-
Patients with heart failure (HF) often have a poor prognosis, with high morbidity and mortality. In the Chinese adult population, the prevalence of HF increased by 44% in the past 15 years, which was 1.3%[1]. HF is often associated with multiple organ disorders[2]. Renal insufficiency is common in patients with HF, and patients with chronic kidney disease (CKD) are significantly more likely to develop HF than patients without CKD. The incidence of HF with CKD ranged from 17% to 21%, with HF being the main cause of mortality and morbidity in patients with CKD. More importantly, in patients with cardiac and renal dysfunction, the presence of one condition tends to worsen the presentation and progression of the other[3]. Patients with HF and CKD associate with remarkably increasing risk of hospitalization, re-hospitalization, kidney replacement therapy, and death, but most patients with renal insufficiency were excluded from HF related studies[3]. Therefore, it is particularly important to identify such high-risk patients.
Shock index (SI) is calculated according to the formula (ratio of heart rate/systolic blood pressure [SBP]) that is easily obtained at the bedside[4]. In previous research, SI was used to evaluate acute circulatory failure and hemorrhage [5,6]. Previous research also found that SI was a useful clinical index for quick risk stratification in various critical care scenarios. A higher SI value was associated with mortality, microvascular damage, and myocardial injury [5,6]. Meanwhile, SI is a more accurate index of hemodynamic status rather than just heart rate or systolic blood pressure alone. In elderly patients, however, SI may be less sensitive for early screening of shock because of the variation of age. In recent years, researchers added new parameters based on SI to improve its prognostic value: such as modified shock index (MSI) and age-adjusted shock index (ASI). MSI is calculated by mean arterial pressure (MAP), which was a major driver of vital organ perfusion and strongly associated with HF and all-cause mortality. During diastolic phase, the myocardium receives blood supply, and previous studies have reported that extremely low dilated blood pressure (DBP) might lead to adverse prognosis. Therefore, it is reasonable and necessary to use MAP instead of SBP to calculate MSI. Meanwhile, the incidence of all-cause mortality is higher in elderly patients due to a higher prevalence of co-morbidity, making age an important predictor in patients with HF. ASI was developed by considering the effect of age [7].
In the present study, we mainly discuss whether SI, MSI, or ASI are effective prognostic indicators in predicting in-hospital mortality in patients with HF and CKD. Additionally, the predictive performance of SI, MSI, ASI are compared.
The current study population was based on a retrospective observational cohort that enrolled consecutive patients with HF in ShengJing Hospital of China Medical University from January 2013 to December 2018. HF as the main diagnosis on admission was defined by the modified Framingham criteria. CKD was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2 according to Kidney Disease Improving Global Outcomes guidelines. Patients’ data were electronically recorded in a database that was created for this purpose specifically. The SI was defined as a ratio of heart rate to SBP on admission. MSI was defined as the ratio of heart rate to MAP; ASI was defined as age multiplied by SI [7,8]. eGFR (mL/min per 1.73 m2) = 175 × standardized creatinine (mg/dL)−1.234 × age (year)−0.179 × 0.79 (if female). Blood pressure and heart rate were measured on admission or during the emergency department visit. Fasting venous blood samples were collected from all patients within 24 h of admission. Exclusion criteria were: severe arrhythmia (such as atrial fibrillation) during blood pressure measurement and unavailable creatinine results. A total of 6,045 HF subjects with CKD were enrolled into the study. The primary endpoint of the study was in-hospital mortality. The study complied with the Helsinki Declaration. Shengjing Hospital of China Medical University Research Ethics Committee ratify this study.
Continuous variables were presented as mean ± SD or median (IQR), depending on whether the data was normally distributed. Categorical variables were presented as counts and proportions (%). Patients were categorized into 2 groups depending on in-hospital mortality. The chi-square test or Fisher’s exact test was used to analyze differences in categorical variables between groups. Students T test or the Kruskal-Wallis H test was applied to analyze differences in continuous variables between groups. Predictors associated with the primary endpoint were analyzed through univariate and multivariate analysis. SI, MSI, ASI were analyzed as continuous variables. Results were represented as odd ratios (OR) with an associated 95% confidence interval (CI). In brief, the area under the curve associated with the primary endpoint through the receiver operating characteristic (ROC) curve was calculated using the statistical software MedCalc (version 18.1.1; MedCalc Software, Ostend, Belgium). The subjective risk of primary endpoint was obtained by entering into a logistic regression model. Each model’s calibration potential was examined by Hosmer-Lemeshow (HL) test and Nagelkerke-R2, which can represent contents of goodness-of-fit in each risk model. Brier scores of SI, MSI, and ASI were calculated additionally. Lower Brier scores represented better accuracy. Absolute integrated discrimination improvement (IDI) and category-free net reclassification improvement (NRI) were used to evaluate the improvement in predictive efficiency of SI, MSI, and ASI. Interaction was tested with a likelihood ratio test, and the ORs with associated 95% CIs were represented by forest plots. Data analyses were conducted by Statistical Analysis Software (SAS Institute Inc, Cary, NC) V.9.4. and statistical significance was set at P < 0.05.
A total of 6,045 patients with HF and CKD were enrolled in our study cohort. Table 1 shows the general characteristics of study participants. The average length of hospitalization was 10.2 ± 5.5 days. In-hospital mortality occurred in 329 (5.4%) patients. Membership in the group that occur in-hospital mortality was associated with older age, worsen NYHA grading, higher heart rate on admission, lower SBP and dilated blood pressure (DBP) on admission, higher SI, MSI ASI, and proportion on the history of coronary artery disease (CAD). Patients with in-hospital mortality also had higher total bilirubin (TBIL), blood urea nitrogen (BUN), N-terminal brain natriuretic peptide (NT-proBNP), and cardiac troponin I (cTNI) and lower albumin, eGFR, hemoglobin, and serum Na on admission. For echocardiographic data, left ventricular ejection fraction (LVEF) was significantly worse in patients with in-hospital mortality. Moreover, the logistic univariate analysis demonstrated that the SI were associated with the in-hospital mortality respectively (OR: 4.406, 95% CI: 2.974–6.528, P < 0.001). Adjustment for multiple confounders did not attenuate the prediction (OR: 2.462, 95% CI: 1.363–4.448, P = 0.003) (Table 2).
Table 1. Baseline characteristics of subjects divided by in-hospital mortality, median (IQR), or n (%), or means ± SD
Variables Overall
(n = 6,045)Patients with in-hospital
mortality (n = 329)Patients without in-hospital
mortality (n = 5,716)P value Age, years 72.0 ± 12.2 76.1 ± 11.4 71.7 ± 12.2 < 0.001 Male, n (%) 2,927 (48.4) 157 (47.7) 2,770 (48.5) 0.794 NYHA grading, n (%) < 0.001 II 1,061 (17.6) 15 (4.6) 1,046 (18.3) III 2,447 (40.5) 84 (25.5) 2,343 (41.3) IV 2,537 (41.9) 230 (69.9) 2,307 (40.4) Heart rate on admission, bpm 86.6 ± 23.5 91.5 ± 23.8 86.3 ± 23.5 < 0.001 SBP on admission, mmHg 136.2 ± 26.1 127.2 ± 28.6 136.8 ± 25.8 < 0.001 DBP on admission, mmHg 80.4 ± 24.4 72.7 ± 15.4 80.9 ± 24.8 < 0.001 SI 0.66 ± 0.23 0.75 ± 0.26 0.66 ± 0.23 < 0.001 MSI 0.90 ± 0.29 1.04 ± 0.32 0.89 ± 0.28 < 0.001 ASI 47.2 ± 17.8 56.9 ± 20.5 46.7 ± 17.5 < 0.001 Albumin, g/L 36.5 ± 4.3 34.6 ± 4.4 36.6 ± 4.3 < 0.001 TBIL, umol/L 15.6 ± 11.5 17.6 ± 14.2 15.5 ± 11.4 0.001 LDL, mmol/L 2.55 ± 0.99 2.44 ± 1.03 2.56 ± 0.99 0.06 BUN, mmol/L 10.9 ± 6.2 16.4 ± 9.3 10.6 ± 5.9 < 0.001 eGFR, mL/min per 1.73 m2 40.8 ± 13.8 32.0 ± 14.2 41.4 ± 13.6 < 0.001 Haemoglobin, g/L 122.2 ± 23.4 113.1 ± 26.8 122.8 ± 23.1 < 0.001 Serum Sodium, mmol/L 138.7 ± 4.1 137.2 ± 5.7 138.8 ± 3.9 < 0.001 cTNI, ng/mL 0.05 (0.02, 0.25) 0.19 (0.04, 3.8) 0.04 (0.02, 0.22) < 0.001 NT-proBNP, pg/mL 4,540 (1,606, 8,127) 6,246 (5,890, 12,976) 4,284 (1,518, 7,743) < 0.001 LVEF, % 49.0 ± 11.0 47.8 ± 8.2 49.0 ± 11.1 0.049 Co-morbidities, n (%) CAD 4,202 (69.5) 253 (76.9) 3,949 (69.1) 0.003 Hypertension 4,064 (67.2) 199 (60.5) 3,865 (67.6) 0.007 AF 1,781 (29.5) 82 (24.9) 1,699 (29.7) 0.063 DM 2,114 (35.0) 129 (39.2) 1,985 (34.7) 0.097 COPD 1,292 (21.4) 63 (19.1) 1,229 (21.5) 0.312 Smoking, n (%) 1,724 (28.5) 79 (24.4) 1,645 (29.3) 0.058 Medications, n (%) ACE-I/ARB/ARNI 4,962 (82.1) 212 (64.4) 4,750 (83.1) < 0.001 Beta blocks 4,650 (76.9) 279 (84.8) 4,371 (76.5) < 0.001 Diuretic 5,703 (94.3) 280 (85.1) 5,423 (94.9) < 0.001 Note. The continuous variable data was expressed as median (IQR) or means ± SD, SBP, systolic blood pressure; DBP, dilated blood pressure; SI, shock index; MSI, modified shock index; ASI, age-adjusted shock index; TBIL, total bilirubin; LDL, low density lipoprotein; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; cTNI, cardiac troponin I; NT-proBNP, n‐terminal brain natriuretic peptide; LVEF, left ventricular ejection fraction; CAD, coronary artery disease; AF, atrial fibrillation; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; ARNI, angiotensin receptor blocker-neprilysin inhibitors. Table 2. Effects of multiple variables on clinical outcomes in univariate and multivariate analysis
Variables Univariate analysis Multivariate analysis OR 95% CI P OR 95% CI P SI 4.406 2.974–6.528 < 0.001 2.462 1.363–4.448 0.003* MSI 3.855 2.843–5.227 < 0.001 2.399 1.533–3.754 < 0.001* ASI 1.025 1.020–1.030 < 0.001 1.011 1.004–1.019 0.004** Note. *Adjusted for age, sex, NYHA grading, Albumin, TBIL, LDL, BUN, creatinine, haemoglobin, serum sodium, cTNI, NT-proBNP, LVEF, CAD, hypertension, AF, DM, smoking, ACEI/ARB/ARNI, beta-blockers, diuretic. ** Adjusted same as * except for age. SI, shock index; MSI, modified shock index; ASI, age-adjusted shock index;TBIL, total bilirubin; LDL, low density lipoprotein; BUN, blood urea nitrogen; cTNI, cardiac troponin I; NT-proBNP, n‐terminal brain natriuretic peptide; LVEF, left ventricular ejection fraction; CAD, coronary artery disease; AF, atrial fibrillation; DM, diabetes mellitus; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; ARNI, angiotensin receptor blocker-neprilysin inhibitors. Although the pathophysiology of SI in HF requires further investigation, the following mechanisms may be responsible for the influence of SI on patient outcomes. SI reflects a consolidation of the central nervous and cardiovascular system, and thus a higher SI indicates a hyperactive status of the sympathetic nervous system, which can influence the progression of HF. Meanwhile, the increased release of sympathetic neurotransmitters in the kidney can accelerate glomerular sclerosis, which may worsen the progression of renal function [9].
MSI and ASI are new models that account for MAP and age, respectively. In our study, we found MSI and ASI were associated with in-hospital mortality in patients with HF and CKD (MSI OR: 2.399, 95% CI: 1.533–3.754, P < 0.001) (ASI OR: 1.011, 95% CI: 1.004–1.019, P = 0.004).
The ROC curve and area under the curve (AUC) of SI, MSI, and ASI were 0.627 (95% CI: 0.614–0.639), 0.652 (95% CI: 0.640–0.664), and 0.659 (95% CI: 0.647–0.671), respectively (Supplementary Figure S1 and Supplementary Table S1, available in www.besjournal.com). The cut-off values for SI, MSI, and ASI for the prediction of in-hospital mortality were 0.63 (specificity 0.539, sensitivity of 0.678), 0.93 (specificity 0.645, sensitivity 0.623), and 45.6 (specificity 0.552, sensitivity 0.699), respectively. Compared with SI, MSI had better prediction efficiency under C-statistic (Z = 5.270, P < 0.001, NRI = 0.4355, P < 0.001; IDI = 0.0031, P < 0.001) (Table 3). ASI also had a better predictive result (Z = 3.869, P < 0.001, NRI = 0.4192, P < 0.001; IDI = 0.0074, P < 0.001) (Table 3). Furthermore, ASI showed a good model calibration with higher Nagelkerke-R2, HL P-value and lower Brier score than the other two parameters (Supplementary Table S1).
Table 3. Comparisons of the predictive performance of SI, MSI, and ASI for the prognosis prediction
Items z for C-statistic P for C-statistic NRI P for NRI IDI P for IDI MSI vs. SI 5.270 < 0.001 0.4355 < 0.001 0.0031 < 0.001 ASI vs. SI 3.869 < 0.001 0.4192 < 0.001 0.0074 < 0.001 MSI vs. ASI 0.812 0.417 − − − − Note. SI, shock index; MSI, modified shock index; ASI, age-adjusted shock index; NRI, net reclassification improvement; IDI, integrated discrimination improvement. 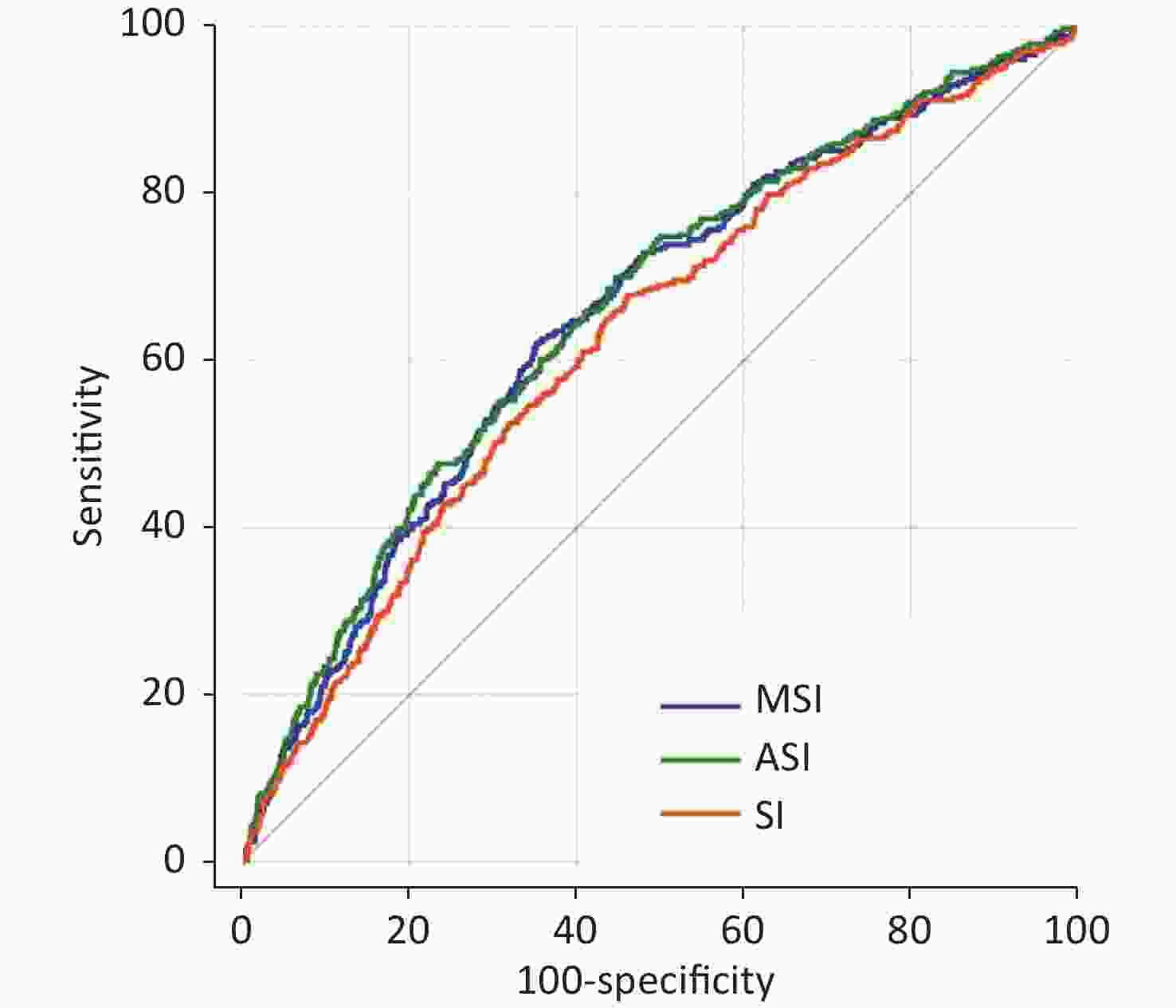
Figure S1. Receiver operating characteristic curves of SI, MSI and ASI for in-hospital death prediction. SI, shock index; MSI, modified shock index; ASI, age-adjusted shock index
Table S1. SI, MSI and ASI for the prognosis prediction
Variables Discrimination Calibration Precision C-statistic SE P value 95% CI HL P value R2 Brier score SI 0.627 0.0159 < 0.001 0.614–0.639 0.163 0.023 0.0511 MSI 0.652 0.0157 < 0.001 0.640–0.664 0.003 0.032 0.0511 ASI 0.659 0.0156 < 0.001 0.647–0.671 0.201 0.041 0.0508 Pourafkari et al. tested the specificity and sensitivity of SI in 554 patients with HF and found that ASI was a better predictor for in-hospital mortality than SI or MSI alone [10]. However, our study found that MSI and ASI had equal predictive performance (Z = 0.812, P = 0.417) (Table 3). This may be due to differences in the overall study population, as we considered patients with CKD. However, further subgroup analysis showed slightly different results. Relevant clinical variables like clinical presentation age (< 65 vs. ≥ 65 years), ejection fraction (EF) (< 50% vs. ≥ 50%), sex (male vs. female) and CAD (yes vs. no) were performed by post-hoc subgroup analysis for the primary endpoint. When the analysis was stratified by clinical presentation, we found that a higher MSI or ASI was significantly associated with an increased risk of the primary endpoint when stratified on the basis of age, sex, and CAD. Studies in recent years have found that the mortality rate of patients with HF and preserved ejection fraction (HFpEF) is not lower than that of patients with HF and reduced ejection fraction (HFrEF), and there is no effective treatment for HFpEF due to its unique pathological mechanism. Therefore, we stratified according to EF and found that MSI also had a good predictive performance in HFpEF and HFrEF patients (interaction P = 0.344). However, ASI only showed association with endpoint in HFrEF patients. (Supplementary Figure S2, available in www.besjournal.com)
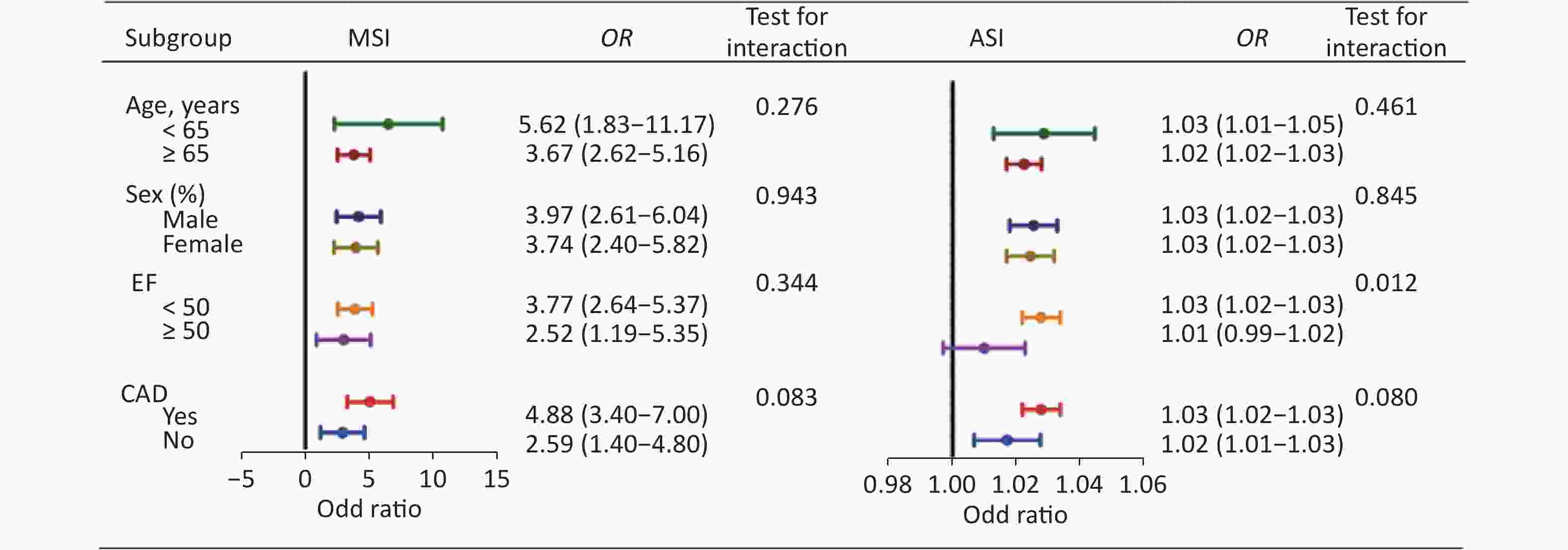
Figure S2. Post-hoc subgroup analysis of MSI and ASI for the primary endpoint. MSI, modified shock index; ASI, age-adjusted shock index; EF, ejection fraction; CAD, coronary artery disease
Our study has several clinical implications. First, monitoring SI, MSI, and ASI can better identify the high-risk patients who may have in-hospital mortality from HF and CKD. Secondly, compared with the SI, MSI, and ASI have better predictive performance. Third, if the patient has HFpEF, MSI may be better than ASI in determining the prognosis.
The present study has some limitations. First, this was a single-center, observational study, so selection bias and potential confounders could not be completely adjusted. Second, because the results analyzed only included the SI, MSI, and ASI on admission, it is not possible to assess whether a decrease in the said indices would improve patient outcomes. Third, according to C-statistic, the specificity and the sensitivity of SI, MSI, and ASI were not extraordinary, so clinical researchers need judgments on the possibility of false positives and negatives.
The results confirmed that SI, MSI, and ASI were all independent prognosticators of in-hospital mortality in patients with HF and CKD. MSI and ASI had better predictive performance than SI. ASI was an effective predictor of in-hospital mortality in HFrEF patients under subgroup analysis, and MSI showed a strong association with prognosis, independent of EF.
Author Contributions All of the authors who contributed towards this article met the criteria for authorship.
Conflict of Interest There was no conflict of interest.
doi: 10.3967/bes2023.030
Shock Index, Modified Shock Index, and Age-Adjusted Shock Index in Predicting the In-Hospital Mortality in Patients with Heart Failure and Chronic Kidney Disease
-
-
Table 1. Baseline characteristics of subjects divided by in-hospital mortality, median (IQR), or n (%), or means ± SD
Variables Overall
(n = 6,045)Patients with in-hospital
mortality (n = 329)Patients without in-hospital
mortality (n = 5,716)P value Age, years 72.0 ± 12.2 76.1 ± 11.4 71.7 ± 12.2 < 0.001 Male, n (%) 2,927 (48.4) 157 (47.7) 2,770 (48.5) 0.794 NYHA grading, n (%) < 0.001 II 1,061 (17.6) 15 (4.6) 1,046 (18.3) III 2,447 (40.5) 84 (25.5) 2,343 (41.3) IV 2,537 (41.9) 230 (69.9) 2,307 (40.4) Heart rate on admission, bpm 86.6 ± 23.5 91.5 ± 23.8 86.3 ± 23.5 < 0.001 SBP on admission, mmHg 136.2 ± 26.1 127.2 ± 28.6 136.8 ± 25.8 < 0.001 DBP on admission, mmHg 80.4 ± 24.4 72.7 ± 15.4 80.9 ± 24.8 < 0.001 SI 0.66 ± 0.23 0.75 ± 0.26 0.66 ± 0.23 < 0.001 MSI 0.90 ± 0.29 1.04 ± 0.32 0.89 ± 0.28 < 0.001 ASI 47.2 ± 17.8 56.9 ± 20.5 46.7 ± 17.5 < 0.001 Albumin, g/L 36.5 ± 4.3 34.6 ± 4.4 36.6 ± 4.3 < 0.001 TBIL, umol/L 15.6 ± 11.5 17.6 ± 14.2 15.5 ± 11.4 0.001 LDL, mmol/L 2.55 ± 0.99 2.44 ± 1.03 2.56 ± 0.99 0.06 BUN, mmol/L 10.9 ± 6.2 16.4 ± 9.3 10.6 ± 5.9 < 0.001 eGFR, mL/min per 1.73 m2 40.8 ± 13.8 32.0 ± 14.2 41.4 ± 13.6 < 0.001 Haemoglobin, g/L 122.2 ± 23.4 113.1 ± 26.8 122.8 ± 23.1 < 0.001 Serum Sodium, mmol/L 138.7 ± 4.1 137.2 ± 5.7 138.8 ± 3.9 < 0.001 cTNI, ng/mL 0.05 (0.02, 0.25) 0.19 (0.04, 3.8) 0.04 (0.02, 0.22) < 0.001 NT-proBNP, pg/mL 4,540 (1,606, 8,127) 6,246 (5,890, 12,976) 4,284 (1,518, 7,743) < 0.001 LVEF, % 49.0 ± 11.0 47.8 ± 8.2 49.0 ± 11.1 0.049 Co-morbidities, n (%) CAD 4,202 (69.5) 253 (76.9) 3,949 (69.1) 0.003 Hypertension 4,064 (67.2) 199 (60.5) 3,865 (67.6) 0.007 AF 1,781 (29.5) 82 (24.9) 1,699 (29.7) 0.063 DM 2,114 (35.0) 129 (39.2) 1,985 (34.7) 0.097 COPD 1,292 (21.4) 63 (19.1) 1,229 (21.5) 0.312 Smoking, n (%) 1,724 (28.5) 79 (24.4) 1,645 (29.3) 0.058 Medications, n (%) ACE-I/ARB/ARNI 4,962 (82.1) 212 (64.4) 4,750 (83.1) < 0.001 Beta blocks 4,650 (76.9) 279 (84.8) 4,371 (76.5) < 0.001 Diuretic 5,703 (94.3) 280 (85.1) 5,423 (94.9) < 0.001 Note. The continuous variable data was expressed as median (IQR) or means ± SD, SBP, systolic blood pressure; DBP, dilated blood pressure; SI, shock index; MSI, modified shock index; ASI, age-adjusted shock index; TBIL, total bilirubin; LDL, low density lipoprotein; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; cTNI, cardiac troponin I; NT-proBNP, n‐terminal brain natriuretic peptide; LVEF, left ventricular ejection fraction; CAD, coronary artery disease; AF, atrial fibrillation; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; ARNI, angiotensin receptor blocker-neprilysin inhibitors. Table 2. Effects of multiple variables on clinical outcomes in univariate and multivariate analysis
Variables Univariate analysis Multivariate analysis OR 95% CI P OR 95% CI P SI 4.406 2.974–6.528 < 0.001 2.462 1.363–4.448 0.003* MSI 3.855 2.843–5.227 < 0.001 2.399 1.533–3.754 < 0.001* ASI 1.025 1.020–1.030 < 0.001 1.011 1.004–1.019 0.004** Note. *Adjusted for age, sex, NYHA grading, Albumin, TBIL, LDL, BUN, creatinine, haemoglobin, serum sodium, cTNI, NT-proBNP, LVEF, CAD, hypertension, AF, DM, smoking, ACEI/ARB/ARNI, beta-blockers, diuretic. ** Adjusted same as * except for age. SI, shock index; MSI, modified shock index; ASI, age-adjusted shock index;TBIL, total bilirubin; LDL, low density lipoprotein; BUN, blood urea nitrogen; cTNI, cardiac troponin I; NT-proBNP, n‐terminal brain natriuretic peptide; LVEF, left ventricular ejection fraction; CAD, coronary artery disease; AF, atrial fibrillation; DM, diabetes mellitus; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; ARNI, angiotensin receptor blocker-neprilysin inhibitors. Table 3. Comparisons of the predictive performance of SI, MSI, and ASI for the prognosis prediction
Items z for C-statistic P for C-statistic NRI P for NRI IDI P for IDI MSI vs. SI 5.270 < 0.001 0.4355 < 0.001 0.0031 < 0.001 ASI vs. SI 3.869 < 0.001 0.4192 < 0.001 0.0074 < 0.001 MSI vs. ASI 0.812 0.417 − − − − Note. SI, shock index; MSI, modified shock index; ASI, age-adjusted shock index; NRI, net reclassification improvement; IDI, integrated discrimination improvement. S1. SI, MSI and ASI for the prognosis prediction
Variables Discrimination Calibration Precision C-statistic SE P value 95% CI HL P value R2 Brier score SI 0.627 0.0159 < 0.001 0.614–0.639 0.163 0.023 0.0511 MSI 0.652 0.0157 < 0.001 0.640–0.664 0.003 0.032 0.0511 ASI 0.659 0.0156 < 0.001 0.647–0.671 0.201 0.041 0.0508 -
[1] Hao G, Wang X, Chen Z, et al. Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012-2015. Eur J Heart Fail, 2019; 21, 1329−37. doi: 10.1002/ejhf.1629 [2] Rangaswami J, Bhalla V, Blair JEA, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American heart association. Circulation, 2019; 139, e840−78. [3] House AA, Wanner C, Sarnak MJ, et al. Heart failure in chronic kidney disease: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) controversies conference. Kidney Int, 2019; 95, 1304−17. doi: 10.1016/j.kint.2019.02.022 [4] Allgöwer M, Burri C. Shock index. Dtsch Med Wochenschr, 1967; 92, 1947−50. doi: 10.1055/s-0028-1106070 [5] Ozsu S, Erbay M, Durmus ZG, et al. Classification of high-risk with cardiac troponin and shock index in normotensive patients with pulmonary embolism. J Thromb Thrombolysis, 2017; 43, 179−83. doi: 10.1007/s11239-016-1443-3 [6] El-Menyar A, Goyal P, Tilley E, et al. The clinical utility of shock index to predict the need for blood transfusion and outcomes in trauma. J Surg Res, 2018; 227, 52−9. doi: 10.1016/j.jss.2018.02.013 [7] Zarzaur BL, Croce MA, Fischer PE, et al. New vitals after injury: shock index for the young and age × shock index for the old. J Surg Res, 2008; 147, 229−36. doi: 10.1016/j.jss.2008.03.025 [8] Liu YC, Liu JH, Fang ZA, et al. Modified shock index and mortality rate of emergency patients. World J Emerg Med, 2012; 3, 114−7. doi: 10.5847/wjem.j.issn.1920-8642.2012.02.006 [9] Rump LC, Wilde K, Schollmeyer P. Prostaglandin E2 inhibits noradrenaline release and purinergic pressor responses to renal nerve stimulation at 1 Hz in isolated kidneys of young spontaneously hypertensive rats. J Hypertens, 1990; 8, 897−908. doi: 10.1097/00004872-199010000-00003 [10] Pourafkari L, Wang CK, Schwartz M, et al. Does shock index provide prognostic information in acute heart failure? Int J Cardiol, 2016; 215, 140–2. -
 22372Supplementary Materials.pdf
22372Supplementary Materials.pdf

-




 下载:
下载:





 Quick Links
Quick Links