HTML
-
Cancer is a major public health issue in China. It is the second leading cause of death, and its incidence and mortality continue to increase [1]. Colon cancer is currently one of the most common malignancies and a leading cause of cancer-related death [2]. Surgery, chemotherapy, radiotherapy, and targeted therapy (immunotherapy, gene therapy, angiogenesis inhibitors, and others) are approaches to treating cancer. Although it is the most common daily treatment, drug treatment is problematic because of its side effects. Cancer patients have a strong desire to seek out alternative diet and nutritional therapies to augment their conventional cancer therapy [3]. Accumulating evidence from the epidemiology, human medicine, and nutrition fields has indicated that dietary agents can safely regulate physiological function and enhance anticarcinogenic activity [4-5]. In addition, natural products have gained popularity in the prevention and treatment of cancer [6-8].
Barley is a cereal that is often associated with beneficial health effects. It is used as animal fodder, as a source of fermentable material for beer and certain distilled beverages, and as a component of various health foods [9]. As it is an excellent source of soluble and insoluble dietary fiber (mainly β-glucan) and other bioactive constituents, such as protein, essential amino acids [10], and phenolic compounds [11-12], consumers have become more inclined to increase their utilization of barley as a source of food. Studies have demonstrated the beneficial free-radical-scavenging, anti-aging, memory-enhancing, anti-obesity, and anti-cancer effects of barley [13-15]. Although barley has obvious anticancer properties [16], its efficacy differs from that of drugs, which require daily intake at a certain dosage. Based on this, new technologies should be explored to improve the efficiency and processing quality of drugs.
Recently, cereal fermentations have shown significant potential in the improvement and design of the nutritional quality and health effects of foods and ingredients [17]. Many biochemical changes occur during fermentation, leading to an altered ratio of nutritive to antinutritive components of plants, which affects product properties, such as bioactivity and digestibility. Barely shochu is traditional Japanese liquor distilled from barley fermented by Saccharomyces cerevisiae. Some studies showed that powder obtained from the remnants of barley shochu distillation showed no acute toxicity in tail vein administration to normal rats. Furthermore, barley powder was shown to have not only an antitumor effect but also an immunostimulatory one [18]. Remarkably high reduction of tumorigenesis and induction of apoptosis in the liver section was obtained in mouse models of hepatocellular carcinoma after oral administration of barely shochu distillation remnants in vivo [19]. Our previous research showed that fermented barley extract could significantly inhibit the growth of tumor cells in nude mice [20]. Although barley fermentation has been studied, there is no information about the apoptosis-regulated mechanisms of barley extract fermented with lactic acid bacteria and no animal experimental results have been reported.
In this study, we used the transplantation tumor model of human HT-29 cells in nude mice to observe the antitumor activities and apoptosis-regulatory mechanisms of LFBE in vivo. Based on previous research, this study investigated the relationship between apoptosis and the expression of Bcl-2: Bax, cyclinD1: and caspase-3 that will be able to provide a theoretical and methodological basis for its future clinical application.
-
We obtained 40 female BALB/c nude mice aged 4-6 weeks and weighing 16-18 g from Shanghai Silaike Laboratory Animal Limited Liability Company. The code number of the animals was SCXK (SU) 2012-0002. The nude mice were caged individually under specific pathogen-free (SPF) conditions in the Laboratory Animal Research Center of Jiangsu University (LARC, Zhenjiang, China) at a temperature of 22 ± 2 ℃ and a relative humidity of 40%-60% and artificially illuminated on an approximate 12 h light/dark cycle. The air exchange rate was approximately 18 times per hour. All nude mice were provided with food and sterile water ad libitum. The Shanghai Medical Experimental Animal Care Commission approved the experimental protocol.
Hulled barley (Yang si mai 3) was purchased from Yancheng of China. The Lactobacillus strain was Lactobacillus plantarum dy-1: which we had previously isolated from pickles. Its preservation number was CGMCC NO.6016 in the Chinese common microbe preservation administration center. HT-29 colon cancer cells were obtained from the Shanghai Institutes for Biological Sciences (SIBS) of the Chinese Academy of Sciences (CAS). Fetal bovine serum and McCoy's 5A medium were purchased from GIBCO. FBS, MTT, and DMSO were purchased from Evergreen. 5-fluorouracil (5-FU) was purchased from Jiangsu Deyuan Pharmaceutical Co. Ltd. Folin-Ciocalteu reagent and bovine serum albumin was purchased from Sigma-Aldrich, Inc. (Supelco, Bellefonte, PA, USA). Mixed-linkage β-glucan kit was purchased from Megazyme. All other biochemical reagents and solvents involved were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China) and were of analytical or chromatographic grade. A fluorescence quantitative PCR-related kit was from treasure biological (Takara) products, and primary and secondary antibodies were purchased from Abcam.
-
Two hundred grams of barley powder, 1.4 L of distilled water, and logarithmic-phase culture of Lactobacillus plantarum dy-1 (viable count reached 4×108 cfu/mL) were shaken and incubated at 30 ℃ for 24 h in a microbiological incubator. After fermentation, the solution was centrifuged at 12, 000 ×g for 15 min at 4 ℃ using a refrigerated centrifuge (Jouan, France), then the supernatant was freeze-dried into a powder (LFBE) using a vacuum freeze dryer (Marin Christ, Germany). In addition, for the unfermented barley, 200 g of powder were extracted with 1.4 L of distilled water at room temperature for 3 h on shaking tables. The supernatant was collected by centrifugation and freeze-dried into a powder (RBE) under the same conditions as the fermented barley. These steps were repeated six times for analysis. The freeze-dried samples were stored in sealed containers at -20 ℃ for further analysis. The extraction yield was calculated according to the following equation: Extraction yield (%) = [weight of freeze-dried powder/weight of barley (g)] × 100.
-
The crude protein content (Kjeldahl method) was measured with a semi-automatic Kjeldahl apparatus (K355: Buchi, Switzerland) and a Metrohm 877 Titrino plus Automatic Titrator (Switzerland). A conversion factor of 5.83 was used.
-
The total phenol content in RB and LFBE was determined using the Folin-Ciocalteu method. Approximately 0.5 g of RB or LFBE was weighed in a 100 mL volumetric flask and dissolved in 40% ethanol aqueous solution. Then, 1 mL of the solution was added to 4 mL of deionized water and 1 mL of Folin-Ciocalteu reagent in glass test tubes. After the mixture was shaken, 10 mL of a 7% aqueous Na2CO3 solution was added and the mixture was shaken once again. The final volume was adjusted to 20 mL with distilled water. After 90 min of reaction at 45 ℃, the absorbance was measured at 765 nm by using a spectrophotometer (model UV-9600: Rayleigh, Beijing, China) with water as a blank and used to calculate the phenol content, using gallic acid as a standard. The total phenol amount was expressed as gallic acid equivalents (GAE, mg gallic acid/g sample) by comparison with a calibration curve of gallic acid. The calibration curve ranged from 20 to 100 μg/mL (R2 = 0.9954).
-
The total flavonoid content was determined according to the aluminum chloride colorimetric method [21]. The total flavonoid content was expressed in milligrams of rutin equivalents per gram of dry material.
-
β-glucan content was measured from at least two independent extracts. The contents of β-glucan in RBE and LFBE were determined using a mixed-linkage β-glucan kit (Megazyme, Wicklow, Ireland). Briefly, RBE or LFBE were pre-extracted with ethanol to remove free sugar, fats, and oil. Then, the samples were incubated with lichenase at 40 ℃ for 1 h followed by further hydrolysis with β-glucosidase at 40 ℃ for 15 min. Finally, the absorbance was measured with an enzyme-linked immunosorbent assay reader at 510 nm after incubation with GOPOD reagent.
-
The total polysaccharide content was determined by using the phenol-sulfuric acid method with slight modifications [22]. First, 2 mL of the sample solution was vortexed with 1 mL of 5% phenol in water before rapidly adding 5 mL of concentrated sulfuric acid. After 30 min of standing at room temperature, the absorbance of the sample solution was measured at 490 nm against the blank, which was prepared by substituting distilled water for the sample solution. Aqueous glucose solutions of different concentrations (5: 10: 20: 40: 80: and 160 μg/mL) were used for the standards. The results were expressed as grams of glucose per 100 g of extract.
-
Human colon cancer cell strain HT-29 was thawed and cultured as monolayers of up to 80% confluence in McCoy's 5A supplemented by 10% heat-inactivated fetal calf serum and 1% penicillin/streptomycin. The insemination and embryo cultures were performed in a CO2 incubator (Forma 310: Thermo Fisher Scientific, MA, USA) under an atmosphere of 5% CO2 in humidified air at 37 ℃. Cell counts were determined using a micro-cell counter CC-108 (Sysmex, Kobe, Japan). Cells in a logarithmic phase of growth were used for the study and are described as follows.
-
HT-29 cells were transplanted via subcutaneous injection of 1 × 107 cells into the right flank of each nude mouse. One week after transplantation, tumors had grown to a volume of approximately 20 mm3 with a model success rate of 100%. The 40 nude mice were equally randomized and divided into four experimental groups, including high- and low-dose LFBE, 5-FU, and control groups.
The high- and low-dose LFBE groups were treated with 2 g/kg and 1 g/kg LFBE by gavage once per day for 30 days, respectively. The control group received equivalent amounts of normal saline in the same way. The 5-FU group received intraperitoneal injections of 25 mg·kg-1·d-1 for 7 days at first, followed by treatment with normal saline by gavage once per day for 30 days to eliminate the effect of gavage (Table 1), similar to the previous design [20].
Group Administration Dosages Days Control Normal saline, 15 mL·kg-1×d-1 30 5-FU 5-FU, 25 mg·kg-1·d-1 7 Low-dose LFBE LFBE, 1 g·kg-1·d-1 30 High-dose LFBE LFBE, 2 g·kg-1·d-1 30 Note. *The 5-FU group received intraperitoneal injections of 25 mg·kg-1·d-1 continued for 7 days at first, followed by treatment with normal saline by gavage once per day for 30 days. Table 1. The Different Groups and the Administration Dosages
The volume of a tumor was calculated by using the following formula: tumor volume (mm3) V = (a2 × b)/2: where a = the shortest diameter and b = the longest diameter of the tumor (in mm), forming the tumor growth curve. In addition, the tumor inhibitory rate = (1 - mean tumor weight of the treatment group/mean tumor weight of the control group) × 100%. At 30 days, 10 animals in each group were sacrificed. Tumor tissue was excised by scalpel for shape observation and gravimetry. In addition, part of the tumor tissue and liver specimens were taken for routine pathology and electron microscopy observation. The rest of the tumor tissue was frozen in liquid nitrogen and kept at -80 ℃ for further use.
-
TUNEL staining was performed using the ApopTag kit (Oncor, Purchase, NY, USA). Sections (4 μm) were briefly deparaffinized in xylene, rehydrated in decreasing concentrations of ethanol, boiled in Citra (Biogenex, San Raman, CA, USA) for 10 min, and digested in 0.5% pepsin for 60 min at 37 ℃. Endogenous peroxidase was then blocked in 3% hydrogen peroxide. Three different dilutions (1:7: 1:11: and 1:16) of terminal deoxynucleotidyl transferase (TDT) in reaction buffer (containing a fixed concentration of digoxigenin-labeled nucleotides) were applied to serial sections for 1 h at 37 ℃. The slides were then placed in a stop/wash buffer for 10 min. Following the washes, a prediluted anti-digoxigenin peroxidase-conjugated antibody was applied for 30 min. Apoptotic cells were detected after incubation in the 3, 3-diaminobenzidine (DAB) chromogen (DAKO, Carpinteria, CA, USA) for approximately 6 min, and the slides were counterstained with methyl green (Sigma, St. Louis, MO, USA). Ten high-power fields were selected for each slice, and the number of positive cells was counted for every 1, 000 cells. The apoptosis rate (%) = number of positive apoptosis cell/1, 000 × 100%.
-
Tumor tissue was pestled in liquid nitrogen and lysed in TRIzol reagent. Total RNA was prepared per the manufacturer's instructions (Takara, Japan). To remove genomic DNA, 5 μg of total RNA was treated with 5 U RNase-free DNaseI for 30 min at 37 ℃. Following DNase treatment, the RNA was incubated at 65 ℃ for 10 min. RNA integrity was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Quantitative PCR was used to generate RNA with two sharp ribosomal 18S and 28S bands. First-strand cDNA synthesis was carried out on 2 μg of the total RNA from each sample using the PrimeScript®RT Master Mix (Takara, Japan) first-strand synthesis kit for RT-PCR per the manufacturer's instructions. Experimental wells containing 25 μL of SYBR Green PCR Master Mix (Takara, Japan) were run using north tube plates. Quantitative PCR was conducted on the iCycler per the SYBR Green method. Forward and reverse primers were designed using the tools available on the MIT Whitehead Institute website. The following primer sequences were used:
Caspase-3(F) AACCTCAGGGAAACATTCAG, caspase- 3(R) GGCTCAGAAGCACACAAAC, Bax(F) AGGATCG- AGCAGGGCGGCGAATG, Bax(R) GACACTCGCTCAGCTT- CTTGG, Bcl-2(F) ATTTCCTGCATCTCATGCCAAGGG, Bcl-2(R) TGTGCTTTGCATTCTTGGACGAGG, CyclinD1 (F) CATGGAACACCAGCTCCTGTG, CyclinD1(R) GTTCAT- GGCCAGCGGGAAGAC, β-Actin(F) AGCGAGCATCCC- CCAAAGTT and β-Actin(R) GGGCACGAAGGCTCATC- ATT. Quantitative PCR amplification for caspases included pre-incubation at 94 ℃ for 6 min, followed by 38 cycles at 95 ℃ for 5 s, 57 ℃ for 45 s, and 72 ℃ for 30 s. The relative expression of mRNA was calculated using the 2-ΔΔCt method.
-
The protein concentration of the tissue extracts was determined by bicinchoninic acid (BCA) protein assay. An aliquot of the tumor tissue extract lysed using cell lysis buffer [0.5 mol/L Tris-HCl, pH7.5, 0.15 mol/L NaCl; 0.001 mol/L EDTA; 2 μg/mL Aprotinin; 0.001 mol/L phenylmethanesulfonyl fluoride (PMSF); and protease inhibitor cocktail (purchased from Bi Yuntian)] was subjected to SDS-PAGE on 10%-12% Tris-glycine gel. The separated proteins were transferred onto PVDF membrane (Millipore) and probed with Bcl-2: Bax, cyclinD1: and caspase-3 antibodies (Abcam Inc., Cambridge, MA, USA) using peroxidase-conjugated appropriate secondary antibodies (Abcam Inc., Cambridge, MA, USA). Signals were visualized using the Chemiluminescence HRP detection system (Millipore) on Versa Doc (Bio-Rad). Membranes were stripped and re-probed with a β-actin antibody (Abcam Inc., Cambridge, MA, USA).
-
Statistical analysis was conducted using SPSS software version 17.0 (SPSS, Chicago, IL, USA). Analysis of variance (ANOVA) was conducted to test for differences between means.
Extract Preparation
Determination of the Protein Content
Determination of Total Phenol Content
Determination of Total Flavonoid Content
Measurement of β-glucan
Determination of Total Sugar Content
Cell Culture
Establishment of the Transplantation Tumor Model and Grouping
Assessment of Apoptosis by TUNEL
Real-time Fluorescence Quantitative PCR Analysis
Western Blotting
Statistical Analysis
-
The main composition of raw barley was as follows: moisture, 10.70% ± 0.91%; crude protein (N×5.83), 11.21% ± 0.32% of dry matter (d.m.); fat, 1.66% ± 0.03% of d.m.; and ash, 1.31% ± 0.09% of d.m. In this experiment, the yields of aqueous extract of fermented barley with Lactobacillus plantarum dy-1 (LFBE) and aqueous extract of raw barley (RBE) were 16.48% ± 0.35% and 7.54% ± 0.28% (Table 2), respectively.
Composition RBE LFBE Extraction ratio (%) 7.54 ± 0.28 16.48 ± 0.35 Protein (%, w/w) 16.1 ± 0.31 25.1 ± 0.25 Total phenols (g gallic acid/100 g) 1.10 ± 0.15 1.26 ± 0.12 Total sugar (%, w/w) 65.61 ± 0.36 34.35 ± 0.32 β-glucan (%, w/w) 5.05 ± 0.14 12.03 ± 0.22 Total flavonoid (mg/g) 0.34 ± 0.01 0.52 ± 0.01 Note. The results are expressed as the mean ± SD, n = 3. Table 2. Main Composition (% Dry-Weight) of LFBE and RBE
Table 2 presents the contents of the main components of RBE and LFBE. As shown, RBE contained 16.10% ± 0.31% crude protein, 65.61% ± 0.36% total sugar, 1.10% ± 0.15% (expressed as GAE, mg gallic acid/g dried extract) total phenols, 0.34 mg/g ± 0.01 mg/g total flavonoid, and 4.83% ± 0.43% β-glucan. Compared with unfermented LFBE, after 24 h of incubation with Lactobacillus plantarum dy-1 at 30 ℃, the protein content of LFBE increased significantly to 25.1% ± 0.25% and the sugar content decreased significantly to 34.35% ± 0.32%. The total phenol and total flavonoid content of LFBE increased to 1.26% ± 0.12% and 0.52 mg/g ± 0.01 mg/g, respectively. In addition, the concentration of β-glucan significantly increased to 12.03% ± 0.22% (Table 2).
-
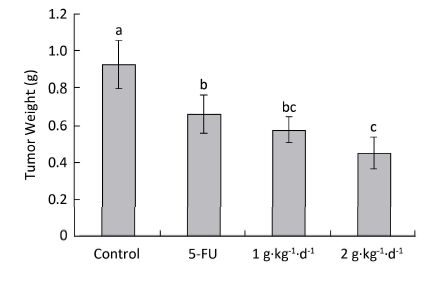
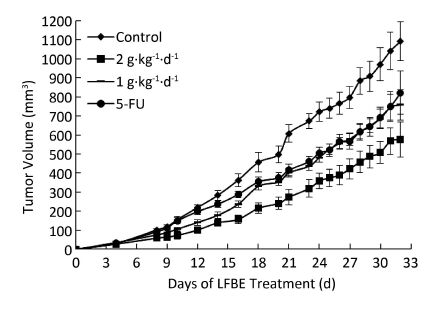
The inhibitory effects of LFBE and 5-FU on the growth of tumors in nude mice were observed and the inhibitory rates were calculated. Figure 1 shows the tumor volume measured at intervals of 3-4 days. After 30 days, the tumor volumes of the control, 5-FU, high-dose LFBE, and low-dose LFBE groups were (1091.97 ± 102.34), (821.70 ± 113.68), (576.60 ± 93.27) and (758.85 ± 78.97) mm3, respectively. Compared with the control group, the tumors of the LFBE and 5-FU groups shrank significantly (P < 0.05), especially in the high-dose LFBE group. The results showed that LFBE had inhibitory effects on the transplantation tumor of human HT-29 cells in BALB/c nude mice, consistent with previous research [20]. At the end of the experiment, all tumors were isolated from the nude mice and weighed. The tumor weights in the nude mice from the control, 5-FU, high-dose LFBE, and low-dose LFBE groups were (0.93 ± 0.13), (0.66 ± 0.10), (0.45 ± 0.08), and (0.57 ± 0.08) g, respectively, as shown in Figure 2. The final tumor weights of the LFBE and 5-FU groups and the final tumor volume decreased compared with those of the control group. Treatment with LFBE and 5-FU significantly inhibited tumor growth. The inhibitory effects of the 5-FU, high-dose LFBE, and low-dose LFBE groups were 29.03%, 51.61%, and 38.71%, respectively. LFBE exhibited a stronger ability to inhibit tumor growth than 5-FU. However, LFBE inhibited HT-29 cell growth in a dose-dependent manner.
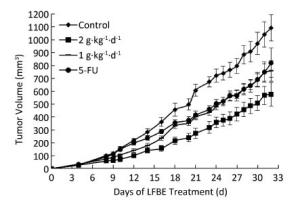
Figure 1. Growth curve of tumor volume in nude mice transplantation tumors during treatment with LFBE (high-dose 2 g·kg-1·d-1; low-dose 1 g·kg-1·d-1) and 5-FU (25 mg·kg-1·d-1). n = 10. Values represent the mean ± SD.
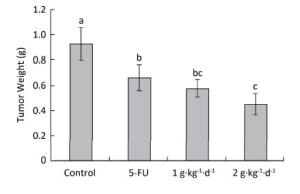
Figure 2. Tumor weights in nude mice transplantation tumors during treatment with LFBE (high-dose 2 g·kg-1·d-1, low-dose 1 g·kg-1·d-1) and 5-FU (25 mg·kg-1·d-1). On day 30: the mice were sacrificed and the tumor weights were measured. n = 10. Values represent the mean ± SD. Different letters indicate a significant difference (P < 0.05).
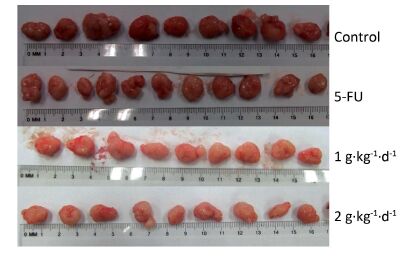
Figure 3 shows the tumors isolated from the nude mice on day 30. All of these tumors had spherical or ellipsoid shapes and smooth surfaces with dendritic protrusions. The surrounding tissues had very clear boundaries and easily or mildly adhered to the skin and subcutaneous tissue. They exhibited a complete coating, a fleshy red surface, a flexible, fish-shaped profile texture, and occasional white bean dregs. The figure also reveals that LFBE significantly inhibited tumor growth and proliferation effects.
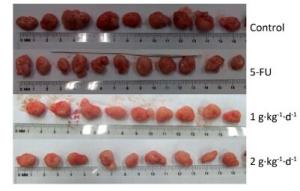
Figure 3. Picture of tumors in nude mice transplantation tumors during treatment with LFBE (high-dose 2 g·kg-1·d-1; low-dose 1 g·kg-1·d-1) and 5-FU (25 mg·kg-1·d-1). n = 10.
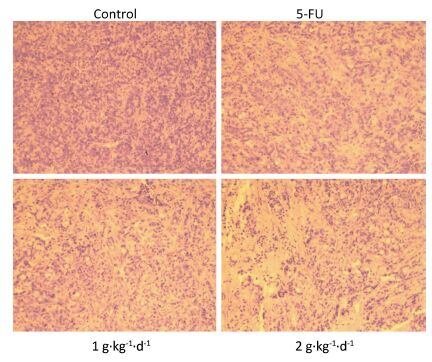
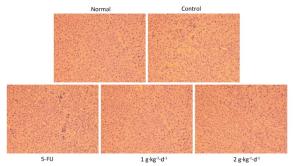
Moreover, from Figure 4: it is clearly noted that significant changes in tumor tissue morphology could be observed when comparing the control group with the 5-FU, LFBE (1 g·kg-1·d-1), and LFBE (2 g·kg-1·d-1) groups.
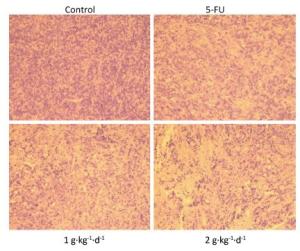
Figure 4. Effects of LFBE (high-dose 2 g·kg-1·d-1; low-dose 1 g·kg-1·d-1) and 5-FU (25 mg·kg-1·d-1) on the morphology of tumor tissues in HT-29 xenograft (× 200).
The results indicated that in HT-29 cells, large nuclear atypia were densely distributed in tumor tissues of the controls. However, HT-29 cells were broken, loosened, and apoptotic in tumor tissues treated with LFBE and 5-FU, consistent with previous research [20].
-
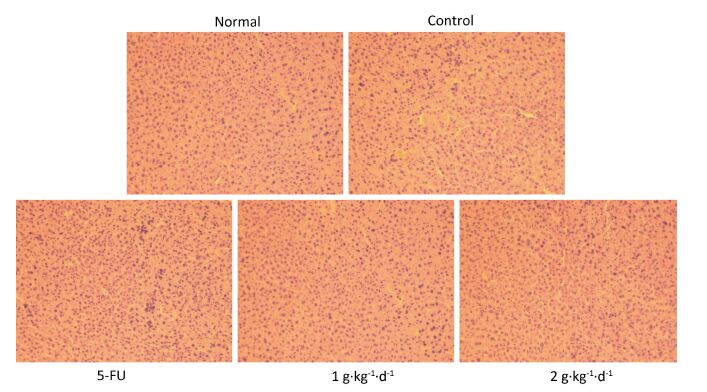
From Figure 5: it is clearly noted that there were no significant differences in the morphology of liver tissues of all groups, consistent with previous research [20]. Moreover, the organ index was determined (organ weight/nude mouse weight), and no significant changes in heart, lung, or kidney could be observed when comparing the control with 5-FU, LFBE (1 g·kg-1·d-1) and LFBE (2 g·kg-1·d-1). These results indicated that LFBE was not toxic to the liver, heart, lung, or kidney.
-
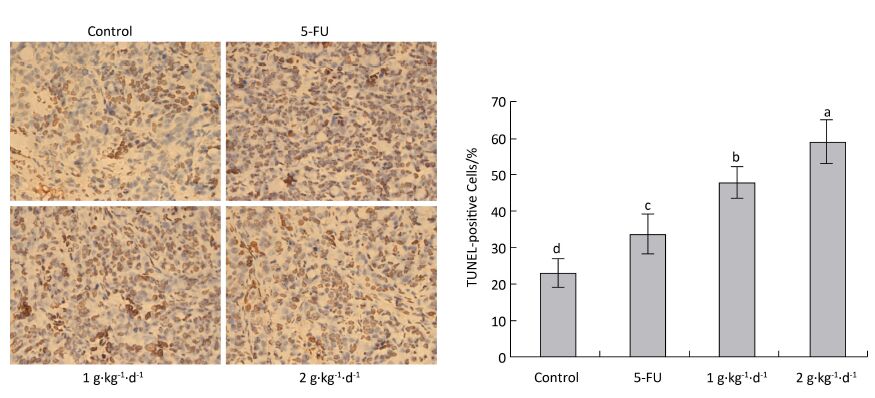
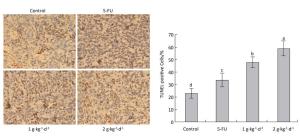
The cell apoptosis rate was determined via TUNEL staining to reveal how LFBE and 5-FU inhibited tumor growth. The nuclei of normal tumor cells were colored dark blue by hematoxylin restaining. According to Figure 6: the following apoptotic features of apoptotic-positive cells were found by TUNEL staining: a shrinking cell body, nuclear pyrosis, chromatin condensation, and tan or brown granules. The cell apoptosis rates of the control, 5-FU, high-dose LFBE, and low-dose LFBE groups were 23.09% ± 4.10%, 33.86% ± 5.68%, 59.37% ± 5.76%, and 48.15% ± 5.76%, respectively (Figure 6). The cell apoptosis rate of the LFBE group was significantly higher than those of the control and 5-FU groups P < 0.05. LFBE induced subcutaneous transplantation tumor apoptosis.
-
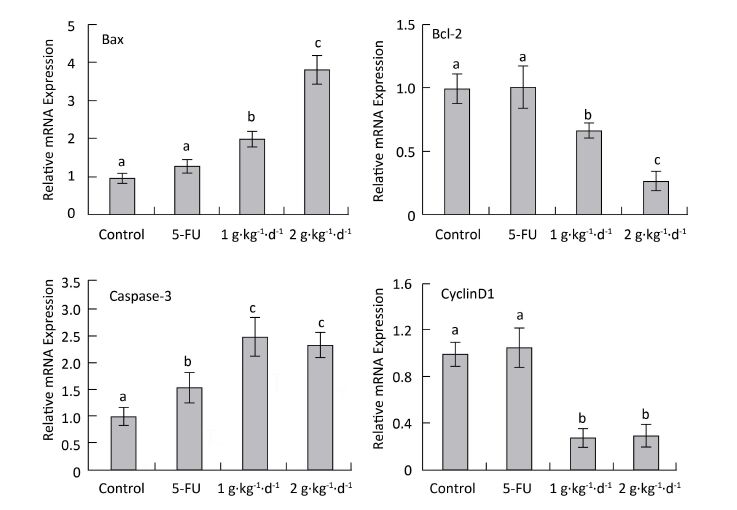
To explore the mechanism of LFBE proapoptotic and antiproliferative activities, fluorescence quantitative PCR was performed to examine the mRNA expression of Bax, Bcl-2: cyclinD1: and caspase-3 in the tumor tissues of nude mice in all groups. RNA purity was quantified by spectrophotometer with an OD260/280 ratio of 1.8-2.0 for all specimens. The RNA integrity was determined by 1% agarose gel electrophoresis, yielding two sharp ribosomal 18S and 28S bands. Figure 7 shows the results.
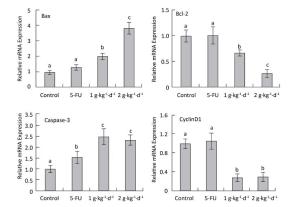
Figure 7. CyclinD1: Bax, Bcl-2: and caspase-3 mRNA expression analysis in nude mice transplantation tumors during treatment with LFBE (high-dose 2 g·kg-1·d-1; low-dose 1 g·kg-1·d-1) and 5-FU (25 mg·kg-1·d-1) n = 6. Values represent the mean ± SD. Different letters indicate a significant difference (P < 0.05).
Compared with the control group, the mRNA-relative contents of cyclinD1 were 1.062: 0.305: and 0.287 in the tumor tissue of the 5-FU, high-dose LFBE, and low-dose LFBE groups, respectively. Furthermore, the relative mRNA contents of Bax were 1.316: 3.824: and 2.007: respectively; the relative mRNA contents of Bcl-2 were 1.005: 0.270: and 0.666: respectively; and the relative mRNA contents of caspase-3 were 1.565: 2.321: and 2.497: respectively (Figure 7). The mRNA expression levels of Bax and caspase-3 in the LFBE group increased significantly compared with those in the control and 5-FU groups after 30 days (P < 0.05).
Moreover, the mRNA expression of Bcl-2 and cyclinD1 decreased significantly (P < 0.05) compared with the control group. However, under 5-FU treatment, the mRNA expression of Bcl-2: Bax, and CyclinD1 showed no significant difference (P > 0.05) and that of caspase-3 was significantly upregulated (P < 0.05). LFBE may have promoted tumor apoptosis by upregulating the mRNA expression of Bax and caspase-3 and downregulating the mRNA expression of Bcl-2 and cyclinD1.
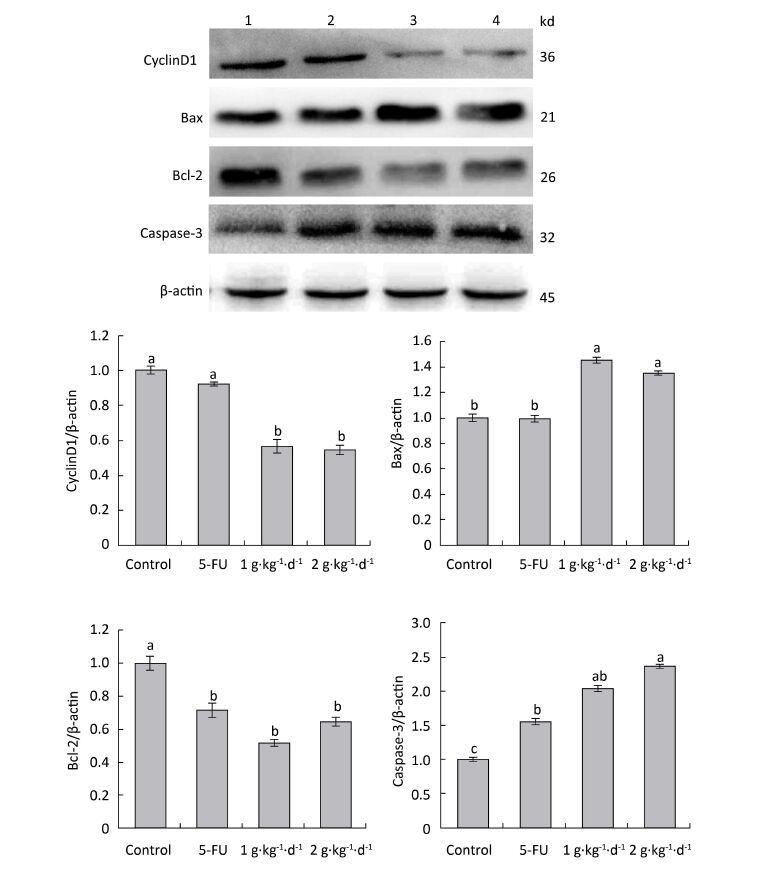
To further explore the mechanisms of proapoptotic and antiproliferative activities in FFBE, western blotting was performed to examine the protein expression of cyclinD1: Bax, Bcl-2: and caspase-3 in tumor tissues of nude mice.
As shown in Figure 8: under LFBE treatment, the protein expressions levels of Bcl-2 and cyclinD1 were significantly downregulated compared with the control group (P < 0.05) and the protein expressions levels of Bax and caspase-3 were significantly upregulated (P < 0.05). However, under 5-FU treatment, the protein expressions levels of Bax and cyclinD1 showed no significant difference (P > 0.05) and that of caspase-3 was significantly upregulated (P < 0.05). However, the protein expression of Bcl-2 showed a significant difference, in contrast to the mRNA result. Different functional components of LFBE may play important roles in promoting apoptosis pathways, indicating the differences between LFBE and 5-FU. In this study, 5-FU mainly activated the expression of caspase-3 to achieve apoptosis. Studies have reported that caspase-3 plays an important role in colorectal cancer. Nevertheless, 5-FU is used as an intermediate to activate caspase-3 in the apoptosis pathway [23]. In renal cell carcinoma, 5-FU can activate caspase-3: 6: 8: and 9 simultaneously [24]. The results of mRNA and protein expression of cyclinD1 and Bax were somewhat disparate; however, the overall trend was the same.
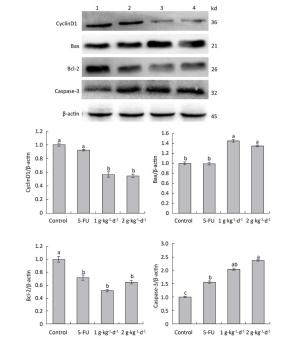
Figure 8. Expression of cyclinD1: Bax, Bcl-2: and caspase-3 proteins in nude mice transplantation tumors during treatment with LFBE (high-dose 2 g·kg-1·d-1; low-dose 1 g·kg-1·d-1) and 5-FU (25 mg·kg-1·d-1) (1: control group; 2: 5-FU group; 3: Low-dose LFBE, 1 g·kg-1·d-1; 4: High-dose LFBE, 2 g·kg-1·d-1) by Western blot n = 6. Values represent the mean ± SD. Different letters indicate a significant difference (P < 0.05).
Finally, the Western blotting results (Figure 8) were consistent with the quantitative PCR results (Figure 7). LFBE inhibited proliferation and induced apoptosis, mainly by downregulating the cyclinD1 and Bcl-2 gene expression levels and upregulating Bax and caspase-3 gene expression.
Yield and Chemical Compositions of the Extracts
Tumor Cell Proliferation in Nude Mice
Histological Examination
Tumor Apoptosis in Nude Mice
Apoptotic Effect of LFBE via Caspase-3 and Bax Activation and Down regulation of Bcl-2 and Cyclin D1 Expression in HT-29 Colon Cancer Cells
-
Barley is a cereal that is often associated with beneficial health effects. Barley fermentation with lactic acid bacteria can lead to a significant increase of free phenolic acidsand β-glucan. Barley fermented with S. cerevisiae has a surprising immunostimulatory and metastasis inhibiting effect when administered as a nutritional supplement to cancer patients [18-19]. Some studies reported that plant phenolic, including phenolic acids and flavonoids, displayed significant anticancer activity [25-26]. β-glucan, as a biological response modifier, has been extensively investigated for both its antitumor activities [27-28].
Based on previous research [20], this study investigated the anticancer effect and the biochemical mechanism of action of LFBE in a subcutaneous transplantation tumor model of human HT-29 cells in nude mice with anticarcinogenic properties. LFBE can obviously inhibit HT-29 cell growth and apoptosis in nude mice, without having toxic side effects.
The main function of cyclinD1 is to promote cell proliferation. CyclinD1 is the key protein regulating the G1 phase of the cell cycle. Many studies have shown that cyclinD1 is excessively expressed in a wide variety of tumor cells and leads to out-of-control and vicious cell proliferation [29]. Gómez-Alonso found that the anti-proliferative colon cancer effects induced by flavonols were, in most cases, preceded by a strong and significant reduction of cyclooxygenase-2 (COX-2) and cyclinD1 expression [30].
Apoptosis is a programmed cell death process that includes cell shrinkage, membrane foaming, chromatin condensation, nuclear division, and ultimately, the formation of apoptotic bodies [31]. Many intracellular molecules involve apoptosis, which plays an important role in maintaining the balance between tissues and organs. Apoptosis can be induced by either the body or external nutrients [32]. Studies have shown that inducing apoptosis has a dietary nutrition effect and does not affect normal cells [23]. The molecular mechanisms of apoptosis have been widely examined. Studies have found a variety of genes involved in apoptosis, such as the ced, Bcl-2: p53: STAT3: and caspase gene families.
It is well known that the Bcl-2 family plays a crucial role in the control of apoptosis [2]. Bcl-2 is an apoptosis inhibitor, and high Bcl-2levels are required to maintain intracellular gene transfer and other changes required blocking apoptosis. Bax is an apoptotic protein [33]. Bax and Bcl-2 can interact and form a complex regulatory network in the regulation of apoptosis. Therefore, with a low level of Bax, induction of apoptosis may require upregulation of Bax expression.
In addition, apoptosis is a well-controlled process involving a programmed set of cellular events partially mediated by caspases [24]. Caspase-3 is a key factor in apoptosis, and its activation is indicative of apoptosis entering an irreversible stage. Caspase family proteases are downstream targets of Bax and Bcl-2 in the mitochondrial apoptosis signaling pathway. The literature indicates that changes in the Bax/Bcl-2 ratio could regulate the activity of caspase proteins [34]. Caspase-3 inactivates essential cellular substrates like DNA-repairing enzyme poly (ADP-ribose) polymerase-1: which is a sterol regulatory element binding protein, by changing the structure of the substrate specificity or affecting a particular signal molecule to induce apoptosis [35]. Polyphenol-rich products have been reported to have potential chemopreventive and chemotherapeutic activities in cancer cells by targeting several apoptosis-regulating pathways [36-37]. We have demonstrated that LFBE inhibited HT-29 cell proliferation by inducing cell apoptosis.
In conclusion, this study clearly shows that LFBE is a regulator of the transplantation tumor of human HT-29 cells in BALB/c nude mice and inhibits cell proliferation and apoptosis induction. Its mechanism may help to increase the gene expression levels of Bax and caspase-3 and decrease those of Bcl-2 and cyclinD1. Based on the data, the development of fermented barley extract with Lactobacillus plantarum dy-1 as the main component of anticarcinogenic function food laid the experimental foundation for this study. Future studies should examine the molecular mechanism of its anticarcinogenic effect in more detail.
-
The authors declare no conflict of interest.
-
YAO Fang designed the study, collected test data, interpreted the results, and wrote the manuscript. ZHANG Jia Yan assisted in animal experiments and collected test data. XIAO Xiang interpreted the results, and revised the manuscript. DONG Ying designed the study, interpreted the results, and wrote the manuscript. ZHOU Xing Hua collected test data and drafted the manuscript. All authors agreed to the final content.







 Quick Links
Quick Links
 DownLoad:
DownLoad: