-
Enterovirus 71 (EV71) and Coxsackievirus A16 (CoxA16) are the major pathogens causing hand, foot, and mouth disease (HFMD) that occurs primarily in children and infants with mild clinical manifestations. HFMD caused by EV71 could develop to a fatal neurological complication.
The 3CD protein is a precursor of mature 3C and 3D proteins. It possesses protease activity, but not polymerase activity. The 3AB protein was digested by the 3CD protein to form Vpg and 3A protein, which could promote the combination of 3D/Vpg and RNA templates. The 3C protein is a cysteine protease that can digest polyprotein to unstructural proteins. It exerts its functions in viral replication and host cell apoptosis. The 3D protein is a viral RNA-dependent RNA polymerase and plays an important role in the replication of EV71[1]. 3D polymerases share similar structures with other enteroviruses[2].
Different strains of EV71 can lead to diverse clinical outcomes. Most mild cases were found to be self-limiting, whereas severe cases could induce neurological complications and even death. This difference may be caused due to the virulence of the virus, host susceptibility, multiple infections with other pathogens, the immune response characteristics, or the environmental factors. Unfortunately, it is not clear why different strains could lead to different outcomes. Therefore, we conducted the present study to determine the roles of 3C and 3CD proteins in the virulence of EV71.
In our previous study, we isolated a severe strain, SDLY107, from a fatal case and a mild strain, SDLY1, from a mild case[3] and found that mice infected with the severe strain exhibited more severe clinical symptoms than those of mice infected with the mild strain. The replication capacity of these strains was different. In the present study, the rescued recombinant 3CRV and 3CDRV strains were used to determine whether the 3C or 3CD protein could be identified by quantitative real-time PCR (qRT-PCR). RD and Vero cells were used to test for cytopathic effect (CPE), replication capacity, cell injury, and temperature sensitivity at 37 ℃ and 39.5 ℃, respectively. Animal experiments using ICR mice were carried out to determine the virulence of the recombinant viruses.
Comparison of the amino acid sequence showed that there were six variations between 3CD regions in the severe and mild strains. One position (Val138/Ile138) was found to be variable in the 3C protein, and five mutations, including Ser37/Asn37, Arg143/Lys143, Ile176/Val176, Glu261/Gly261, and Gly360/Ala360, were found in the 3D protein. Among these variations, only the character of the 261st amino acid site was changed from polar negative to neutral.
Microscope observations revealed no differences of CPE induced by 3CRV and severe strains in RD and Vero cells at 37 ℃ or 39.5 ℃. However, the 3CDRV strain triggered a milder CPE on RD or Vero cells than that induced by the severe strain at 37 ℃ and 39.5 ℃. Interestingly, the CPE induced by the 3CDRV strain was more severe than that induced by the mild strain at 37 ℃; however, at 39.5 ℃, there was no statistically significant difference in the CPE induced by 3CDRV and mild strains.
Results of the LDH assay showed no significant differences between the rate of cell injury induced by the severe and 3CRV strains (P > 0.05) under the four conditions, namely, RD cells at 37 ℃, Vero cells at 37 ℃, RD cells at 39.5 ℃, and Vero cells at 39.5 ℃. The rate of injury induced by the 3CDRV strain was significantly higher than that induced by the mild strain (P < 0.05) in RD and Vero cells at 37 ℃. However, there were no significant differences in the rate of cell injury between 3CDRV and mild strains at 39.5 ℃ (P > 0.05). The rate of cell survival after infection was evaluated by CCK-8 assay. Statistical analysis of the CCK-8 assay results showed similar findings as those of the LDH assay. These results indicated that the virulence of the recombinant 3CDRV virus strain was decreased and the cell injury induced by EV71 was sensitive to temperature.
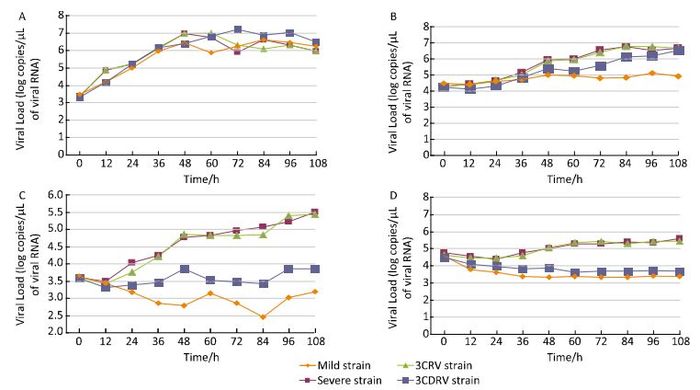
Growth characteristics of the viruses were determined by qRT-PCR, which showed that Vero cells could be quantitatively determined every 12 h post infection by real-time PCR. The RT-PCR results also showed that the 3CRV and mild strains had similar growth curves. In RD cells at 37 ℃ (Figure 1A), the 3CRV and severe strains showed a slightly decreasing growth trend after 60 h of infection, while the growth of the 3CDRV strain was slow and persistent. In Vero cells at 37 ℃ (Figure 1B), the growth curve of the 3CDRV strain was slightly lower than that of the severe and 3CRV strains. This difference became more obvious at 39.5 ℃, with inhibition of growth of the 3CDRV strain. Altogether, these in vitro data suggest that the virulence of the 3CDRV strain was attenuated and its replication capacity was more sensitive to temperature than that of the severe strain.
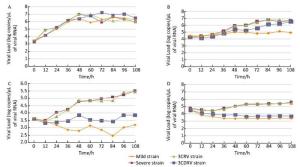
Figure 1. Growth curves. (A) in RD cells at 37 ℃; (B) in Vero cells at 37 ℃; (C) in RD cells at 39.5 ℃; (D) in Vero cells at 39.5 ℃.
To test whether the virulence of the recombinant 3CRV and 3CDRV virus strains was changed, we intraperitoneally inoculated severe, mild, and 3CRV and 3CDRV strains into 1-day-old ICR mice. The mice injected with severe and 3CRV strains exhibited symptoms, including arched back and limb paralysis at day 3 post infection. The mice injected with the 3CDRV strain were more active than the mice injected with severe and 3CRV strains. The ICR mice injected with the 3CDRV strain showed no paralysis, no death, and milder symptoms. The severe and 3CRV strains exhibited more severe virulence, with one dead mouse each among the mice injected with the severe and 3CRV strains. In addition, compared with the control group, mice injected with EV71 showed different levels of weight loss. Comparison of the body weight showed that mice infected with the 3CDRV strain were heavier than mice infected with the severe or 3CRV strain but slightly lighter than mice infected with the mild strain. These results indicated that the 3CDRV strain-infected mice grew more quickly than those infected by the severe or 3CRV strain but slightly slower than the mild strain-infected mice.
Immunohistochemical staining was performed to detect the distribution of EV71 in mouse tissues. EV71-specific antigens were detected in muscle tissues of the 1-day-old mice infected with the strain 4 days post infection, indicating the distribution of EV71 in the muscle tissues of ICR mice.
To determine whether replication of EV71 in ICR mice was affected by replacement of the 3C or 3CD protein, qPCR assay was performed to detect the growth curves of the four viral strains in vivo (Figure 2). Hindlimb muscles of ICR mice were collected for quantitative detection of EV71 using qPCR assays, based on the result of immunohistochemical staining. The growth curves showed that the four strains shared similar virus replication tendency in the 1-day-old ICR mice. However, the peaks of the growth curves of the severe and 3CRV and 3CDRV strains appeared earlier than those of the mild strain. The growth curve of the EV71 3CRV strain was not different from that of the severe strain. Although the peaks of the growth curves of the severe and 3CDRV strains appeared on the same day, the viral load of the 3CDRV strain was slightly lower than that of the severe strain. In addition, the viral load of the 3CDRV strain was slightly higher than that of the mild strain.
Figure 3 shows the pathological changes observed in the muscles and lungs of the ICR mice. The muscles of ICR mice infected with the severe and 3CRV and 3CDRV strains exhibited texture disorder at day 3 post infection, whereas mice injected with the mild strain showed texture disorder at day 4 post infection. In addition, the pathological changes in the 3CDRV strain-infected mice were less serious than those in the mice infected with the severe and 3CRV strains. The lung tissues primarily showed pneumorrhagia in ICR mice injected with the severe (Figure 3D, H) and 3CRV (Figure 3E, I) and 3CDRV (Figure 3F, J) strains at day 3 post infection, but mice injected with the mild strain exhibited pneumorrhagia at day 4 post infection (Figure 3G). Thickening of alveolar septa and a decrease in the number of alveoli were also observed in the lung tissues of ICR mice injected with the severe strain (Figure 3H) and the 3CRV (Figure 3I) and 3CDRV (Figure 3J) strains. Pathological changes in the 3CDRV strain-infected mice were more serious than those observed in the mild strain-infected mice but less serious than those in mice infected with the severe and 3CRV strains. Furthermore, lymphocyticinflammatory infiltration and exudation of edema fluid were also observed in the alveolar cavities.
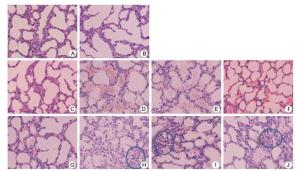
Figure 3. Representative histology of lung tissues of ICR mice infected with EV71. (A) and (B): negative control; (C) and (G): mild strain; (D) and (H): severe strain; (E) and (I): 3CRV strain; (F) and (J): 3CDRV strain; (A, C, D, E, F): Day 3 post infection; (B, G, H, I, J): Day 4 post infection. Magnification: 200×.
All these in vitro and in vivo results indicated that the virulence of the 3CDRV strain was decreased and there were virulent determinants in the 3CD region of EV71.
Although several studies have investigated the mechanism of EV71 pathogenesis for several years, the pathogenesis of EV71 is still obscure. Multifactors were associated with diverse clinical manifestations[4], and the evolution of the virus was a key determinant of its virulence. Some researchers suggested that serm antibody levels pre-epidemic EV71 were associated with mortality rates of HFMD[5]. Variation of EV71 might be one of the primary contributors to serious epidemics. Huang et al.[6] and Chua et al.[7] reported that a G to E mutation at VP1-145 enhanced the virulence of EV71. Kung et al.[8] suggested that I251T mutations in the 3D protein might attenuate the virulence of EV71.
Till date, the most serious epidemic of EV71 has occurred in Taiwan in 1998, with 78 deaths and 405 severe cases. Before that, two mild EV71 epidemics occurred in 1980 and 1986, with no reports of death[9]. Kung et al.[8] suggested that the evolution of EV71 might have led to this serious epidemic in 1998 in Taiwan and that I251T mutation in the 3D protein might be a determinant of the virulence of EV71. In addition, the I251T mutation in the 3D protein rendered EV71 sensitive to temperature. These findings were consistent with our results partly because no mutation was observed in position 251 of the 3D protein in our study. Among these five mutation sites in the 3D region, changes in the property of amino acids occurred only in position 261, which is located in the conserved palm subdomain. This suggested that the sites of amino acids might act together or independently, being responsible for temperature sensitivity and replication. Temperature sensitivity of EV71 might be related to the diversity of manifestations induced by different EV71 strains. Some researchers found that patients with neurological complications were more inclined to have a higher and more durable fever than those with no neurological complications[10]. In our study, replacement of the 3CD region in EV71 decreased mortality, pathological injury of lungs, and replication capacity in hindlimb muscles, but replacement of the 3C region did not exhibit such changes. Other mutations of the 3C protein in EV71 will be analyzed in our future study.
In conclusion, the virulence of the recombinant 3CDRV virus strain was decreased, whereas the virulence of the 3CRV strain showed no difference compared with that of the severe strain. This result indicated that replacement of the 3C protein (Val138/Ile138) could not change the virulence of EV71 independently, and the 3CD protein might be responsible for the virulence of EV71. Furthermore, the in vitro results indicated that the 3CD protein of EV71 contributed to temperature sensitivity of EV71. Further studies on the interaction between 3C and 3D regions are necessary.
doi: 10.3967/bes2017.103
-
Abstract: Enterovirus 71 is a neuroinvasive virus that is associated with severe neurological complications. We had earlier suggested that the replication capacity of a severe strain was higher than that of a mild strain. The recombinant 3CRV and 3CDRV virus strains were successfully rescued in our previous study. In the present study, we found no difference in virulence between 3CRV and severe strains. However, the capacity of replication and to cause cell injury of 3CDRV strain decreased in vitro, especially at 39.5 ℃. Replacement of 3CD region in the severe strain led to milder symptoms, less body weight loss, and lower viral load in ICR mice. Histopathological findings indicated less severe injury in mice infected with 3CDRV strain. This study suggests that the 3CD region contributes to the attenuation of the severe strain, including its replication capacity and temperature sensitivity.
-
Figure 3. Representative histology of lung tissues of ICR mice infected with EV71. (A) and (B): negative control; (C) and (G): mild strain; (D) and (H): severe strain; (E) and (I): 3CRV strain; (F) and (J): 3CDRV strain; (A, C, D, E, F): Day 3 post infection; (B, G, H, I, J): Day 4 post infection. Magnification: 200×.
-
[1] Richards OC, Spagnolo JF, Lyle JM, et al. Intramolecular and intermolecular uridylylation by poliovirus RNA-dependent RNA polymerase. J Virol, 2006; 80, 7405-15. doi: 10.1128/JVI.02533-05 [2] Gruez A, Selisko B, Roberts M, et al. The crystal structure of coxsackievirus B3 RNA-dependent RNA polymerase in complex with its protein primer VPg confirms the existence of a second VPg binding site on Picornaviridae polymerases. J Virol, 2008; 82, 9577-90. doi: 10.1128/JVI.00631-08 [3] Wen HL, Si LY, Yuan XJ, et al. Complete genome sequencing and analysis of six enterovirus 71 strains with different clinical phenotypes. Virol J, 2013; 10, 115. doi: 10.1186/1743-422X-10-115 [4] Chang LY, King CC, Hsu KH, et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics, 2002; 109, e88. doi: 10.1542/peds.109.6.e88 [5] Lu CY, Lee CY, Kao CL, et al. Incidence and case-fatality rates resulting from the 1998 enterovirus 71 outbreak in Taiwan. J Med Virol, 2002; 67, 217-23. doi: 10.1002/(ISSN)1096-9071 [6] Huang SW, Wang YF, Yu CK, et al. Mutations in VP2 and VP1 capsid proteins increase infectivity and mouse lethality of enterovirus 71 by virus binding and RNA accumulation enhancement. Virology, 2012; 422, 132-43. doi: 10.1016/j.virol.2011.10.015 [7] Chua BH, Phuektes P, Sanders SA, et al. The molecular basis of mouse adaptation by human enterovirus 71. J Gen Virol, 2008; 89, 1622-32. doi: 10.1099/vir.0.83676-0 [8] Kung YH, Huang SW, Kuo PH, et al. Introduction of a strong temperature-sensitive phenotype into enterovirus 71 by altering an amino acid of virus 3D polymerase. Virology, 2010; 396, 1-9. doi: 10.1016/j.virol.2009.10.017 [9] Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med, 1999; 341, 929-35. doi: 10.1056/NEJM199909233411301 [10] Li CC, Yang MY, Chen RF, et al. Clinical manifestations and laboratory assessment in an enterovirus 71 outbreak in southern Taiwan. Scand J Infect Dis, 2002; 34, 104-9. doi: 10.1080/00365540110077119 -




 下载:
下载:



 Quick Links
Quick Links