-
Tuberculosis (TB) is a significant infectious disease caused by Mycobacterium tuberculosis (MTB). In 2017, 10.0 million new TB cases and 1.3 million deaths were reported globally, according to World Health Organization (WHO)[1]. The Mycobacterial culture test is the gold standard for diagnosis but the positive rate is only about 30%[1], and it is even lower with extrapulmonary tuberculosis. For sputum-negative pulmonary and extrapulmonary TB, pathological examination plays an important role in diagnosis. The detection of MTB in formalin-fixed paraffin-embedded (FFPE) tissues is critical for the definite pathological diagnosis of culture-negative TB. Accurate diagnosis is essential in reducing TB-related morbidity and mortality[2]. Molecular pathological tests detecting MTB DNA have shown advantages in improving diagnostic sensitivity.
The automatic diagnostic test, Xpert MTB/RIF assay (Xpert), that simultaneously detects MTB and rifampin resistance, has been recommended by the WHO in 2011[3]. Xpert improved the positive rate of detecting TB greatly, compared with the traditional culture method[4]. This technology has been applied to diagnose TB globally, especially in regions with a high burden of TB[5]. The applications of Xpert for culture-negative TB have been previously reported[6]; however, the sensitivities are not satisfactory. The sensitivity of Xpert is still not adequate for detecting paucibacillary specimens.
The new Xpert MTB/RIF Ultra assay (Ultra) has been developed by Cepheid. It adopts nested nucleic acid amplification, more rapid thermal cycling, and improved fluidics and enzymes. Most importantly, Xpert detects the rpoB gene, whose copy number is only one in each genome while Ultra detects IS6110 and IS1081, whose copy number ranges from 10 to 12 in various MTB strains[7]. The limit of detection (LOD) of Ultra has been reduced to 16 colony forming units (CFU) of MTB per mL; the LOD of Xpert is 114 CFU per mL[8]. As an improved technology, Ultra may be more sensitive for culture-negative TB, however, no data has been reported yet. Here, we performed the first retrospective study comparing the accuracy of Ultra with that of Xpert in detecting MTB and rpoB gene mutations, using FFPE tissues (pulmonary and extrapulmonary).
This study was approved by the ethical and institutional review boards for human investigation at the Beijing Chest Hospital, Capital Medical University. In 2017, we collected 164 FFPE specimens from 164 patients who were suspected to have TB and underwent surgery. Fifty-one cases with no definite diagnosis were excluded because they did not meet the following diagnostic standards for TB or for ‘not’ TB (Figure 1). The standard for the diagnosis of TB is to meet any of the following: 1) either mycobacterial culture or Xpert for sputum or bronchial lavages was positive; or 2) granulomatous inflammation with both acid fast staining and a TB-PCR positive outcome. Patients were classified as ‘not’ TB with any definite diagnosis of another disease. According to these standards, among the 113 eligible patients, 83 were classified as definite TB and 30 were classified as ‘not’ TB.
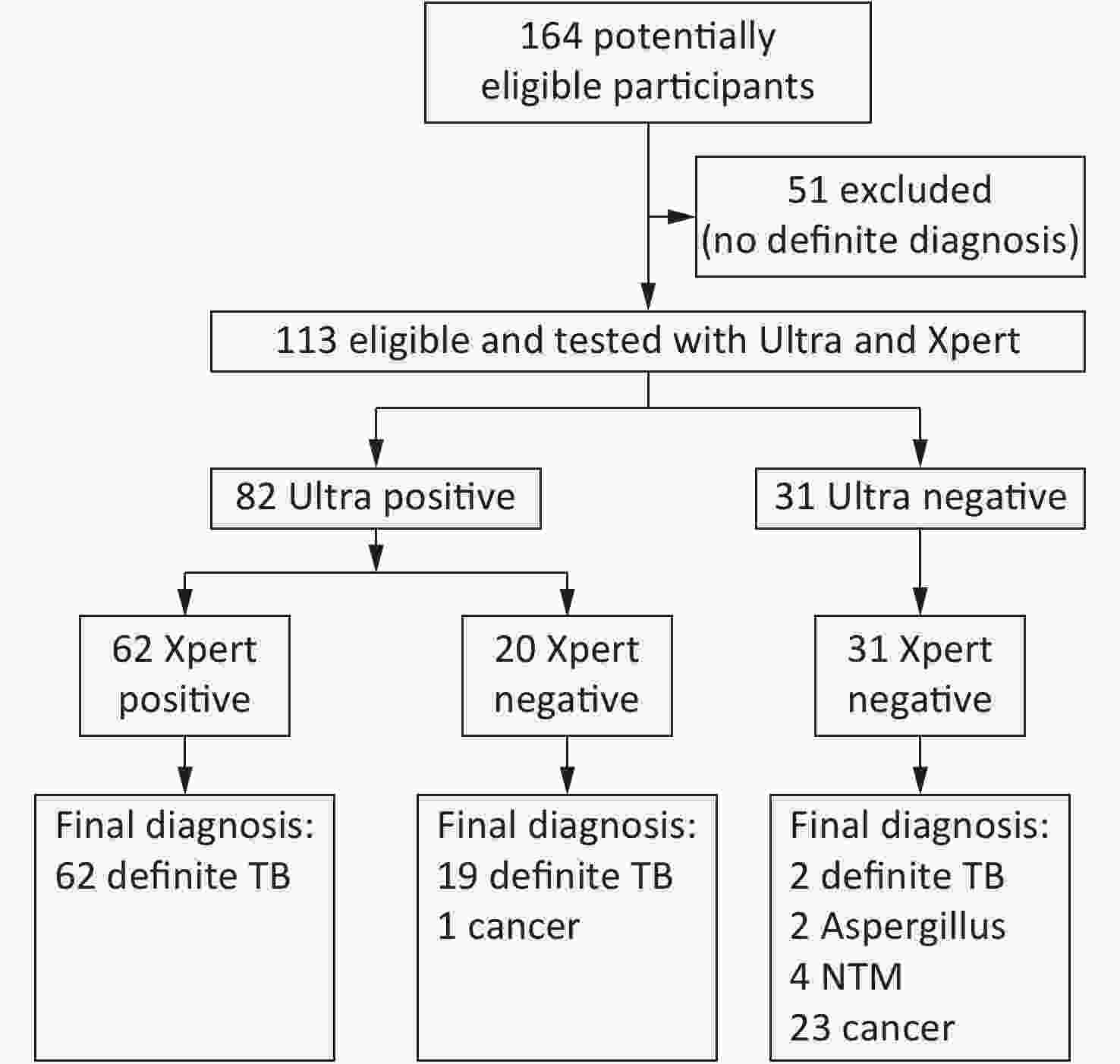
Figure 1. Test profile of all cases in this study. The standard for the diagnosis of TB is to meet any of the followings: (1) Mycobacterial culture positive and further confirmed by TB-PCR, (2) Acid fast stain positive and further confirmed by TB-PCR, or (3) Xpert for sputum, pus or bronchial lavage
The FFPE specimens were processed according to the following procedures. The Leica RM2135 rotation microtome (Leica, Germany) was used to cut paraffin-embedded tissues (it was thoroughly cleaned before the usage). For each specimen, 6 to 10 FFPE sections, with thickness of 4 μm, were used for Ultra and Xpert tests. The tissue sections were placed into a 1.5 mL clean centrifuge tube and incubated with 320 μL of deparaffinization solution (QIAGEN cat # 19093) at 56 ℃ for 3 min. Then, 180 μL ATL lysis buffer (QIAGEN cat # 19076) was added into the centrifuge tube, vortexed and centrifuged at 11,000 ×g for 1 min. After centrifugation, a clarified liquid layer was formed at the bottom of the microcentrifuge. Into this layer, 20 μL Proteinase K (QIAGEN cat # 19133) was added and incubated at 56 ℃, for more than 1 h, until the tissue sections were digested thoroughly. The mixture was further incubated at 90 ℃ for 1 h and was centrifuged; about 200 μL of a clarified liquid layer was obtained at the bottom of tube.
The final obtained clarified liquid layer for each sample was aspirated into a 10 mL centrifuge tube. Then, 2 mL of commercial NaOH– and an isopropanol-containing sample reagent (SR; Cepheid, USA) were added into the tube; this mixture was settled for 15 min. Ultra and Xpert tests were simultaneously performed with the whole obtained mixtures, according to the manufacturer’s instructions. If contamination or errors were reported, the assay was repeated using the same FFPE specimen.
The sensitivity and specificity of both methods were analyzed using 95% confidence intervals (CIs). Statistical comparisons for the categorical variables were determined using the McNemar test. Differences were considered statistically significant when P < 0.05. The Kappa coefficient test was performed to evaluate the agreement of categorical variables between the two methods. The above analyses were performed using SPSS software packages (V.21.0, SPSS Inc, Chicago, Illinois, USA).
Among the 113 patients included in this study, 83 were TB patients (48 males/35 females, mean age: 38.0 ± 17.5 years) and 30 were ‘not’ TB patients (18 males/12 females, mean age: 56.0 ± 12.5 years; Figure 1). TB FFPE specimens included lung (n = 49), bone (n = 16), pleura (n = 7), lymph node (n = 5), thoracic wall (n = 4) and pyothorax (n = 2). FFPE specimens of ‘not’ TB included nontuberculous mycobacteria (NTM) disease (n = 4; 3 were infected with Mycobacterium intracellulare and 1 was infected with M. abscessus), Aspergillus infection (n = 2) and several malignancies (n = 24; 11 lung, 4 bone, 4 mediastina, 3 lymph node, and 2 pleura). The histological changes of the TB patients included 4 granuloma, 4 necrosis, and 55 granuloma with necrosis. Among the TB patients, 20 cases were TB culture or Xpert positive, with respiratory samples, and 63 cases were both acid-fast staining and TB-PCR positive, with FFPE tissues.
The sensitivity of Ultra was significantly higher than Xpert (97.6% vs. 74.7%, P < 0.001), not only in pulmonary tissues (98.0% vs. 79.6%, P = 0.004) but also in extrapulmonary tissues (97.1% vs. 67.6%, P < 0.001); this was similar to previous studies[4]. The most significant increase in sensitivity with extrapulmonary tissues was observed with bone specimens (93.8% vs. 56.2%, P = 0.030; Table 1). The 62 Xpert positive samples were also positive with Ultra. However, Ultra detected 20 positive samples that were found to be negative with Xpert (Table 2). The one false-positive case reported using Ultra with trace was repeated and the result came out to be the same. There was no granulomatous histology; acid-fast staining and TB-PCR were both negative. This patient was diagnosed with lung cancer 14 years prior and experienced recurrence three times thereafter. His immunity may have been deprived due to his extensive medical history so he may have had a latent MTB infection.
Table 1. Sensitivities and specificities of Ultra and Xpert MTB/RIF for all cases
Diagnostic categories (No.) Sensitivity (%, 95 CI) P value Specificity (%, 95 CI) P value Ultra Xpert Ultra Xpert Total (113) 97.6
(92.6−99.5)74.7
(64.3−82.9)< 0.001 96.7
(81.9−99.9)100.0
(86.5−100.0)NS Pulmonary (65) 98.0
(88.3−99.9)79.6
(66.2−88.7)0.004 93.7
(69.7−99.9)100.0
(77.3−100.0)NS Extrapulmonary (48) 97.1
(83.8−99.9)67.7
(50.1−81.0)< 0.001 100.0
(76.1−100.0)100.0
(76.1−100.0)NS Lymph node (8) 100.0
(51.1−100.0)80.0
(36.0−98.0)NSa 100.0
(38.3−100.0)100.0
(38.3−100.0)NS Pleura (10) 100.0
(59.6−100.0)85.7
(46.7−99.5)NS 100.0
(38.3−100.0)100.0
(38.3−100.0)NS Bone (20) 93.8
(69.7−99.99)56.2
(33.1−76.9)0.030 100.0
(45.4−100.0)100.0
(45.4−100.0)NS Othersb (10) 100.0
(55.7−100.0)66.7
(29.6−90.8)NS 100.0
(45.4−100.0)100.0
(45.4−100.0)NS Note. aNot significant. bOthers: 4 mediastina, 4 thoracic wall , and 2 pyothorax. Table 2. Detection of MTB using Ultra and Xpert with all specimens
Specimen
(Total No.)No. of specimens with indicated results by Ultra/Xpert testa Pos/pos Neg/neg Pos/neg Neg/pos Pulmonary (65) 39 16 10 0 Lymph node (8) 4 3 1 0 Pleura (10) 6 3 1 0 Bone (20) 9 5 6 0 Othersb (10) 4 4 2 0 Note. aPos: positive result; Neg: negative result. bOthers: 4 mediastina, 4 thoracic wall, and 2 pyothorax. The specificity of Ultra was 96.7% (29/30) and 100% (30/30) for Xpert (Table 1). Ultra produced one false-positive result in trace category lung cancer case, who had no history of TB treatments. Both methods showed acceptable specificities in all types of tissues. Four NTM and 2 Aspergillus infection specimens produced negative results via the two methods, indicating that Ultra and Xpert were reliable in distinguishing MTB from other mycobacterial or fungal diseases. The specificity of Ultra was a little lower than what was found in a previous study that used a sputum sample[4]; this may be due to the difference in sample types.
The positive predictive values (PPV) for Ultra and Xpert were similar: 98.8% (95% CI: 92.8%–99.99%) and 100% (95% CI: 93.0%–100%), respectively. However, the negative predictive value (NPV) of Ultra (93.5%, 95% CI: 78.3%–99.2%) was much higher than Xpert (58.8%, 95% CI: 45.2%–71.3%).
With regard to detecting rifampin susceptibility in the 83 definite TB cases, Ultra revealed 50 sensitive and 13 resistant results; while Xpert revealed 49 and 12 results, respectively (Supplementary Table S1 available in www. besjournal.com). The efficiencies of the two methods were similar and did not show significant differences (P = 0.827). Fifty cases (39 sensitive, 11 resistant) had valid results using both Ultra and Xpert (Supplementary Table S1 ). The two methods showed perfect agreement with no discrepancies in these cases.
Table S1. Determination of Rifampin resistance by Xpert and Ultra in definite TB specimens
Ultra Xpert S R N S 39 0 11 R 0 11 2 N 10 1 9 Note. S: Sensitive, R: Resistant, N: No effective result. However, the trace category of Ultra provided no rifampin susceptibility results, because the rpoB fluorescence signal in this category is very low. In this study, rifampin susceptibility results were reported in only 24 cases by either Ultra or Xpert; no rifampin susceptibility results were reported in 9 cases (via either test; Supplementary Table S1). The Kappa coefficient for Ultra and Xpert regarding the detection of rifampin susceptibility, calculated from all the 83 TB cases, was considered as ‘moderate’ agreement (Kappa = 0.482). These inconsistent results may be due to the different rpoB detection principles of the two methods; Ultra incorporates melting temperature-based analysis[9] while Xpert utilizes molecular beacon technology[10].
This study has several limitations. First, there were only 12 culture positive cases as pathological diagnosis is usually performed for difficult cases like culture negative patients. Due to the small number of culture positive cases, comparison of Xpert and Ultra based on culture was not performed. Second, the phenotypic drug susceptibility test (pDST) was unapproachable in this study as none of culture positive cases performed pDST. Consequently, genotypic rifampin resistance could not be further analyzed with pDST. Third, the amount of the extrapulmonary samples (pleura: n = 10, lymph node: n = 8 and others: n = 10) was limited. Comparisons of sensitivities for extrapulmonary specimens (excluding bone) should be further confirmed using more specimens.
In conclusion, Ultra is more feasible and accurate than Xpert for paucibacillary FFPE specimens and in detecting MTB, regardless of the tissue type. Ultra and Xpert showed similar efficiency in detecting rifampin resistance.
Contributors CHEN NY and DU WL designed this study and CHEN NY supervised all the experiments; SONG J, WANG JG, LIU ZC, LI K, DONG YJ, and WANG YX. acquired the data; DU WL drafted the manuscript; DU WL and CHEN NY critically revised the manuscript for important intellectual content; and DU WL performed the statistical analysis assisted by WANG YX.
Competing Interests None declared.
Patient Consent Obtained
Ethics Approval Ethical and institutional review boards for human investigation at Beijing Chest Hospital, Capital Medical University.
Provenance and Peer Review Not commissioned; externally peer reviewed.
doi: 10.3967/bes2019.115
Performance of Xpert MTB/RIF Ultra and Xpert MTB/RIF for the Diagnosis of Tuberculosis through Testing of Formalin-fixed Paraffin-embedded Tissues
-
-
Figure 1. Test profile of all cases in this study. The standard for the diagnosis of TB is to meet any of the followings: (1) Mycobacterial culture positive and further confirmed by TB-PCR, (2) Acid fast stain positive and further confirmed by TB-PCR, or (3) Xpert for sputum, pus or bronchial lavage
Table 1. Sensitivities and specificities of Ultra and Xpert MTB/RIF for all cases
Diagnostic categories (No.) Sensitivity (%, 95 CI) P value Specificity (%, 95 CI) P value Ultra Xpert Ultra Xpert Total (113) 97.6
(92.6−99.5)74.7
(64.3−82.9)< 0.001 96.7
(81.9−99.9)100.0
(86.5−100.0)NS Pulmonary (65) 98.0
(88.3−99.9)79.6
(66.2−88.7)0.004 93.7
(69.7−99.9)100.0
(77.3−100.0)NS Extrapulmonary (48) 97.1
(83.8−99.9)67.7
(50.1−81.0)< 0.001 100.0
(76.1−100.0)100.0
(76.1−100.0)NS Lymph node (8) 100.0
(51.1−100.0)80.0
(36.0−98.0)NSa 100.0
(38.3−100.0)100.0
(38.3−100.0)NS Pleura (10) 100.0
(59.6−100.0)85.7
(46.7−99.5)NS 100.0
(38.3−100.0)100.0
(38.3−100.0)NS Bone (20) 93.8
(69.7−99.99)56.2
(33.1−76.9)0.030 100.0
(45.4−100.0)100.0
(45.4−100.0)NS Othersb (10) 100.0
(55.7−100.0)66.7
(29.6−90.8)NS 100.0
(45.4−100.0)100.0
(45.4−100.0)NS Note. aNot significant. bOthers: 4 mediastina, 4 thoracic wall , and 2 pyothorax. Table 2. Detection of MTB using Ultra and Xpert with all specimens
Specimen
(Total No.)No. of specimens with indicated results by Ultra/Xpert testa Pos/pos Neg/neg Pos/neg Neg/pos Pulmonary (65) 39 16 10 0 Lymph node (8) 4 3 1 0 Pleura (10) 6 3 1 0 Bone (20) 9 5 6 0 Othersb (10) 4 4 2 0 Note. aPos: positive result; Neg: negative result. bOthers: 4 mediastina, 4 thoracic wall, and 2 pyothorax. S1. Determination of Rifampin resistance by Xpert and Ultra in definite TB specimens
Ultra Xpert S R N S 39 0 11 R 0 11 2 N 10 1 9 Note. S: Sensitive, R: Resistant, N: No effective result. -
[1] World Health Organization. 2018. Global tuberculosis report. Geneva, Switzerland. [2] Dowdy DW, Chaisson RE, Moulton LH, et al. The potential impact of enhanced diagnostic techniques for tuberculosis driven by HIV: a mathematical model. AIDS, 2006; 20, 751−62. doi: 10.1097/01.aids.0000216376.07185.cc [3] World Health Organization. Policy statement: automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. 2011. [4] Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis, 2018; 18, 76−84. doi: 10.1016/S1473-3099(17)30691-6 [5] Bahr NC, Nuwagira E, Evans EE, et al. Diagnostic accuracy of Xpert MTB/RIF Ultra for tuberculous meningitis in HIV-infected adults: a prospective cohort study. Lancet Infect Dis, 2018; 18, 68−75. doi: 10.1016/S1473-3099(17)30474-7 [6] Rindi L, Ali G, Fabiani B, et al. Detection of Mycobacterium tuberculosis from paraffin-embedded tissues by GeneXpert MTB/RIF. Tuberculosis, 2017; 106, 53−5. doi: 10.1016/j.tube.2017.06.005 [7] Cave MD, Eisenach KD, McDermott PF, et al. IS6110: conservation of sequence in the Mycobacterium tuberculosis complex and its utilization in DNA fingerprinting. Mol Cell Probes, 1991; 5, 73−80. doi: 10.1016/0890-8508(91)90040-Q [8] World Health Organization. WHO meeting report of a technical expert consultation: Non-inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. 2017. [9] Ramaswamy S, Musser JM. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis. Tubercle Lung Dis, 1998; 79, 23−9. [10] Lawn SD, Nicol MP. Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol, 2011; 6, 1067−82. doi: 10.2217/fmb.11.84 -




 下载:
下载:



 Quick Links
Quick Links