-
Hordeolum, a common inflammatory eyelid disease affecting the meibomian gland (MG, internal hordeolum) or the glands of Zeis or Moll (external hordeolum), is characterized by warm, tender, swollen red eyelid and nodules[1]. Blepharitis signs such as thickened eyelid margins and telangiectatic blood vessels can also occur. Occasionally, internal hordeolum can also cause corneal abrasion[2]. Patients may experience eye pain and blurred vision. Hordeolum is thought to develop from bacterial infection of the meibomian gland or the glands of Zeis or Moll leading to inflammation and suppuration. Generally, hordeolum can be resolved with heated compresses, lid scrubs or antibiotics, but some patients experience a recurrent process with granuloma or cyst formation, causing subacute or chronic inflammation of the eyelid[3]. Traditional treatments for hordeolum include warm compresses[4], lid scrubs[4], antibiotics[5], steroids[4], acupuncture[6], etc., which have poor effects on chronic hordeolum. For patients with frequent recurrences, multiple operations are difficult to tolerate.
As a new technology, intense pulsed light (IPL) therapy plays a therapeutic role through the effects of light energy, and is widely used in the treatment of dermatological diseases, e.g., irregular pigmentation, vascular lesions, hypertrichosis and acne vulgaris[7]. In 2002, Dr. Toyos first found that IPL treatment exerted a therapeutic effect on meibomian gland dysfunction (MGD)[8]. Since then, IPL has gradually been applied in the treatment of ophthalmic diseases. Several clinical studies have demonstrated that IPL therapy effectively improves the symptoms and signs of MGD and dry eye disease[9-12]. Additionally, IPL is used to cure chronic inflammatory diseases in ophthalmology, including blepharitis-associated keratoconjunctivitis and hidradenitis suppurativa[13,14]. Several possible mechanisms may contribute to the therapeutic effects of IPL. It has been suggested that IPL treatment could induce the shrinkage of small vessels around the eyelid margins, improving local inflammation by decreasing the levels of inflammatory factors[15-17]. Photobiomodulation is also an important mechanism of action of IPL, by which light in the visible and infrared portions of the electromagnetic spectrum causes intracellular changes at the gene and protein levels, ultimately reducing local inflammation[18]. In addition, IPL could decrease bacterial and parasite growth, providing a temporary local warming effect and mediating selective photothermolysis, which could contribute to the treatment of chronic hordeolum[19]. There are a number of different types of filters available for IPL that can help filter unwanted light for precise treatment and avoid unnecessary damage. The acne filter is a dual-band filter that filters out light at wavelengths of 400–600 nm and 800–1,200 nm. The shorter band (400–600 nm) mainly acts on porphyrins and haemoglobin in superficial blood vessels, and the longer band (800–1,200 nm) mainly acts on sebaceous glands and deep blood vessels. The middle band (600–800 nm) mainly acts on melanocytes and is hardly absorbed by haemoglobin; thus, it is filtered out to ensure therapeutic effects and avoid unnecessary melanin deposition. Acne filters have been shown to be effective in treating acne and telangiectasia[20,21].
The clinical manifestation of chronic hordeolum includes the appearance of nodules in the eyelid with bacterial infection, and irregularity and hyperaemia of the eyelid margin, resulting in subacute or chronic inflammation. The pathological mechanisms is similar to that of acne vulgaris, as both involve inflammation of the sebaceous glands and bacterial infections. However, there have been no studies on the changes in hordeolum before and after IPL treatment. Therefore, we collected data from patients suffering from chronic hordeolum who received IPL treatment with an acne filter and conducted this retrospective study to observe the effect.
-
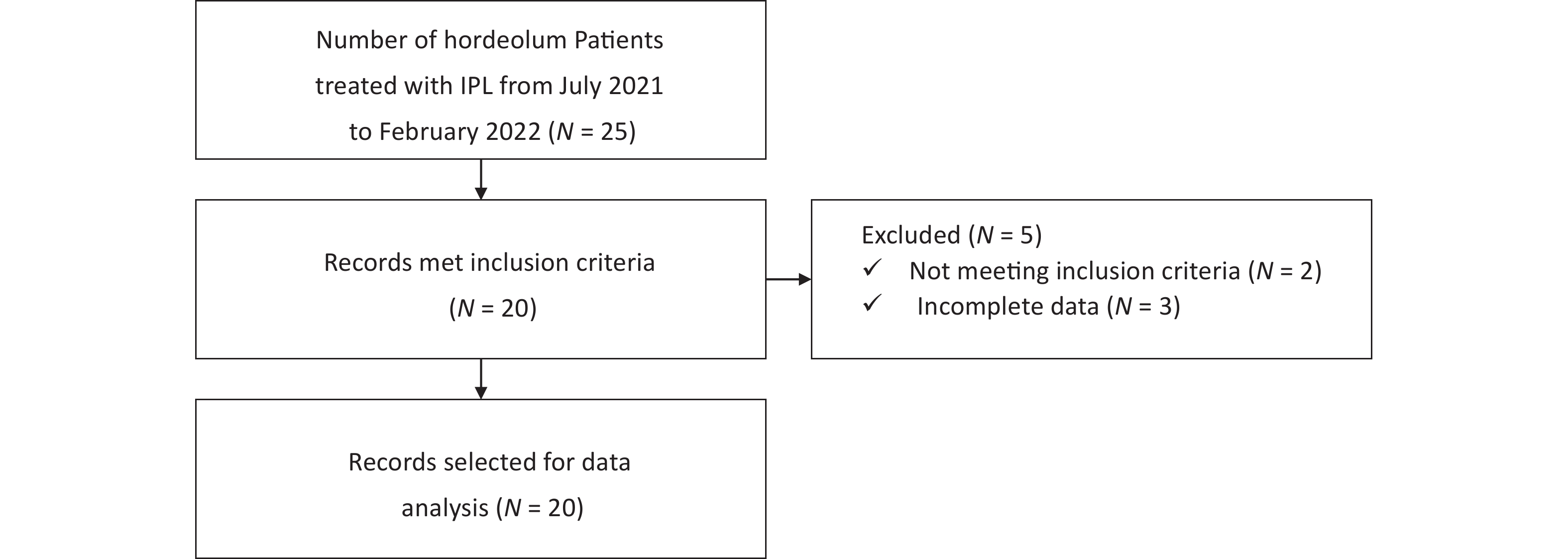
This was a single-centre, retrospective, self-controlled study performed at the Ophthalmology Department of Beijing Tongren Hospital, Capital Medical University from July 2021 to February 2022 (Figure 1). Patients with chronic hordeolum unwilling to undergo surgery were administered IPL treatment once every two weeks. The course of IPL treatment ranged from 3 to 5 sessions depending on patient outcomes and tolerance. In the course of this study, all patients were not treated with any medication and only recieved IPL treatment. All patients had received topical compound antimicrobial therapy before IPL treatment and showed no significant improvement for at least two weeks. The drugs used were gatifloxacin eye gel (5.0 g, Diyou, Sinqi, China), tobramycin eye ointment (3.5 g, Tobrex, Alcon, Belgium) or tobramycin dexamethasone eye ointment (3.5 g, TobraDex, Alcon, Belgium). This study was approved by the Ethics Committee of Beijing Tongren Hospital, Capital Medical University (no.: TRECKY2017-063). All examination and treatment procedures were carried out following the Declaration of Helsinki and associated ethical standards. All patients signed an informed consent form prior to the start of the study.
Changes in subjective symptoms, nodules, the ocular surface and the MG were examined and recorded by the same senior specialist before and after treatment. Visual acuity (VA), intraocular pressure (IOP), the anterior segment, the fundus, and adverse events were observed during treatment to assess safety.
-
The patients enrolled in this study met all of the following inclusion criteria: (1) hordeolum with secondary chronic inflammatory nodules, with manifestations including warm, tender, swollen, and red eyelid lumps and painful nodules, and granulomatous and/or thickened lid margins with telangiectatic blood vessels, no abscess or chalazion; (2) congested and swollen nodules showing no significant remission after at least 2-weeks of nonsurgical treatments before IPL treatment; (3) received at least 3 IPL treatments.
-
Patients with a diagnosis not consistent with chronic hordeolum or any of the following conditions were excluded: (1) hordeolum in the acute phase (usually less than 2 weeks); (2) history of hordeolum excision surgery; (3) incomplete clinical data.
-
Before IPL treatment, the patients were typed by Fitzpatrick skin typing[22]. Energy density level depended on skin type, and lower energy levels were applied for patients with darker skin. The settings were as follows: energy density, 15–19 J/cm2; optical filter, acne filter; pulse quantity, 3; pulse time, 5 ms; pulse delay, 40 ms.
-
After dropping 0.5% proparacaine hydrochloride eye drops (15 mL/75 mg, Alcaine, Alcon, Belgium) in the conjunctival sac and cleaning the ocular surface, both patient eyes were closed and sealed with a sterilized cornea-protective shield to protect them from the emitted therapeutic light. The physician also wore protective glasses for protection.
-
IPL treatment was conducted by a single physician using M22 OPT (Lumenis Medical Laser Co., Ltd). A medical ultrasonic couplant (250 g, Jinnuote, China) was applied to the treatment area, including the forehead and upper and lower eyelids, to conduct the applied light and help spread the energy homogeneously. In both patients with single and multiple hordeola, the tip was sited on 1–2 mm away from the upper and lower lid margins, and four shots were separately applied. A small tip (6 mm × 6 mm) was applied to a single nodule to ensure treatment accuracy and avoid damage to surrounding normal skin. For multiple nodules, a medium tip (8 mm × 15 mm) was applied. A large tip (15 mm × 35 mm) was used to treat the forehead near the eyelids in patients with both single and multiple hordeola (the energy density was lower than that applied to nodules). The ultrasonic couplant was gently removed from the treated skin area after treatment. The eyelid margins and nodules were cleaned after every IPL treatment. An eye mask stored in the refrigerator was used to cover the eyes to cool the skin of the treated area.
-
A visual analogue scale (VAS) was applied to evaluate the discomfort of each patient and reflect the degree of subjective symptoms[23]. The VAS is a 10-centimetre (100-mm) continuous scale comprising a horizontal (HVAS, used in this study) or vertical (VVAS) line. The scale ranged from “no discomfort” (0 points) to “discomfort as bad as it could be” or “worst discomfort” (100 points). The patients were asked to place a line perpendicular to the VAS line at the point indicating the intensity of their discomfort in the past 2 weeks. The degree of discomfort was evaluated by the distance (mm) between the “no discomfort” anchor and the patient’s mark.
-
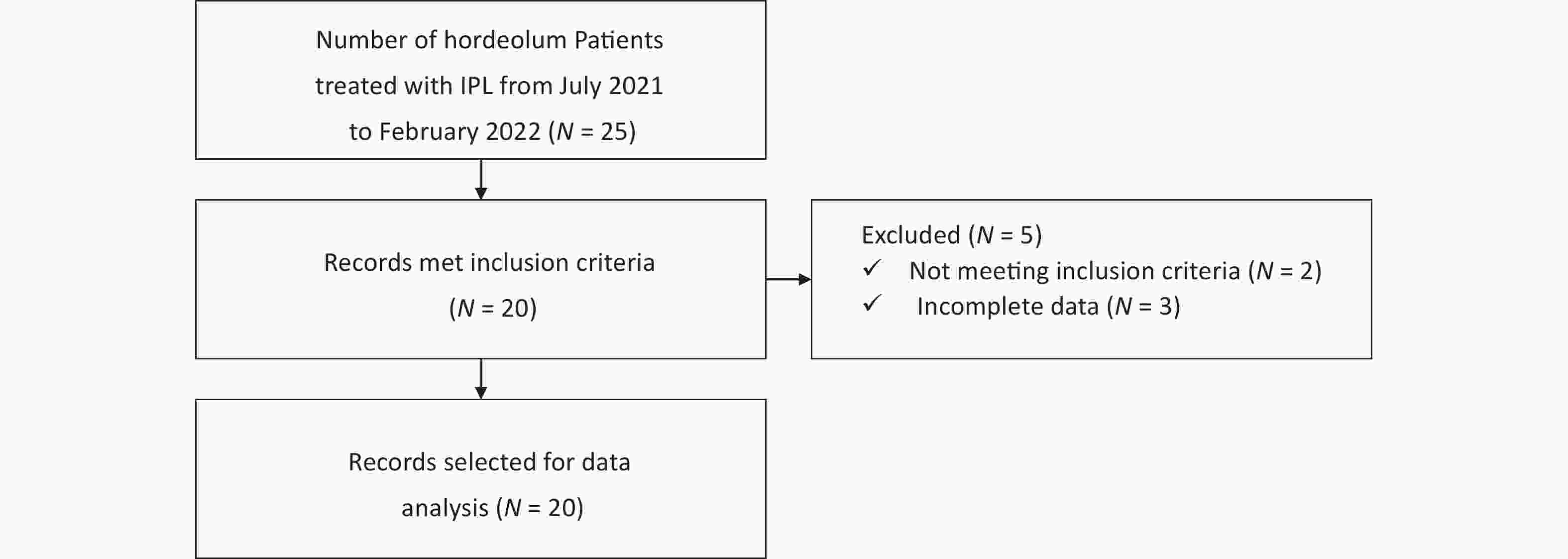
The number of inflammatory nodules was counted and recorded before and after IPL treatment. The size of the nodule could be calculated indirectly by comparison with the transverse diameter of the cornea[24]. In the same picture, the transverse diameter of the cornea, and the long and short diameters of each nodule were measured using the image processing software Photoshop independently (Figure 2). The relative size of each nodule was calculating by determining the number of pixels of the transverse diameter of cornea occupied by the long and short diameters. Therefore, the value was the ratio of the length and diameter of the nodule to the transverse diameter of the cornea. The nodules were approximated as ellipses, and the relative area was calculated using formula for calculating the area of an ellipse. By regarding the cornea as approximately round, the relative size of the nodule relative to the cornea could be calculated. All values are kept to 2 decimal places. The Clinician Erythema Assessment (CEA) scale was used to score the degree of congestion of each nodule[25]. The scoring criteria were as follows: 0, clear skin with no signs of erythema; 1, almost clear skin and slight redness; 2, mild erythema and definite redness; 3, moderate erythema and marked redness; 4, severe erythema and intense redness. All hordeolum examinations were conducted by the same doctor.
-
The composite eyelid score was used to evaluate the eyelid margin signs[26], including blunt rounding of the eyelid margin, thickening of lid margin, hyperkeratinization of the lid margin, congestion of the anterior lid margin, and vascularity and telangiectasia around MG orifices. Each clinical symptom was given a score of 1 point, on a scale of 0–5 points.
-
The central five glands of the lower eyelids were pressed with Meibomian Gland Evaluator (MGE; Tear Science, Inc.)[27]. MG expressibility was scored according to a previous method[28]: 0, all glands expressible; 1, 3–4 glands expressible; 2, 1–2 glands expressible; 3, no glands expressible. A score ranging from 0 to 3 was then assigned to each eye.
-
The liquid extracted from the central five MGs by gently pressing 1–2 mm below the eyelid margin was scored according to a previously proposed method[29]: 0, clear fluid; 1, cloudy fluid; 2, cloudy particulate fluid; 3, inspissated, toothpaste-like discharge. A score ranging from 0 to 3 was then assigned to each eye.
-
Using the Meibo-Scan of Oculus Keratograph 5M (K5M, Oculus Optikgerate GmbH, Germany), the structure of the MG was observed with an infrared light source, and loss of the glands was divided into four grades[30]: grade 0, no loss of meibomian glands; grade 1, loss of meibomian glands below 1/3 of the total area; grade 2, loss of meibomian glands accounting for 1/3 to 2/3 of the total area; grade 3, loss of meibomian glands accounting for 2/3 or more of the total area.
-
The TMH was also measured using the K5M. On a natural light background, the computer system was used to acquire images and measure the TMH in millimeters (mm). Triplicate measurements were obtained by the same person and averaged.
-
Fluorescent dye was dropped into the patient's conjunctival sac and observed on the background of cobalt blue light using a slit lamp. The cornea was divided into five regions and graded based on the American NEI scale[31]. The total CFS score was between 0 and 15, ranging from 0 to 3 in each region as follows: 0, no staining; 1, 1–30 punctate staining; 2, punctate staining > 30; 3, diffuse staining, filaments, ulcer, etc.
-
A standard logarithmic visual acuity chart was used to evaluate the visual acuity (VA) and intraocular pressure (IOP) was monitored using a noncontact tonometer. Both eyes in all patients were monitored and the results recorded during treatment. At the same time, the patients’ anterior segment and fundus, discomfort symptoms and other adverse reactions were monitored.
-
Statistical analysis was performed via SPSS 20.0 software (IBM, Armonk, NY, USA). None of the data fit a normal distribution. As a result, they were presented as the median, the first quartile and third quartile M (Q1, Q3). As the VAS score was not binocular data, the Wilcoxon paired test was used for comparison. The clustered Wilcoxon rank sum test was used to compare changes in each nodule, MG and ocular surface signs before and after treatment since they were related to both eyes[32]. P < 0.05 was considered to indicate a statistically significant difference.
-
In total, 55 nodules in 20 hordeolum patients underwent treatment with IPL from July 2021 to February 2022 were enrolled in this study. Data were collected before and after treatment, and the completed form was filed in the medical record. The patients’ demographic and clinical data included age, sex, type of hordeola, number of recurrences in the past year and related factors before treatment (Table 1). Among the 13 women (65%) and 7 men (35%), there were 6 cases of single monocular hordeolum (30%), 2 cases of multiple monocular (10%) and 12 cases of multiple binocular hordeola (60%). All patients experienced more than one recurrence.
Table 1. Demographic data, clinical characteristics and recurrent times of patients before treatment
Case Age Sex Eye Type Quantity and position Duration of disease (days) Recurrent Times With blepharitis With acne vulgaris 1 44 F OD SGL 1 in upper eyelid 15 2 No No 2 35 M OD SGL 1 in lower eyelid 20 3 No No 3 51 M OS SGL 1 in upper eyelid 21 1 No No 4 33 F OS SGL 1 in lower eyelid 16 1 No No 5 32 F OS SGL 1 in lower eyelid 18 3 No No 6 57 M OS SGL 1 in upper eyelid 22 2 No No 7 28 F OD MULT 1 in lower eyelid
1 in upper eyelid28 3 No No 8 52 F OS MULT 2 in upper eyelid 15 1 No No 9 42 M BO MULT 1 in right upper eyelid
1 in left upper eyelid21 3 No No 10 24 F BO MULT 1 in right upper eyelid
1 in right lower eyelid
2 in left upper eyelid16 2 No No 11 38 F BO MULT 2 in right lower eyelid
1 in left upper eyelid18 1 Yes No 12 31 F BO MULT 2 in right upper eyelid
1 in left upper eyelid24 2 Yes Yes 13 55 F BO MULT 2 in right upper eyelid
1 in right lower eyelid
1 in left upper eyelid
2 in left lower eyelid21 5 Yes No 14 44 M BO MULT 2 in right lower eyelid
1 in left lower eyelid15 3 No Yes 15 48 F BO MULT 1 in right upper eyelid
2 in left lower eyelid20 1 Yes No 16 54 F BO MULT 2 in right upper eyelid
1 in left upper eyelid
1 in left lower eyelid28 3 Yes No 17 26 F BO MULT 2 in right upper eyelid
1 in right lower eyelid
2 in left upper eyelid
2 in left lower eyelid40 2 No No 18 45 M BO MULT 2 in right upper eyelid
2 in left upper eyelid25 1 No No 19 42 M BO MULT 2 in right upper eyelid
1 in left lower eyelid18 4 Yes No 20 52 F BO MULT 1 in right upper eyelid
2 in left upper eyelid14 3 No No Note. M, male; F, female; OD, right eye; OS, left eye; BO, binoculus; SGL, single; MULT, multiple. Changes in the VAS score were recorded and analysed. After IPL treatment, the VAS score of discomfort was reduced to 5.45 ± 8.79, which was significantly different from the score of 25.33 ± 7.97 before treatment (P < 0.05). Changes in nodules are presented in Table 2. There were 55 nodules before treatment, and the number decreased to 23 after IPL treatment. The size of nodules was also reduced. Changes in the MG and ocular surface signs are shown in Table 3. All of the signs except MGDR showd significant improvement.
Table 2. Changes of each nodule before and after treatment
Time Number of nodules CEA Score Relative long diameter of nodules Relative short diameter of nodules Relative area of nodules Before 55 3 (2, 3) 0.26 (0.17, 0.43) 0.26 (0.17, 0.26) 0.07 (0.03, 0.11) After 20# 1 (0, 1) 0.09 (0.00, 0.26) 0.09 (0.00, 0.17) 0.01 (0.00, 0.06) P* < 0.001 < 0.001 < 0.001 < 0.001 Note. #The relative diameter and area of missing nodules were marked the as 0 and included in the statistics. *P value of clustered wilcoxon rank sum test to compare data before and after treatment. CEA, clinician erythema assessment. Table 3. Changes of meibomian gland and the signs of ocular surface before and after treatment
Time Number of
sick eyesEyelid margin
signsMG
expressibilityMeibum
qualityMGDR
(upper eyelid)MGDR
(lower eyelid)TMH
(mm)CFS score Before 32 3 (3, 4) 1 (0, 2) 2 (2, 3) 3 (2, 3) 2 (2, 3) 0.16 (0.11, 0.20) 2 (1, 2) After 32 2 (1, 2) 1 (0, 1) 2 (1, 2) 3 (2, 3) 2 (2, 3) 0.21 (0.18, 0.24) 0 (0, 1) P* < 0.001 < 0.001 < 0.001 1.000 0.564 < 0.001 < 0.001 Note. *P value of clustered wilcoxon rank sum test to compare data before and after treatment. MG, meibomian gland; MGDR, meibomian gland dropout; TMH, tear meniscus height; CFS, corneal fluorescein staining. Images acquired under the slit lamp for single monocular, multiple monocular, and multiple binocular patients are shown in Figures 3, 4, and 5. Their subjective symptoms and objective signs improved significantly.
There were no significant changes in VA and IOP before and after treatment. The anterior segment and fundus in all patients also showed no significant changes. None of the patients complained of related side effects.
-
As an inflammatory disease, hordeolum has a typical inflammatory appearance in the acute phase; the main presentation is single or multiple swollen red nodules, accompanied by pain and burning sensation[33]. The pathological manifestations are acute suppurative inflammation involving the MG or the glands of Zeis or Moll, and inflammatory cells such as neutrophils can be concentrated in the lesion area[34]. It is usually caused by bacterial infections, especially Staphylococcus aureus infections. If properly treated, hordeolum can typically resolve spontaneously within a few days or weeks. However, in some cases, disease exacerbation occurs, with progression to an acute abscess, which requires operative treatment[35]. As the disease progresses, the nodules can be absorbed. If hordeolum recurs often, the nodule can also become subacute or chronic and form an inflammatory granuloma[36]. Sometimes, hordeolum can form localized fibrous wraps, leading to chalazion formation. Therefore, taking measures to promote the absorption of hordeolum that do not respond well to traditional treatment is necessary.
The beneficial effects of IPL as a treatment modality for MGD have been previously reported in many investigations, with significant improvement eyelid margin signs, meibum quality, CFS scores, and TMH values[37-39]. IPL treatment has been shown to reduce ocular surface inflammation and improve the secretory ability of the MG. The anti-inflammatory, antimicrobial and antitelangiectatic properties of IPL are naturally related to the underlying causes of the hordeolum. IPL treatment is minimally invasive compared to traditional surgery, and more easily accepted by patients. However, the therapeutic effect of IPL on chronic hordeolum remains poorly investigated. Therefore, we collected data from patients with hordeolum treated by IPL to observe the efficacy.
All patients in this study had chronic inflammatory nodules excluding abscessed and chalazia formation. The manifestations, included swollen, red eyelid lumps and painful nodules, and granulomatous and/or thickened lid margins with telangiectatic blood vessels. Hordeolum influences the function of MG to some extent, especially in patients with multiple hordeola and dry eyes. Thus, we observed the signs of the MGs and ocular surface.
During therapy, we applied different IPL treatment methods for different conditions in patients. The energy density was set at 15–19 J/cm2 according to the Fitzpatrick skin type of the patient, which was higher than that in other studies. The reason is that patients with hordeolum often have swollen eyelids and a more extensive range of inflammation, which make it difficult to promote nodules absorption using routine energy density. A slight increase in the energy density could solve this problem, with no obvious damage to the skin. The choice of the IPL system tip also differed from other studies. In both patients with single and multiple hordeola, the tip was sited on 1–2 mm away from the upper and lower lid margins, and four shots were separately applied. A small tip (6 × 6 mm) was applied to a single nodule. For multiple nodules, a medium tip (8 × 15 mm) was applied. A large tip (15 × 35 mm) was used to treat the forehead in patients with both single and multiple hordeola. Regarding the choice of the treatment course, because of individual differences among patients, we adjusted the treatment frequency flexibly between 3 and 5. The observation endpoints were the lack of new nodules, distinctly decreased nodules and significantly improved subjective symptoms. We also observed that some patients showed a less pronounced reduction in nodule number than others. This may be associated with the severity of local inflammation. If an inflammatory wrap or cyst is formed, the treatment effect may be greatly reduced. Therefore, we recommend that IPL treatment should be initiated when inflammation has just entered the subacute or chronic stage.
The improvement in the eyelids and nodules after IPL treatment are associated with the anti-capillary and anti-inflammatory effects of IPL. Chronic inflammation of the eyelid induced by hordeolum recurrence is an important reason for new capillaries and telangiectasia in the eyelid margin. Inflammatory mediators leak from the new dilated capillaries and aggravate inflammatory reactions, constituting a vicious cycle. It has been reported that IPL targets chromophores in haemoglobin and selectively removes superficial vessels[40] and reduces the amounts of bacteria, fungi and demodex by selective photothermolysis[41], which not only relieves congestion but also reduces the release of inflammatory mediators, eventually resulting in improvement in eyelid margin signs, infections and hyperaemia.
Improvements in the amount and quality of meibum excretion were observed, including an increased amount, clarified colour and thinner texture. Inflammatory factors produced by chronic inflammation of the MG result in an altered meibum composition, which is associated with clinically observable signs affecting meibum quality and MG structure[42]. IPL decreases inflammatory cytokines and improves local inflammation by reducing the number of capillaries[15]. The meibum improvements reflected alleviation of the MG inflammation. A study has indicated that relative hypoxia significantly increases DNase II activity and stimulates the expression of SREBP-1 in differentiating immortalized human MG epithelial cells (IHMGECs), thereby promoting the function of MG[43]. IPL helps reduce local capillaries and maintain hypoxia. The use of IPL can increase skin temperature[9], help liquefy meibum and improve its secretion[44]. We concluded that IPL treatment is an effective method to enhance secretion and reduce inflammation of the MG.
CFS scores reflect the degree of ocular surface damage, which has a positive correlation with ocular surface inflammation[45]. The improvements in the CFS score and TMH indicated the amelioration of MG secretion and ocular surface inflammation after treatment, reflecting therapeutic efficacy.
There were no significant changes in VA, IOP, the anterior segment or the fundus after treatment. During the experiment, the subjects showed adaptability and only a small number of patients complained of burning pain. No skin scalding or burning was observed after treatment. These results indicate that IPL could be considered a safe treatment for hordeolum.
Of course, this study also has limitations. Because of the lack of a control group, small sample size, and inconsistent degree of disease among patients. In addition, there are many other factors including systemic diseases (diabetes, high blood sugar etc.) or other medications patients may be taking that could affect the result. Despite these limitations, it can still be concluded from clinical observation and data analysis of objective indicators that IPL is effective in the treatment of hordeolum. In future work, we plan to enlarge the sample size, increase the observation indexes and clinical data, include a blank control, and address other limitations, to further prove the validity of IPL for chronic hordeolum.
-
IPL is beneficial for the treatment of chronic hordeolum by reducing the area of nodules and relieving ocular surface symptoms and signs.
-
Ethical approval and consent to participation: This study was approved by the Ethics Committee of Beijing Tongren Hospital, Capital Medical University (no.: TRECKY2017-063) and all methods were carried out in accordance with relevant guidelines and regulations. All patients’ consent was obtained before the study. All examination and treatment procedures were carried out following the Declaration of Helsinki and ethical standards.
Consent to publication (Only if applicable): Not applicable.
Availability of data and material: The datasets used and/or analysed during the current study are available from the corresponding author professor Ying Jie on reasonable request.
Competing interest: The author(s) declare no competing interests.
Funding: no funding or grant support.
Acknowledgement: There are no acknowledgements.
Author contribution: JIE Ying, TIAN Lei contributed to the conception of the study; YANG Ke, WEN Ya, ZHU Lei performed the experiment; BAO Jia Yu, LI Shang contributed significantly to analysis and manuscript preparation; WANG Ying Hui, FENG Jun helped perform the analysis with constructive discussions.
Informed: All participants provided signed informed consent before the study.
doi: 10.3967/bes2023.131
-
Abstract:
Objective To evaluate the effect of intense pulsed light (IPL) in the treatment of chronic hordeolum. Methods Patients with chronic hordeolum who underwent IPL treatment were enrolled in this study. According to the severity of hordeolum, the patients were treated with IPL 3 to 5 times. Patients' satisfaction and visual analog scale scores for ocular discomfort symptoms before and after treatment were collected. The number, congestion, long diameter, short diameter and area of nodules were also recorded and measured. Finally, eyelid margin signs, meibum quality, meibomian gland expressibility, meibomian gland dropout, tear meniscus height, and corneal fluorescein staining were scored. Results 20 patients were enrolled in this study. The eyelid margins were congestive and swollen, with blunt rounding or irregularity. The meibum was cloudy or toothpaste-like. The meibomian gland expressibility, meibomian gland dropout and tear meniscus height were reduced. The cornea showed scattered fluorescein staining. After treatment, score of visual analog scale, congestion and size of nodules were significantly reduced. Eyelid margin signs, meibum quality, meibomian gland expressibility, tear meniscus height and corneal fluorescein staining scores were improved. Meibomian gland dropout had no significant change. No side effects occurred during treatment. Conclusions IPL is beneficial for the treatment of chronic hordeolum. -
Key words:
- Intense pulsed light /
- Hordeolum /
- Chronic
注释: -
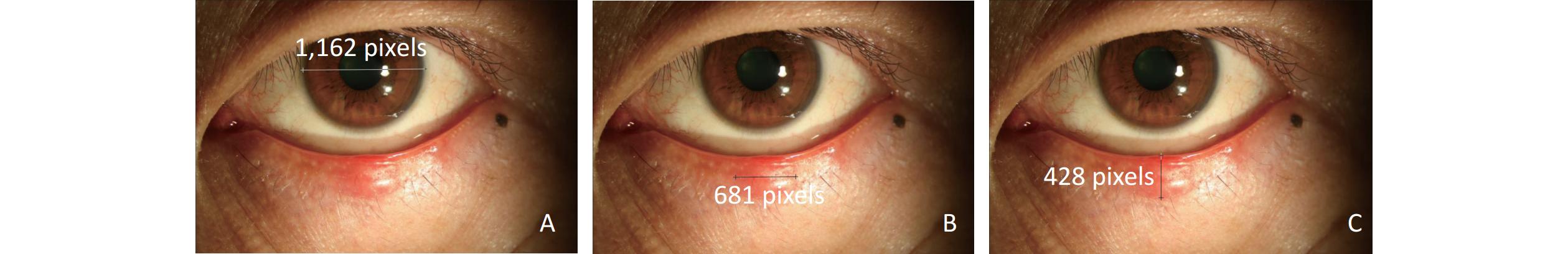
Figure 3. Changes in a patient with a single nodule in the left eye after IPL treatment.
This patient was a middle-aged man who had experienced once recurrence. He underwent IPL treatment 3 times. (A) Before IPL treatment, there was a painful red nodule in the middle of the left lower eyelid. The eyelid margin was bluntly rounded. (B) Two weeks after the 1st treatment, the size of the nodule and degree of hyperaemia had decreased. (C) One month later (2 weeks after the 2nd treatment), the nodule had disappeared and the hyperaemia and swelling had subsided; however, the eyelid margin still showed blunt rounding. (D) Six weeks later (2 weeks after the 3rd treatment), the eyelid margin was completely smooth.
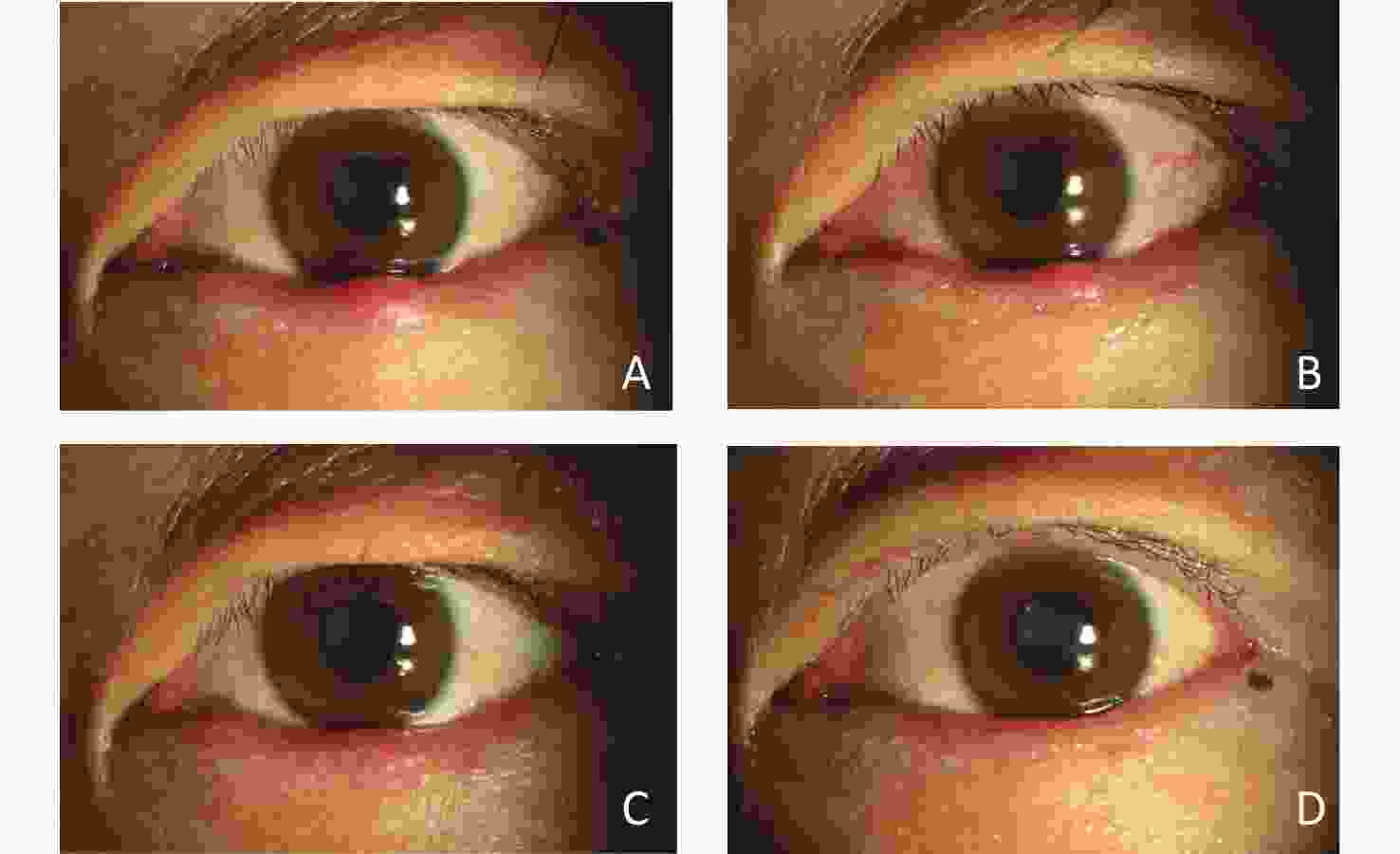
Figure 4. Changes in a patient with multiple hordeola in the right eye after IPL treatment.
This patient was a young woman who had experienced 3 recurrences. She underwent IPL treatment 3 times. (A) Before IPL treatment, there was a red nodule in the middle of the right lower eyelid, and another in the middle of the right upper eyelid. The eyelid margins were bluntly rounded. (B) Two weeks later (2 weeks after the 1st treatment), the size of the nodule on the lower eyelid and the hyperaemia had decreased. (C) One month later (2 weeks after the 2nd treatment), the size of the nodule was had decreased, and hyperaemia had been notably alleviated. (D) Six weeks later (2 weeks after the 3rd treatment), the nodule on the lower eyelid had almost disappeared, and the hyperaemia on the upper eyelid had subsided.
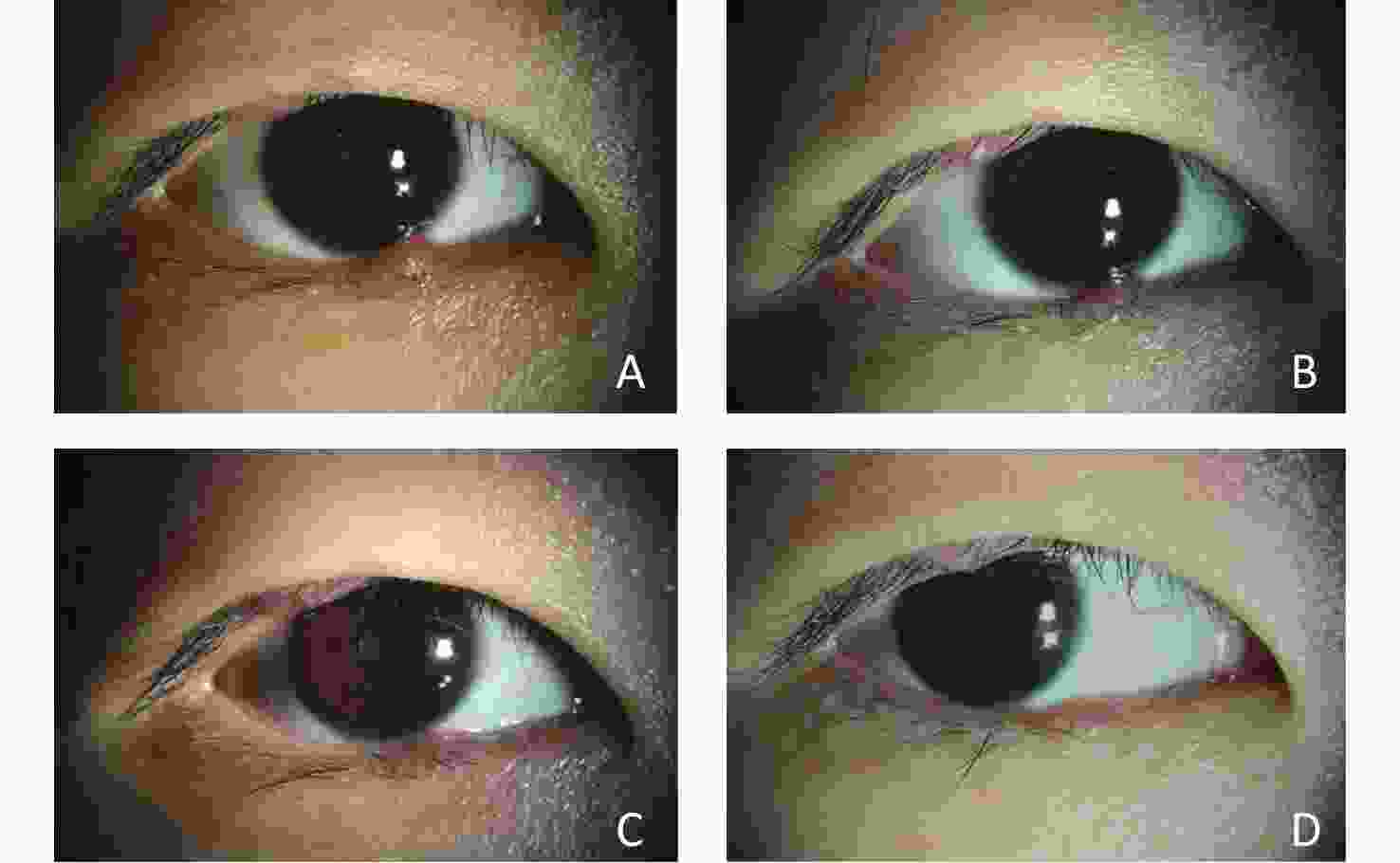
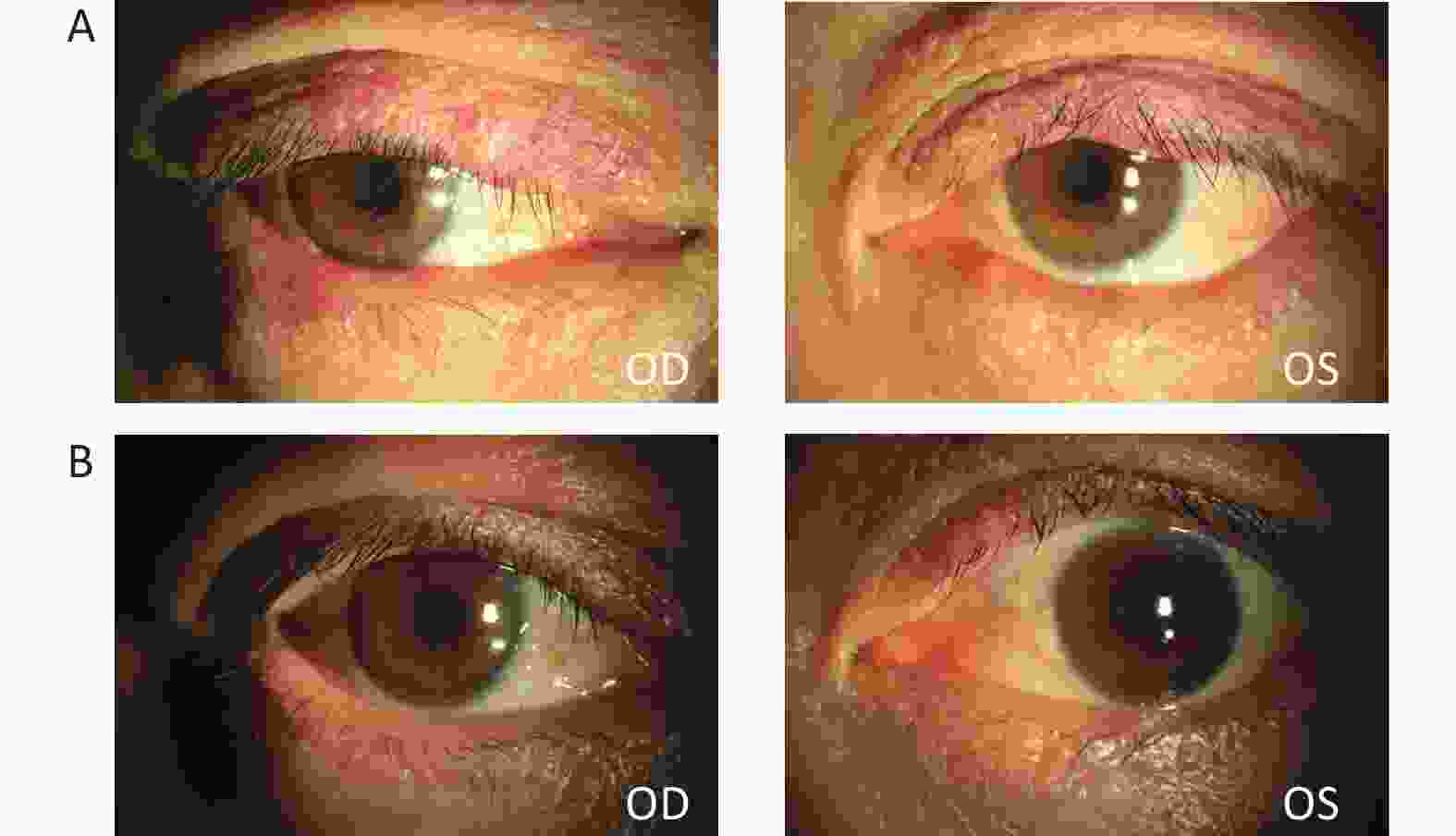
Figure 5. Changes in a patient with multiple hordeola in both eyes and blepharitis after IPL treatment.
This patient was a middle-aged woman who experienced 5 recurrences. She underwent 5 rounds of IPL treatment. (A) Before IPL treatment, the patient’s eyelids were red and swollen, with multiple nodules; the eyelid margins were bluntly rounded and congested. (B) After 5 courses of treatment, the nodules had disappeared and the hyperemia had subsided.
Table 1. Demographic data, clinical characteristics and recurrent times of patients before treatment
Case Age Sex Eye Type Quantity and position Duration of disease (days) Recurrent Times With blepharitis With acne vulgaris 1 44 F OD SGL 1 in upper eyelid 15 2 No No 2 35 M OD SGL 1 in lower eyelid 20 3 No No 3 51 M OS SGL 1 in upper eyelid 21 1 No No 4 33 F OS SGL 1 in lower eyelid 16 1 No No 5 32 F OS SGL 1 in lower eyelid 18 3 No No 6 57 M OS SGL 1 in upper eyelid 22 2 No No 7 28 F OD MULT 1 in lower eyelid
1 in upper eyelid28 3 No No 8 52 F OS MULT 2 in upper eyelid 15 1 No No 9 42 M BO MULT 1 in right upper eyelid
1 in left upper eyelid21 3 No No 10 24 F BO MULT 1 in right upper eyelid
1 in right lower eyelid
2 in left upper eyelid16 2 No No 11 38 F BO MULT 2 in right lower eyelid
1 in left upper eyelid18 1 Yes No 12 31 F BO MULT 2 in right upper eyelid
1 in left upper eyelid24 2 Yes Yes 13 55 F BO MULT 2 in right upper eyelid
1 in right lower eyelid
1 in left upper eyelid
2 in left lower eyelid21 5 Yes No 14 44 M BO MULT 2 in right lower eyelid
1 in left lower eyelid15 3 No Yes 15 48 F BO MULT 1 in right upper eyelid
2 in left lower eyelid20 1 Yes No 16 54 F BO MULT 2 in right upper eyelid
1 in left upper eyelid
1 in left lower eyelid28 3 Yes No 17 26 F BO MULT 2 in right upper eyelid
1 in right lower eyelid
2 in left upper eyelid
2 in left lower eyelid40 2 No No 18 45 M BO MULT 2 in right upper eyelid
2 in left upper eyelid25 1 No No 19 42 M BO MULT 2 in right upper eyelid
1 in left lower eyelid18 4 Yes No 20 52 F BO MULT 1 in right upper eyelid
2 in left upper eyelid14 3 No No Note. M, male; F, female; OD, right eye; OS, left eye; BO, binoculus; SGL, single; MULT, multiple. Table 2. Changes of each nodule before and after treatment
Time Number of nodules CEA Score Relative long diameter of nodules Relative short diameter of nodules Relative area of nodules Before 55 3 (2, 3) 0.26 (0.17, 0.43) 0.26 (0.17, 0.26) 0.07 (0.03, 0.11) After 20# 1 (0, 1) 0.09 (0.00, 0.26) 0.09 (0.00, 0.17) 0.01 (0.00, 0.06) P* < 0.001 < 0.001 < 0.001 < 0.001 Note. #The relative diameter and area of missing nodules were marked the as 0 and included in the statistics. *P value of clustered wilcoxon rank sum test to compare data before and after treatment. CEA, clinician erythema assessment. Table 3. Changes of meibomian gland and the signs of ocular surface before and after treatment
Time Number of
sick eyesEyelid margin
signsMG
expressibilityMeibum
qualityMGDR
(upper eyelid)MGDR
(lower eyelid)TMH
(mm)CFS score Before 32 3 (3, 4) 1 (0, 2) 2 (2, 3) 3 (2, 3) 2 (2, 3) 0.16 (0.11, 0.20) 2 (1, 2) After 32 2 (1, 2) 1 (0, 1) 2 (1, 2) 3 (2, 3) 2 (2, 3) 0.21 (0.18, 0.24) 0 (0, 1) P* < 0.001 < 0.001 < 0.001 1.000 0.564 < 0.001 < 0.001 Note. *P value of clustered wilcoxon rank sum test to compare data before and after treatment. MG, meibomian gland; MGDR, meibomian gland dropout; TMH, tear meniscus height; CFS, corneal fluorescein staining. -
[1] Gordon AA, Danek DJ, Phelps PO. Common inflammatory and infectious conditions of the eyelid. Dis Mon, 2020; 66, 101042. doi: 10.1016/j.disamonth.2020.101042 [2] Bragg KJ, Le PH, Le JK. Hordeolum. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC. 2020. [3] Lindsley K, Nichols JJ, Dickersin K. Interventions for acute internal hordeolum. Cochrane Database Syst Rev, 2013; 4, CD007742. [4] Lindsley K, Nichols JJ, Dickersin K. Non-surgical interventions for acute internal hordeolum. Cochrane Database Syst Rev, 2017; 1, CD007742. [5] Maldonado MJ, Juberías JR, Moreno-Montañés J. Extensive corneal epithelial defect associated with internal hordeolum after uneventful laser in situ keratomileusis. J Cataract Refract Surg, 2002; 28, 1700−2. doi: 10.1016/S0886-3350(01)01271-8 [6] Cheng K, Law A, Guo MH, et al. Acupuncture for acute hordeolum. Cochrane Database Syst Rev, 2017; 2, CD011075. [7] Babilas P, Schreml S, Szeimies RM, et al. Intense pulsed light (IPL): a review. Lasers Surg Med, 2010; 42, 93−104. doi: 10.1002/lsm.20877 [8] Vora GK, Gupta PK. Intense pulsed light therapy for the treatment of evaporative dry eye disease. Curr Opin Ophthalmol, 2015; 26, 314−8. doi: 10.1097/ICU.0000000000000166 [9] Craig JP, Chen YH, Turnbull PRK. Prospective trial of intense pulsed light for the treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci, 2015; 56, 1965−70. doi: 10.1167/iovs.14-15764 [10] Xue AL, Wang MTM, Ormonde SE, et al. Randomised double-masked placebo-controlled trial of the cumulative treatment efficacy profile of intense pulsed light therapy for meibomian gland dysfunction. Ocul Surf, 2020; 18, 286−97. doi: 10.1016/j.jtos.2020.01.003 [11] Arita R, Fukuoka S, Morishige N. Therapeutic efficacy of intense pulsed light in patients with refractory meibomian gland dysfunction. Ocul Surf, 2019; 17, 104−10. doi: 10.1016/j.jtos.2018.11.004 [12] Wladis EJ, Aakalu VK, Foster JA, et al. Intense pulsed light for meibomian gland disease: a report by the American academy of ophthalmology. Ophthalmology, 2020; 127, 1227−33. doi: 10.1016/j.ophtha.2020.03.009 [13] Ruan F, Zang YX, Sella R, et al. Intense pulsed light therapy with optimal pulse technology as an adjunct therapy for moderate to severe blepharitis-associated keratoconjunctivitis. J Ophthalmol, 2019; 2019, 3143469. [14] Lyons AB, Townsend SM, Turk D, et al. Laser and light-based treatment modalities for the management of hidradenitis suppurativa. Am J Clin Dermatol, 2020; 21, 237−43. doi: 10.1007/s40257-019-00491-1 [15] Toyos R, McGill W, Briscoe D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction; a 3-year retrospective study. Photomed Laser Surg, 2015; 33, 41−6. doi: 10.1089/pho.2014.3819 [16] Taylor M, Porter R, Gonzalez M. Intense pulsed light may improve inflammatory acne through TNF-α down-regulation. J Cosmet Laser Ther, 2014; 16, 96−103. doi: 10.3109/14764172.2013.864198 [17] Jiang XD, Lv HB, Song H, et al. Evaluation of the safety and effectiveness of intense pulsed light in the treatment of meibomian gland dysfunction. J Ophthalmol, 2016; 2016, 1910694. [18] Zhu Q, Xiao SY, Hua ZJ, et al. Near infrared (NIR) light therapy of eye diseases: a review. Int J Med Sci, 2021; 18, 109−19. doi: 10.7150/ijms.52980 [19] Dell SJ. Intense pulsed light for evaporative dry eye disease. Clin Ophthalmol, 2017; 11, 1167−73. doi: 10.2147/OPTH.S139894 [20] Han JY, Lee Y, Nam S, et al. Effect of intense pulsed light using acne filter on eyelid margin telangiectasia in moderate-to-severe meibomian gland dysfunction. Lasers Med Sci, 2022; 37, 2185−92. doi: 10.1007/s10103-021-03482-z [21] Ryu SI, Suh DH, Lee SJ, et al. Efficacy and safety of intense pulsed light using a dual-band filter for the treatment of facial acne vulgaris. Lasers Med Sci, 2022; 37, 531−6. doi: 10.1007/s10103-021-03292-3 [22] Roberts WE. Skin type classification systems old and new. Dermatol Clin, 2009; 27, 529−33. [23] Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: visual analog scale for pain (VAS Pain), numeric rating scale for pain (NRS Pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res, 2011; 63, S240−52. doi: 10.1002/acr.20543 [24] Larsen SDH, Heegaard S, Toft PB. Histological and clinical evaluation of the hard palate mucous membrane graft for treatment of lower eyelid retraction. Acta Ophthalmol, 2017; 95, 295−8. doi: 10.1111/aos.13321 [25] Tan J, Liu H, Leyden JJ, et al. Reliability of clinician erythema assessment grading scale. J Am Acad Dermatol, 2014; 71, 760−3. doi: 10.1016/j.jaad.2014.05.044 [26] Yan XM, Hong J, Jin XM, et al. The efficacy of intense pulsed light combined with meibomian gland expression for the treatment of dry eye disease due to meibomian gland dysfunction: a multicenter, randomized controlled trial. Eye Contact Lens, 2021; 47, 45−53. doi: 10.1097/ICL.0000000000000711 [27] Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea, 2008; 27, 1142−7. doi: 10.1097/ICO.0b013e3181814cff [28] Pflugfelder SC, Tseng SC, Sanabria O, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea, 1998; 17, 38. doi: 10.1097/00003226-199801000-00007 [29] Bron AJ, Benjamin L, Snibson GR. Meibomian gland disease. Classification and grading of lid changes. Eye, 1991; 5, 395−411. doi: 10.1038/eye.1991.65 [30] Srinivasan S, Menzies K, Sorbara L, et al. Infrared imaging of meibomian gland structure using a novel keratograph. Optom Vis Sci, 2012; 89, 788−94. doi: 10.1097/OPX.0b013e318253de93 [31] Amparo F, Wang HB, Yin J, et al. Evaluating corneal fluorescein staining using a novel automated method. Invest Ophthalmol Vis Sci, 2017; 58, BIO168−73. doi: 10.1167/iovs.17-21831 [32] Rosner B, Glynn RJ, Lee MLT. Incorporation of clustering effects for the Wilcoxon rank sum test: a large-sample approach. Biometrics, 2003; 59, 1089−98. doi: 10.1111/j.0006-341X.2003.00125.x [33] Carlisle RT, Digiovanni J. Differential diagnosis of the swollen red eyelid. Am Fam Physician, 2015; 92, 106−12. [34] George JL. Eyelid pathology: stye, chalazion, ectropion, entropion. Diagnosis. Rev Prat, 1990; 40, 1619−20. [35] McAlinden C, González-Andrades M, Skiadaresi E. Hordeolum: Acute abscess within an eyelid sebaceous gland. Cleve Clin J Med, 2016; 83, 332−4. doi: 10.3949/ccjm.83a.15012 [36] Akal A, Goncu T, Kocarslan S, et al. Hemorrhagic pyogenic granuloma after internal hordeolum. Int J Crit Illn Inj Sci, 2014; 4, 317−8. doi: 10.4103/2229-5151.147540 [37] Arita R, Fukuoka S, Mizoguchi T, et al. Multicenter study of intense pulsed light for patients with refractory aqueous-deficient dry eye accompanied by mild meibomian gland dysfunction. J Clin Med, 2020; 9, 3467. doi: 10.3390/jcm9113467 [38] Suwal A, Hao JL, Zhou DD, et al. Use of intense pulsed light to mitigate meibomian gland dysfunction for dry eye disease. Int J Med Sci, 2020; 17, 1385−92. doi: 10.7150/ijms.44288 [39] Cote S, Zhang AC, Ahmadzai V, et al. Intense pulsed light (IPL) therapy for the treatment of meibomian gland dysfunction. Cochrane Database Syst Rev, 2020; 3, CD013559. [40] Farrell HP, Garvey M, Cormican M, et al. Investigation of critical inter-related factors affecting the efficacy of pulsed light for inactivating clinically relevant bacterial pathogens. J Appl Microbiol, 2010; 108, 1494−508. doi: 10.1111/j.1365-2672.2009.04545.x [41] Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science, 1983; 220, 524−7. doi: 10.1126/science.6836297 [42] Paranjpe V, Tan J, Nguyen J, et al. Clinical signs of meibomian gland dysfunction (MGD) are associated with changes in meibum sphingolipid composition. Ocul Surf, 2019; 17, 318−26. doi: 10.1016/j.jtos.2018.12.006 [43] Liu Y, Chen D, Chen XM, et al. Hypoxia: A breath of fresh air for the meibomian gland. Ocul Surf, 2019; 17, 310−7. doi: 10.1016/j.jtos.2018.12.001 [44] Dell SJ, Gaster RN, Barbarino SC, et al. Prospective evaluation of intense pulsed light and meibomian gland expression efficacy on relieving signs and symptoms of dry eye disease due to meibomian gland dysfunction. Clin Ophthalmol, 2017; 11, 817−27. doi: 10.2147/OPTH.S130706 [45] Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: the role of gland dysfunction in dry eye disease. Ophthalmology, 2017; 124, S20−6. doi: 10.1016/j.ophtha.2017.05.031 -




 下载:
下载:



 Quick Links
Quick Links