-
Prolactinoma is the most common kind of intracranial tumor that accounts for about 40% of clinical adenomas[1]. In vitro rat experiments also show that a large number of estrogen receptor α (ERα)-positive cells exist in the pituitary, suggesting that ERα plays a major role in cell proliferation and hormone secretion. Estrogen plays a critical role in the development of the anterior pituitary and homeostatic regulation, particularly prolactin (PRL).
Leukemia related protein 16 (LRP16; GenBank Number: AF202922), which was cloned from human leukemic cells in 1999, is comprised of an open reading frame encoding 325 amino acids; however, its relevant functional significance remains ambiguous. Some studies show that expression of the LRP16 protein is positively correlated with expression of ERα[2-3]. In this study, Sprague-Dawley (SD) rats became prolactinoma models, and silenced LRP16 expression impaired the secretory function of GH3 cells, which may provide a novel medical therapy in clinical trials.
Fifty male SD rats (age, 30-day-old; mean weight, 240.72 ± 6.11 g) were purchased from the Laboratory Animal Center of No. 302 Hospital of PLA (SPF level). The animals were randomly divided into five groups of 10 rats each: 1) sham group: subcutaneous embedding of silicone rubber without 17-β estradiol (E2; Sigma-Aldrich, St. Louis, MO, USA); 2) E2 (10 mg/kg) treatment; 3) E2 with Faslodex (ICI182780, College of Military Medicine Science of China, Beijing, China) low-dose (10 mg/kg); 4) E2 with Faslodex medium-dose (20 mg/kg); and 5) E2 with Faslodex high-dose (40 mg/kg).
A 0.125 cm3 portion of pituitary gland tissue was harvested from the animals and subjected to protein extraction; the pieces were lysed with 400 μL detergent lysis buffer and homogenized. The homogenate was centrifuged (12, 000 rpm, 20 min at 4 ℃), and the supernatant was collected and centrifuged again. An equivalent of 30 µg sample was resolved by 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to PVDF membranes. The membranes were probed with primary antibodies: 1:200 mouse anti-PRL monoclonal antibody, 1:500 rabbit anti-ERα monoclonal antibody, 1:400 rabbit anti-LRP16 polyclonal antibody and 1:500 rabbit anti-GADPH polyclonal antibody, respectively. The secondary antibodies were as follows: 1:1, 000 goat anti-mouse anti-PRL, 1:5, 000 goat anti-rabbit anti-ERα and 1:1, 000 goat anti-rabbit anti-LRP16, followed by the development of immunoreactive bands using a diaminobenzidine (DAB) kit (Invitrogen, Carlsbad, CA, USA). The luminescence signal was captured and analyzed using Image J.
Immunohistochemistry (IHC) staining kit: Histostain-Plus Mouse Primary (Invitrogen) and Histostain-Plus Rabbit Primary (Invitrogen) antibodies. Fixed pituitary gland tissue stored in liquid nitrogen was flushed with PBS (3 min × 3) and then incubated in 3% hydrogen peroxide in deionized water. The samples were flushed again with PBS. The excess reagent was removed and the samples were incubated with anti-ERα (1:50), anti-LRP16 (1:100), and anti-PRL (1:100) in a humid chamber at 4 ℃ overnight. Subsequently, the samples were incubated, sequentially at room temperature for 15 min, followed by washes in PBS. The sections were visualized by DAB and counterstained with haematoxylin. The stained cells and tissues were viewed under an Olympus FV1000 confocal laser-scanning microscope (Olympus, Tokyo, Japan) at 488 nm (green) and 594 nm (red) (magnification 400×). Axiotis scoring criteria were utilized: including the positive cell percentages of the total number of cells and cell staining intensity. '-' (negative) (no or < 5% positive cells), '+' (5%-25% positive cells), '++' (26%-50% positive cells), and '+++' (> 50% positive cells); staining intensity was scored: 0, negative; 1, weak; 2, moderate; and 3, strong.
GH3 cells were cultured in HyClone® Improved RPMI-1640 Medium. The cells were passaged three times, washed with PBS, and plated in two 6-well plates. (a) When density reached 80%, the cells were divided into three groups: untreated (blank) group, cDNA3.1 group and the cDNA3.1+LRP16 group; (b) the siRNAs L668, L374, and the negative control (NC) were solubilized in 250 µL diethyl pyrocarbonate. When density reached 70%, the cells were divided into four groups: untreated (blank) group, NC group, L374 group, and L668 group, while siRNA was categorized into 30, 60, and 90 nmol/L groups. At 48 h post-transfection, the cells were centrifuged at maximum speed to harvest the supernatant for screening efficiency using a PRL enzyme-linked immunosorbent assay (ELISA) kit. We used GADPH as a reference gene, and all samples were assayed in triplicate. Analyses were conducted using the 2−ΔΔCt method.
ERα, LRP16, and GAPDH mRNA expression levels were analyzed by real-time polymerase chain reaction (RT-PCR). cDNA was subjected to quantitative PCR using specific primers for ERα (5'-TTTCTTTAAG AGAAGCATTCAAGG-3' and 5'-TTATCGATGGTGCATTG GTTTGT-3'), LRP16 (5'-ATCCCCATGTGGAAGGAGA CA-3' and 5'-CTTATACTTGGGGTTCTCCAC-3'), and GADPH (5'-TCATTGACCTCAACTACATGG-3' and 5'-TCGCTCCTGGAAGATGGTG-3'). Total RNA was isolated from cultured GH3 cells using TRIzol reagent and chloroform according to the manufacturer's protocol. RT-PCR was performed using GoTaq® Q-PCR Master Mix A6001 (Promega, Madison, WI, USA) according to the manufacturer's recommendations.
PRL was obtained after transfection. The supernatants from the L374-60 nmol/L and L668-90 nmol/L groups showed maximum inhibition efficiency using the ELISA kit, according to the manufacturer's recommendations. Absorbance was measured at 450 nm on a microplate reader (Heros Technology Co., Ltd., Beijing, China). The plot consisted of absorbance on the Y-axis and concentration on the X-axis; PRL level was calculated from the standard curve.
Data are expressed as mean ± standard deviation and analyzed by t-test, F-test, one-way analysis of variance and Dunnett's method. A P-value < 0.05 was considered significant using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA)
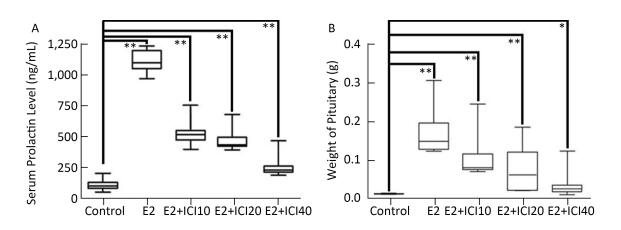
The effect of varying the ICI182780 dose (10, 20, and 40 mg/kg, respectively) on the prolactinoma were investigated using flow cytometry. We compared pituitary weight and serum PRL level. Notably, tumor size, weight, cell secretion, and cell cycle functions decreased as the ICI dose was increased. In addition, the serum PRL level and pituitary weights decreased significantly, which were associated with the increase in ICI dosage, thereby indicating that ICI may have a converse dose-dependent effect on tumourigenicity (Supplemental Figure, available in www.besjournal. com).
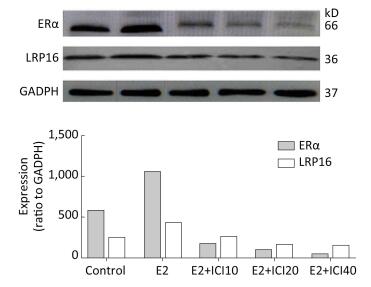
To explore whether the models and the association between LRP16 and ERα was successfully established, Western blot, and IHC were employed to determine the mechanism underlying the LRP16-mediated regulation of ERα by long-term E2 administration. The results showed a significant increase in LRP16 expression in the E2 groups, whereas ERα expression tended to vary. Administration of ICI gradually decreased ERα expression, and, thus, LRP16 expression. In contrast, the variations in LRP16, Erα, and PRL expression were associated with E2 administration and were coincident with dosage (P < 0.01, Figure 1).
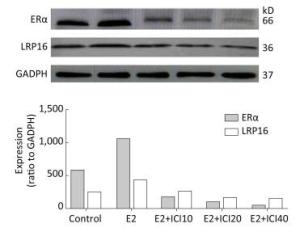
Figure 1. Estrogen receptor (ER)α and leukemia-related protein 16 (LRP16) expression assessed by the 2−ΔΔCt method and Western blot. The results show that interference from ICI reduced the level of ERα in the 17β-estradiol (E2), E2+ICI10, E2+ICI20, and E2+ICI40 groups. LRP16 expression decreased simultaneously compared to the control group; values are means ± standard errors (n = 10 rats/group).
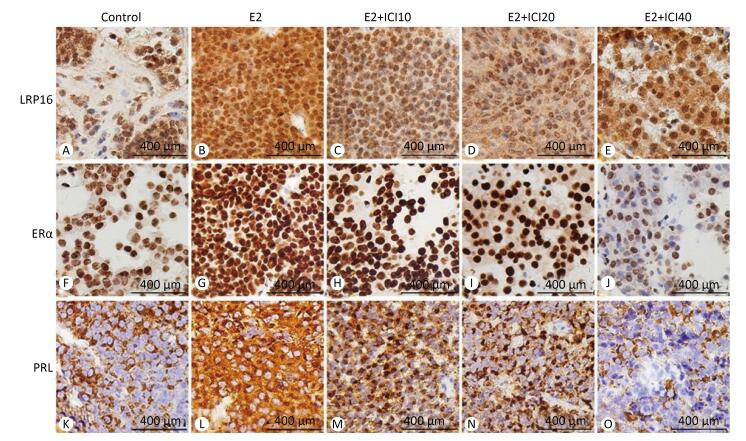
The anti-LRP16, anti-ERα, and anti-PRL antibodies were immunohistochemically positive in the E2 group (Figure 2) in response to the different doses of ICI. The lesions regarded as tumors showed a gradual decrease of expression in the E2+ICI10, E2+ICI20, and E2+ICI40 groups (Figure 2H-J). PRL plasma-positive cells were brown and encompassed the entire visual field (Figure 2L); however, they declined gradually in the E2+ICI10, E2+ICI20, and E2+ICI40 groups (Figure 2M-O). IHC demonstrated the sequential order. LRP16, ERα, and PRL were highest in the E2 group and lowest in the E2+ICI40 group. Therefore, we concluded that long-term E2 administration might promote the development of prolactinoma; the results demonstrate that the SD rat prolactinoma models were successfully established.
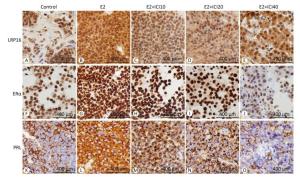
Figure 2. Immunohistochemistry analysis of leukemia-related protein 16 (LRP16), estrogen receptor (ER)α, and prolactin (PRL). (A), (F), (K) were obtained from the sham group. LRP16 in the nuclei of the prolactinoma stained brownish yellow in the E2 group (B) and diminished gradually in the E2+ICI10, E2+ICI20, and E2+ICI40 groups (C-E). A large number of brownish yellow granule clumps appeared in the nuclei representing ERα and PRL (G, L), and expression decreased gradually (H-J, M-O).
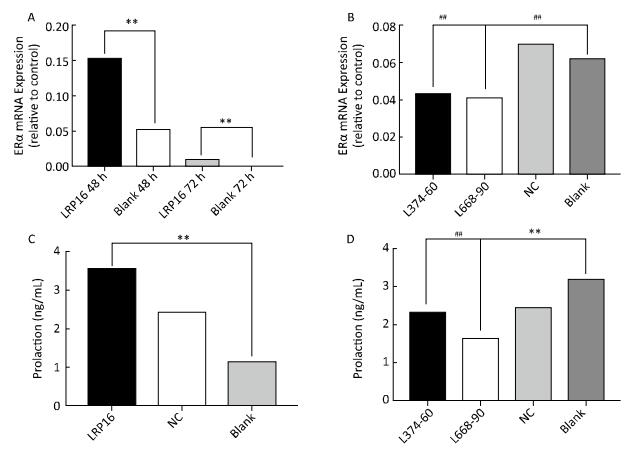
Notably, ERα and PRL paralleled the changes in LRP16; that is, our in vitro studies suggest that the siRNA transfection strategy up and downregulated the LRP16 expression level by specific siRNA. Elevated LRP16 resulted in elevated ERα and PRL expression (P < 0.01, Figure 3A and C). Suppressed LRP16 resulted in impaired secretion, together with a decrease in ERα level. The L374-60 nmol/L and L668-90 nmol/L groups exhibited maximum inhibition compared to the NC and blank, so LRP16 reached a minimum in the two groups. ERα and PRL mRNA expression was at their lowest at the same time (P < 0.05, Figure 3B and D).
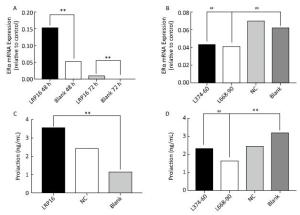
Figure 3. Estrogen receptor (ER)α expression was tested by real-time-polymerase chain reaction, and serum prolactin levels were assessed by enzyme-linked immunosorbent assay. Results obtained from overexpression are shown in A (ERα) and C (prolaction), suppressed expression is shown in B (ERα) and D (prolaction). Bars denote relative expression levels. Vertical bars represent standard deviations (SDs) of triplicate cultures. The ERα mRNA expression levels in the leukemia-related protein 16 (LRP16)-overexpressing groups were higher than that in the blank group (**P < 0.01), and ERα mRNA expression levels in the L374-60 nm, L668-90 nm and NC-90 nm groups were lower than those in the blank and PEI groups. (##P < 0.05). Serum prolactin (PRL) levels in the LRP16-overespressing groups were higher than that in the blank group (**P < 0.01), and serum PRL level in the L374-60 nm and L668-90 nm groups were lower than that in the blank group. (##P < 0.05).
Hitherto, in this study, we have successfully established the male SD rat prolactinoma model using exogenous estrogen.
Estrogen promotes the proliferation of GH3 cells, which in turn, releases PRL through the ER signal transduction pathway, especially ERα[4]. Han et al.[5] argued that LRP16 harbors two separate reaction zones located upstream of the translation initiation site: region A and region B; the latter is comprised of a GC-rich sequence. After estrogen binding with ERα, not only the downstream cascades vary but also the ERα conformation is altered, which enhances transcription of LRP16 mainly through region B[6]. ERα-mediated transcription activation is enhanced or suppressed by synchronous enhancement and suppression-mediated changes in LRP16. Takekoshi et al.[7] proposed that a mitogenic effect is exerted on pituitary tumors through ERα during long-term estrogen administration. In our study, the ER antagonist decreased ERα expression as assessed by IHC. Moreover, our in vitro studies suggest that the siRNA transfection strategy up and downregulated LRP16 expression, which was corroborated by the increase and decrease in the levels of PRL, indicating enhanced and impaired secretion function due to siRNA. Notably, the ERα paralleled the changes in LRP16; that is, LRP16 could not produce the effect alone but was likely to act in synergy with ERα.
In summary, pituitary adenomas showed high expression of LRP16, providing novel insight into prolactinomas, as well as accumulating new evidence on estrogen-induced tumors associated with LRP16 expression. These results could potentially lead to an understanding of the underlying pathogenesis and may provide insight into future clinical use of LRP16 in prolactinoma therapy.
The authors greatly appreciate the staff of the Endocrinology Division of Chinese PLA General Hospital for their excellent cooperation.
-

Figure Supplemental. Serum prolactin and pituitary weight are shown after different ICI dose treatments in all groups. The pituitary weight and serum prolactin level decreased proportionally as the level ICI administration increased, in an ICI dose-dependent manner. No significant differences were observed between the control and E2+ICI40 groups (n = 10; **P < 0.05; *P > 0.05).
Up-and Down-regulated Leukemia-related Protein 16 Affects ERα Expression and Prolactin Secretion by GH3 Cells
doi: 10.3967/bes2017.127
the National Natural Science Foundation of China and Hainan Province 81570705
the National Natural Science Foundation of China and Hainan Province 20168353
the National Natural Science Foundation of China and Hainan Province 81270866
- Received Date: 2017-06-11
- Accepted Date: 2017-11-07
Abstract: Prolactinoma is an estrogen-related tumor and leukemia-related protein 16 (LRP16) is correlated with the progression of estrogen-related tumors, but the regulatory mechanism between LRP16 and prolactinoma remain unclear. This study demonstrates a variation in LRP16 with estrogen receptor α (ERα) in prolactinoma models and the up and downregulation effects of LRP16 on prolactin secretion of pituitary adenomas cells (GH3 cells). In our study, 50 male SD rats (30-day-old) were randomly divided into five groups of 10 rats each. After 120 days of treatment, the rats were sacrificed, and the expression of LRP16 and ERα were examined by Western blot and immunohistochemistry to explore the changes in ERα, LRP16, and prolactin. After siRNA transfection of the respective genes, the GH3 cells were cultured, and their secretory function as well as the expression of ERα mRNA and prolactin were analyzed by enzyme-linked immunosorbent assay and real-time-polymerase chain reaction analysis. The results show that secretion of prolactin by GH3 cells can be affected by up and downregulating LRP16 expression, which may provide a novel medical therapy in clinical trials.
| Citation: | SU Xing, WU Zhi Qiang, YANG Xiao Jiang, WANG An Ping, ZHAO Kun, WU Tao Guang, SUN Yi, SUN Jie, CHANG Zheng Yao, GUO Xiao Yong, GUO Qing Hua, MU Yi Ming. Up-and Down-regulated Leukemia-related Protein 16 Affects ERα Expression and Prolactin Secretion by GH3 Cells[J]. Biomedical and Environmental Sciences, 2017, 30(12): 938-942. doi: 10.3967/bes2017.127 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: