastroenteritis in Fujian, 2009-2017.
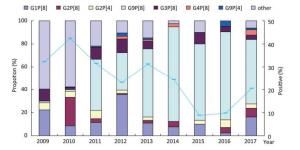
Figure 2. Prevalence of rotavirus genotypes among hospitalized children with acute gastroenteritis aged < 5 years in Fujian, 2009-2017.
Based on the difference in VP7 and VP4, RVA could be further divided into the genotypes of G and P, respectively. Among the detected RVA in this study, six G genotypes (G1, G2, G3, G4, G8, and G9) were determined in 624 (96.74%) samples, and six P genotypes (P[1], P[4], P[6], P[8], P[9], and P[10]) were determined in 566 (87.75%) samples. Among the G genotypes, G9 (269/624, 43.11%) was predominant, followed by G1 (139/624, 22.28%), G2 (108/624, 17.31%), G3 (54/624, 8.65%), G4 (14/6242.24%), G8 (6/624, 0.96%), and other mixed strains (34/624, 5.45%). Among the P genotypes, P[8] (477/566, 84.28%) was detected most frequently throughout 2009-2017, followed by P[4] (50/566, 8.83%), P[6] (5/566, 0.88%), P[9] (4/566, 0.71%), P[10] (3/566, 0.53%), P[1] (1/566, 0.18%), and other mixed strains (26/566, 4.59%). In Mainland China, the most prevalent G-P combinations were G3P[8] (32.1%), G1P[8] (23.0%), and G2P[4] (7.9%) before 2011[8]. Overall, more than 26 combinations of the genotypes G and P were assigned to 551 (85.43%) of the 645 RVA-positive samples. A total of 242 (43.92%) of these strains were G9P[8], 93 (16.88%) were G1P[8], 52 (9.44%) were G2P[8], and 41 (7.44%) were G3P[8]. G1P[8] and G2P[8] were the common combinations detected in 2009 and 2010, respectively. The most predominant genotype of RVA had transformed into G9P[8] since 2011, except the proportion of G1P[8] (35.53%) that was slightly higher than that of G9P[8] (32.89%) in 2012 (Figure 2). However, the genotype of G3P[8] had never predominated among the hospitalized children during our study period. Because Rotarix or RotaTeq vaccine recommended by the WHO had not been introduced in China and due to the limited use of Lanzhou lamb rotavirus vaccine belonging to G10P[15][9] during the study period, we inferred that the genotype substitution of RVA in Fujian would not be due to selective pressure related to vaccination. Considering the high prevalence and the rapid evolutionary rate of G9P[8], our results suggest that the proportion of G9P[8] has increased gradually and had been in its peak epidemic situation. For a better understanding of endemic dynamics and genotypic distribution of RVA, continuous epidemic and etiological surveillance is necessary.
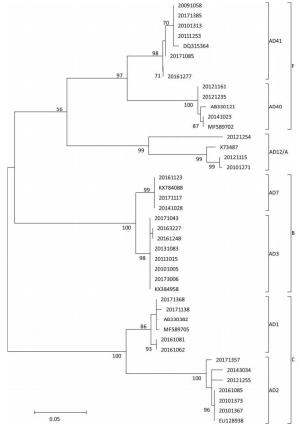
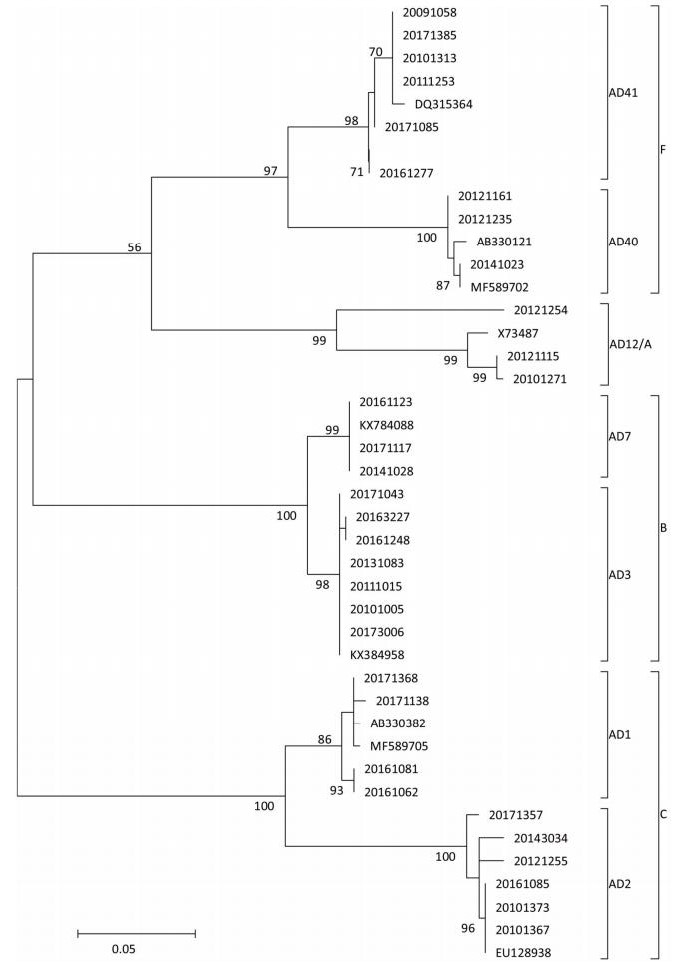
Figure 3. Phylogenetic tree constructed based on hexon gene of adenovirus strains collected in hospitalized children aged < 5 years with acute gastroenteritis in Fujian, 2009-2017. Specimen was assigned an 8-digit ID based on a naming convention, year, institution code, and serial number. Phylogenetic tree was constructed using the method of maximum likelihood, and the number of bootstrap replications was 1, 000.
HAdVs are double-stranded, linear DNA viruses with a genome of 34-37 kb. HAdVs belong to the family Adenoviridae, genus Mastadenovirus, which contains seven known species from A to G. In addition, at least 60 serotypes of HAdV have been reported till date based on traditional hemagglutination and serum neutralization reaction or modern molecular biological techniques. Although the majority of publications have reported HAdV40 and HAdV41 as the primary agents causing viral diarrheal disorders, especially HAdV41[10], to our surprise, of the successfully determined adenovirus-positive specimens, HAdV3 was found to be the most common serotype (23/64, 35.94%), followed by HAdV41 (16/64), HAdV2 (10), HAdV1 (6), HAdV40 (3), HAdV7 (3), and HAdV12 (3). The constituent ratio of non-enteric HAdV exceeded 68%. These seven serotypes of HAdV belonging to the species of A, B, C, and F were identified by phylogenetic analysis (Figure 3). The genotype composition varied significantly from year to year. This study provides evidence about the contribution of adenovirus in causing gastroenteritis in young children. Non-enteric HAdV might play an important role in causing diarrhea in children, although it is primarily caused due to respiratory tract infections.
In conclusion, RVA is an important etiology of acute gastroenteritis in hospitalized children aged < 5 years in Fujian. The genotypic distribution of RVA varied over the years. Therefore, further research is required to determine the distribution of the RV strain genotypes, which will contribute toward establishing vaccine development strategies for RV in the world. Although enteric adenovirus was the frequently detected adenovirus among children in the present study, the role of non-enteric adenoviruses should not be ignored in children with diarrhea. Our study enrolled only hospitalized children, excluding those who had visited the outpatient department due to diarrhea. However, the larger sample size (2, 745) of our study spanning from 2009 to 2017 may represent a part of real epidemiological data of the viral diarrheal disease in Fujian, although a broader etiological spectrum should be further included to clarify the epidemiology.
Our results should prove useful for patient management, especially regarding antibiotic usage and vaccination.
We thank relevant personnel of the designated hospitals for their assistance in data and specimen collection, CHENG ZH, LIN L, LIU ZJ, TU JJ, LI Q.
No conflict of interests declared.
This article does not contain any studies with human participants or animals performed by any of the authors.
Prevalence and Genotypes of Rotavirus A and Human Adenovirus among Hospitalized Children with Acute Gastroenteritis in Fujian, China, 2009-2017
doi: 10.3967/bes2019.028
major project granted by the Science and Technology Ministry of China 2017ZX10104001
- Received Date: 2018-12-12
- Accepted Date: 2019-03-04
| Citation: | WU Bing Shan, HUANG Zhi Miao, WENG Yu Wei, CHEN Feng Qin, ZHANG Yun Lin, LIN Wei Dong, YU Ting Ting. Prevalence and Genotypes of Rotavirus A and Human Adenovirus among Hospitalized Children with Acute Gastroenteritis in Fujian, China, 2009-2017[J]. Biomedical and Environmental Sciences, 2019, 32(3): 210-214. doi: 10.3967/bes2019.028 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: