-
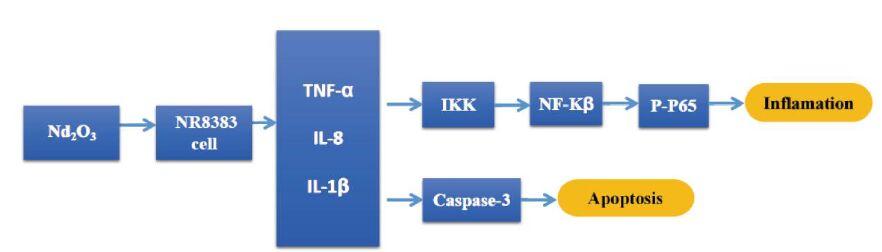
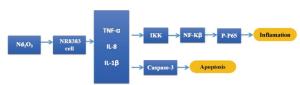
We investigated whether Nd2O3 treatment results in cytotoxicity and other underlying effects in rat NR8383 alveolar macrophages. Cell viability assessed by the MTT assay revealed that Nd2O3 was toxic in a dose-dependent manner, but not in a time-dependent manner. An ELISA analysis indicated that exposure to Nd2O3 caused cell damage and enhanced synthesis and release of inflammatory chemokines. A Western blot analysis showed that protein expression levels of caspase-3, nuclear factor-κB (NF-κB) and its inhibitor IκB increased significantly in response to Nd2O3 treatment. Both NF-κB and caspase-3 signaling were activated, suggesting that both pathways are involved in Nd2O3 cytotoxicity.
Rare earth elements have a wide range of applications in agriculture and animal husbandry, fisheries, industry, environmental protection, and medicine. Products containing rare earth compounds ingested via the food web inevitably find their way into the environment and can affect human health. Therefore, more attention should be paid to the effects of rare earth compounds on the environment and ecology.
Neodymium is widely used in industrial materials, and most of these materials are produced in China. Although neodymium is a rare earth metal and occurs widely in nature, its toxicity has not been thoroughly investigated. Therefore, it is important to determine the effects induced by neodymium oxide (Nd2O3) treatment and its possible underlying mechanism. Cerium or lanthanum dust causes marked eye and mucous membrane irritation and moderate skin irritation. Inhaling this dust can cause a lung embolism and accumulated exposure causes liver damage [1]. This dust enters the body mainly via inhalation and adheres to the surface of the alveolar cavity, where it is engulfed by alveolar macrophages (AMs). AMs are a natural barrier that prevents invasion by exogenous microbes and are also important effectors in the body’s natural immune system. AMs ingest dust and initiate the inflammatory response by releasing inflammatory cytokines, such as tumor necrosis factor α (TNF-α), interleukin 8 (IL-8), and IL-1β [2].
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a protein complex that controls DNA transcription, cytokine production, and cell survival [3] and plays a key role regulating the immune response of pro-inflammatory stimuli, such as TNF-α, IL-8, and IL-1β. NF-κB is usually in an inactive state in the cytoplasm by associating with an endogenous inhibitor protein of the IκB (inhibitor of NF-κB) family [4]. When NF-κB is activated, IκB is phosphorylated and disassociates with NF-κB, allowing NF-κB to enter the nucleus and initiate transcription. Dysregulation of NF-κB has been linked to cancers, inflammatory and autoimmune diseases, viral infection, and improper immune development.
The aim of the present study was to investigate the effect of Nd2O3 on rat NR8383 AMs by measuring cell viability and the expression of cytokines and transcription factors following treatment with different concentrations of Nd2O3. These findings may provide further insight into the cytotoxicity of rare earth Nd2O3 and the roles of Nd2O3 in respiration, which could benefit the Nd2O3 industrial standard and safety standard formulation. Our results may also uncover the potential mechanism of Nd2O3-induced acute lung injury in vitro.
The 3-(4, 5-dimethyl-2-yl)-2, 5-diphenyltetrazolium bromide method (MTT) was used to determine cell viability and cytotoxicity of Nd2O3. The concentrations of Nd2O3 used in the assay were determined in a preliminary experiment. An enzyme-linked immunosorbent assay (ELISA) was used to detect the levels of cytokines in the cell culture supernatant. A Western blot analysis was performed to assay p-IKKβ, p-p65, and caspase-3 expression with the indicated antibodies.
Results are presented as mean ± standard deviation. Statistical tests were performed using SPSS 17.0 software (IBM Corp., Armonk, NY, USA). Unpaired Student’s t-tests were used to compare the means of two groups. A P-value < 0.05 was considered significant.
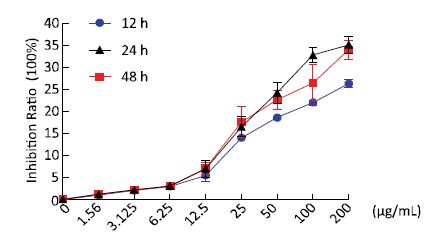
To investigate the effects of Nd2O3 exposure on rat AMs (NR8383), we measured the cell growth inhibition ratio using the MTT assay after 12, 24, and 48 h treatment with Nd2O3 at final concentrations of 0, 1.56, 3.125, 6.25, 12.5, 25, 50, 100, and 200 μg/mL. The cell growth inhibition ratio increased significantly in a dose-dependent manner at 12, 24, and 48 h, but not in a time-dependent manner (Figure 1). The cell inhibition ratios did not increase in the 1.56, 3.125, and 6.25 μg/mL groups (Figure 1), suggesting that these low concentrations were not toxic to NR8383 cells. However, the cell inhibition ratio did not change at 100 μg/mL compared with that of the 200 μg/mL group after a 48 h incubation with Nd2O3, indicating that 100 μg/mL may be the highest toxic concentration and that concentrations > 100 μg/mL maintained the cell growth inhibition ratio at the same level. Taken together, these MTT results demonstrate that Nd2O3 decreased cell viability in a dose-dependent manner. An Nd2O3 treatment duration of 24 h and a series of concentrations (0, 1.56, 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL) were chosen for subsequent experiments.
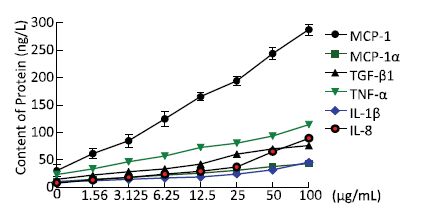
To examine the inflammatory changes in NR8383 cells exposed to Nd2O3, we performed an ELISA to evaluate cytokine levels. The levels of TNF-α, IL-8, and IL-1β secreted into the cell supernatant increased significantly (P < 0.05) compared with those in the control group (0 μg/mL) (Figure 2). As our data suggest that Nd2O3 induced secretion of pro-inflammatory cytokines by NR8383 cells, we hypothesized that inflammatory cytokines would be activated and secreted. Consistent with our hypothesis, the levels of inflammatory cytokines, such as monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, and transforming growth factor-β1, were higher in the group treated with Nd2O3 than those in the control group (0 μg/mL) (Figure 2). All cytokines increased in a dose-dependent manner, and cell viability decreased in a dose-dependent manner, implicating cytotoxicity in the inflammatory response.
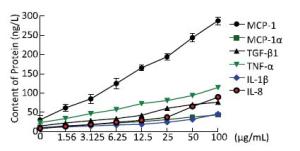
Figure 2. Inflammatory cytokines collected from the supernatant of NR8383 cells treated in vitro with neodymium oxide for 24 h at final concentrations of 0, 1.56, 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL.
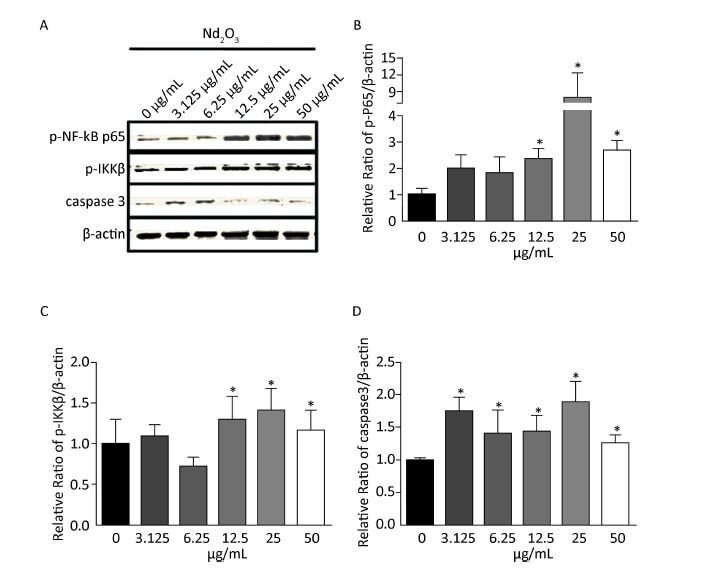
As the levels of the pro-inflammatory cytokines, such as TNFα, IL-8, and IL-1β, increased after Nd2O3 treatment, and TNFα, IL-8, and IL-1β are targets of the NF-κB signaling pathway, we determined whether the NF-κB signaling pathway was activated. Total protein was extracted from NR8383 cells harvested 24 h post Nd2O3 treatment. Following the immunoblot analysis, the lysates were used to determine the expression of phosphorylated p65 (p-p65) which is a marker of NF-κB activation, and p-IKKβ, which is a marker for inactivation of the repressor. The p-p65 and p-IKKβ levels increased gradually in the 3.125 and 6.25 μg/mL groups (P > 0.05). p-p65 and p-IKKβ expression increased significantly (P < 0.05) as the Nd2O3 dose was increased. p-p65 expression was higher in the 25 μg/mL group than that in any other group, and the same result was observed for the expression of p-IKKβ (Figure 3A-C). Changes in protein levels were quantified and normalized to β-actin expression to verify protein induction (Figure 3B-C).
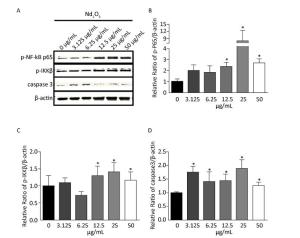
Figure 3. The nuclear factor kappa beta (NF-κB) signaling and caspase-3 pathways changed following Nd2O3 exposure. (A) NR8383 cells were incubated with neodymium oxide for 24 h at final concentrations of 0, 3.125, 6.25, 12.5, 25, and 50 μg/mL, collected, and lysed. The cell lysates were immunoblotted with anti-p-p65, anti-p-IKKβ, anti-caspase-3, and anti-β-actin. (B) The intensity of p-p65 was quantitatively normalized to that of β-actin. (C) The intensity of p-IKKβ was quantitatively normalized to that of β-actin. (D) The intensity of caspase-3 was quantitatively normalized to that of β-actin. Results are mean ± standard deviation (n = 3). P < 0.05 was considered significant.
Previous studies have indicated that lead exposure-induced cytotoxicity in the hippocampus is mediated through increased caspase-3 activity [5]. Apoptosis is the main pathway causing the decrease in cell number and cytotoxicity, thus, we further analyzed the level of caspase-3 and the apoptosis index by immunoblot. Expression of caspase-3 was shifted towards the normal value by Nd2O3 compared with that in the control group. Interestingly, the level of caspase-3 was slightly attenuated by 50 μg/mL Nd2O3 (Figure 3A and D). The changes in protein levels were quantified and normalized to that of β-actin expression to verify protein induction.
The interest in using Nd2O3 in industry, consumer goods, and biomedical applications has triggered a significant effort to understand the potential toxicity of this compound. Over the last decade, some other rare earth compounds, such as cerium oxide (CeO2) and titanium dioxide, have been shown to reduce cell viability and cause apoptosis at high concentrations [6].
In the present study, we initially examined the toxic effects of Nd2O3 in rat AMs by the MTT assay. A marginal change in cell proliferation was observed in the 1.56, 3.125, and 6.25 μg/mL groups. However, cell viability decreased in a dose-dependent manner in response to concentrations ≥ 12.5 μg/mL. These findings are consistent with studies on other rare earth materials.
Some studies have reported that lung macrophages can ingest nanoparticles and induce an inflammatory response. NF-κB is an important participant in inflammatory networks. Our findings were similar to those published previously, as NR8383 cells ingested Nd2O3 and activated the NF-κB pathway. However, some studies have shown that the cytotoxicity induced by CeO2 occursvia activation of the mitogen activated protein kinase (MAPK) and Janus kinase-signal transducer of activators of transcription (JAK-STAT) signaling pathways [7]. Further investigations are required to determine whether there is crosstalk among the NF-κB, MAPK, or JAK-STAT pathways.
Previous studies have shown that CeO2 and Y2O3 nanoparticles decrease cell viability and activate apoptosis by upregulating caspase-3 expression [8]. In addition, exposure to lanthanum chloride promotes activation of caspase-3 [9]. Caspase-3 executes the terminal steps of apoptosis and regulates upstream induction of cell destruction. Our studies confirmed these results, as upregulation of caspase-3 was shifted towards normal by Nd2O3 and the level of caspase-3 was slightly attenuated by 50 μg/mL Nd2O3.
This decrease in caspase-3 suggests that other mechanisms contribute to Nd2O3 cytotoxicity. Besides apoptosis, some evidence shows that nanoparticles cause injury by inducing autophagic cell death through the AKT-TCS2-mTOR pathway [10]. In addition, CeO2 nanoparticles are activators of autophagy and promote clearance of autophagic cargo. In this study, the cytotoxicity induced by Nd2O3 may be associated with the activation of NF-κB and caspase-3 signaling (Figure S1 in the website of BES, www.besjournal.com). However, more studies are required to better understand the cell mechanisms in response to Nd2O3.
Neodymium can be used in glass, magnets, and medical devices, such as magnetic braces, as well as in bone repair. Neodymium also acts as an anticoagulant, particularly when given intravenously. Our findings will provide a basis for the Nd2O3 industrial and safety standard formulations. We also suggest that a safe and critical concentration of < 6.25 μg/mL can be used in animal experiments or a clinical trial based on our Nd2O3 cytotoxicity experimental results.
Neodymium Oxide Induces Cytotoxicity and Activates NF-κB and Caspase-3 in NR8383 Cells
doi: 10.3967/bes2017.010
This project was supported by the National Natural Science Foundation of China No. 81660532, 81260426
and the Scientific Research Foundation of Baotou Medical College BYJJ-YF201613
the Doctoral Scientific Research Foundation of Baotou Medical College BSJJ201621
the Science and Technology Plan Project in Inner Mongolia No. 201502080
the Natural Science Foundation of Inner Mongolia No. 2016MS(LH) 0822
- Received Date: 2016-07-22
- Accepted Date: 2016-12-29
| Citation: | HUANG Li Hua, YANG Huan, SU Xin, GAO Yan Rong, XUE Hai Nan, WANG Su Hua. Neodymium Oxide Induces Cytotoxicity and Activates NF-κB and Caspase-3 in NR8383 Cells[J]. Biomedical and Environmental Sciences, 2017, 30(1): 75-78. doi: 10.3967/bes2017.010 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: