-
This prospective study was designed to examine the combined influence of insulin resistance (IR) and inflammatory biomarker levels on type 2 diabetes mellitus (T2DM) among 1, 903 Inner Mongolians. During follow-up, 205 (10.77%) participants developed T2DM, and the incidence of T2DM was higher among subjects with IR, elevated C-reactive protein (CRP), elevated sICAM-1, elevated sE-selectin, or the coexistences of IR with elevated CRP, elevated sICAM-1, elevated sE-selectin, and elevated angiotensin Ⅱ (all P < 0.05) compared with patients without IR or any elevated biomarkers. In multivariate analysis, the odd ratios [OR, (95% confidence intervals)] for these conditions were 1.944 (1.405-2.691), 2.003 (1.449-2.767), 1.706 (1.232-2.362), 1.560 (1.123-2.165), 2.708 (1.809-4.054), 1.885 (1.155-3.078), 2.101 (1.340-3.295), and 2.260 (1.426-3.582), respectively. Our findings demonstrated that IR and elevated inflammatory biomarkers were associated with T2DM, and that the coexistence of IR and elevated inflammatory biomarkers increased the risk of T2DM.
Type 2 diabetes mellitus (T2DM) is a major worldwide public health problem. T2DM rates have rapidly risen in China over the last few decades, increasing disease burden. Previous studies have shown that insulin resistance (IR) is a risk factor for T2DM[1]. It is also generally accepted that chronic low-grade inflammation may play an important role in IR pathogenesis; thus, increased levels of inflammatory markers might predict future T2DM development[2]. Endothelial progenitor cells are important for maintaining normal endothelial function and vascular repair in T2DM patients, especially for reducing the peripheral vascular complications of T2DM[3]. In contrast, endothelial dysfunction aggravates glycaemia during the diabetic phase. Additionally, the coexistence of IR and inflammation can predict cardiac disease among T2DM patients[4]; however, few studies have evaluated the combined influence of IR, inflammation biomarkers and endothelial dysfunction on T2DM development. In this study, we examined the combined influence of inflammatory biomarkers [circulating C-reactive protein (CRP), soluble intercellular cell adhesion molecule-1 (sICAM-1), soluble E-selectin (sE-selectin) and angiotensin Ⅱ], endothelial dysfunction, and IR on T2DM among Inner Mongolians.
This prospective cohort study was conducted from 2002 to 2013 in 32 villages of two adjacent townships, Kezuohou banner (country) and Naiman banner, Inner Mongolia, China. The methods for participant recruitment and baseline data collection have been described elsewhere. Briefly, 2, 589 Mongolians ≥ 20-years-old were recruited from Inner Mongolia. All participants signed informed consent and completed a baseline questionnaire. Individuals with cardiovascular diseases, endocrine diseases, infectious diseases within the last 2 weeks and antihypertensive drug users were excluded. Additionally, 94 T2DM patients and 36 individuals without complete key variables were excluded. Finally, 2, 459 Mongolians who have lived in this region for many generations and maintained a traditional diet and lifestyle, were included at baseline. This study was approved by the Soochow University Ethics Committee (Suzhou, China).
A standard questionnaire was administered by trained staff to obtain demographic information, personal characteristics, lifestyle risk factors and personal medical histories. Cigarette smoking was defined as having at least one cigarette per day for 1 year or more. Alcohol drinking was defined as having consumed at least 50 g alcohol per day for 1 year or more. Each subject's height and weight were measured while wearing light clothing and without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters (kg/m2). Waist circumference was measured at a level 1 cm above the umbilicus. Blood pressure was measured using a standard mercury sphygmomanometer according to standard protocols. A fasting condition of at least 10 h was required for all subjects before blood samples were collected for laboratory examinations. Plasma glucose was measured using a modified hexokinase enzymatic assay. Total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) concentrations were analyzed enzymatically on a Beckman Synchrony CX5 Delta Clinical System (Beckman Coulter, Fullerton, CA, USA) using commercial reagents. Low-density lipoprotein cholesterol (LDL-C) concentrations were calculated using the Friedewald equation. Serum insulin was measured using a radioimmunoassay method, and the homeostasis model assessment (HOMA) index was calculated by the following formula: HOMA-IR = fasting insulin (mU/L) × fasting glucose (mmol/L) ÷ 22.5. Subjects with IR were defined as a HOMA-IR ≥ 75th percentile (≥ 3.28). The CRP concentration was measured by immunoturbidimetry on a Beckman Synchrony CX5 Delta Clinical System using commercial reagents. sICAM-1 and sE-selectin were measured with an enzyme-linked immunosorbent assay (R & D Systems, Minneapolis, MN, USA) that employed the quantitative sandwich enzyme immunoassay technique. Angiotensin Ⅱ was measured with a double antibody radioimmunoassay. Elevated CRP, sICAM-1, sE-selectin and angiotensin Ⅱ were defined as 10.64 mg/L or greater, 391.14 ng/mL or greater, 24.17 ng/mL or greater, and 68.90 pg/mL or greater (the upper quartile), respectively.
For follow-up, the study population was re-investigated from 2013 to 2014. After an overnight fast of at least 10 h, fasting plasma glucose was measured using the same hexokinase assay within 24 h. Participants without a history of T2DM were instructed to maintain their usual physical activity and diet at least 3 d before the oral glucose-test. All participants were given a standard 75-g glucose solution and required to drink it within 5 min; plasma glucose was measured 2 h after administering the during the oral glucose tolerance test. Incident T2DM was defined based on the 1999 World Health Organization criteria (≥ 7.0 mmol/L fasting or ≥ 11.1 mmol/L 2-h glucose) or validated physician diagnosis or the use of antidiabetic medication at any investigation or diagnosed as T2DM in medical records or death certificates.
Continuous variables that showed a normal distribution were expressed as means ± standard deviations or medians (quartile intervals) for variables with non-normal distribution. Differences between groups were analyzed with independent t test (for continuous variables), Wilcoxon rank test (for non-normally distributed variables), or chi-squared test (for proportions). Unconditional logistic regression was used to evaluate the association of T2DM with IR, elevated inflammatory biomarkers, endothelial dysfunction, and for the coexistence of IR and elevated inflammatory biomarkers. Age, gender, systolic blood pressure, diastolic blood pressure, smoking, drinking, waistline, TC, TG and HDL-C were used as adjustments for repeating the analyses. All statistical analyses were performed with SAS 9.2 statistical software (SAS Institute, Cary, NC, USA), all P values were two-tailed, and the statistical significance was set at P < 0.05.
Among the 2, 459 participants, 1, 903 participated in the follow-up, 274 were deceased, and 282 were lost to follow-up. The mean follow-up period was 10.48 years, and a total of 19, 402 person-years were observed. There were 205 (10.77%) cases of incident T2DM during follow-up.
Table 1 shows the baseline characteristics of subjects stratified into two groups: non-T2DM at follow-up (reference group) and T2DM at follow-up. Compared with the reference group, subjects with T2DM were more likely to be older, and have higher drinking rates, systolic blood pressure, diastolic blood pressure, BMI, waist circumference, fasting plasma glucose, LDL-C, HDL-C, TC, TG, HOMA-IR, CRP, sICAM-1, and sE-selectin. There were no significant differences in gender, smoking or angiotensin Ⅱ.
Variables Non-T2DM at Follow-up T2DM at Follow-up P Value N 1, 698 205 - Age (years) 43.71 ± 10.93 47.74 ± 11.16 < 0.001 Men (%) 38.31 40.98 0.425 SBP (mmHg) 120 (110-134) 130 (120-145) < 0.001 DBP (mmHg) 80 (75-90) 87 (80-96) < 0.001 BMI (kg/m2) 21.60 (19.83-23.92) 23.70 (21.00-26.84) < 0.001 WC (cm) 78.0 (73.0-85.0) 86.0 (78.0-93.0) < 0.001 FPG (mmol/L) 4.7 (4.2-5.3) 5.3 (4.9-5.9) < 0.001 LDL-C (mmol/L) 2.10 (1.57-2.74) 2.41 (1.82-3.17) < 0.001 HDL-C (mmol/L) 1.17 (0.96-1.38) 1.00 (0.86-1.24) < 0.001 TC (mmol/L) 3.51 (2.94-4.23) 3.82 (3.12-4.81) 0.003 TG (mmol/L) 0.90 (0.62-1.30) 1.24 (0.79-2.10) < 0.001 HOMA-IR 2.48 (1.72-3.20) 3.11 (2.31-4.35) < 0.001 Smoking (%) 42.76 40.98 0.626 Drinking (%) 29.80 38.05 0.016 CRP (mg/L) 5.44 (3.64-9.76) 9.39 (4.86-16.62) < 0.001 sICAM-1 (ng/mL) 322.55 ± 95.83 354.87 ± 100.99 < 0.001 sE-selectin (ng/mL) 18.15 (14.48-23.84) 20.69 (16.05-27.06) < 0.001 Angiotensin Ⅱ (ng/mL) 48.00 (40.00-68.55) 49.00 (40.60-71.00) 0.201 Elevated IR (%) 22.85 43.41 < 0.001 Elevated CRP (%) 22.38 45.37 < 0.001 Elevated ICAM-1 (%) 21.97 35.12 < 0.001 Elevated E-selectin (%) 22.26 32.68 < 0.001 Elevated angiotensin Ⅱ (%) 24.79 26.83 0.525 IR + elevated CRP (%) 6.77 23.41 < 0.001 IR + elevated ICAM-1 (%) 5.42 12.68 < 0.001 IR + elevated E-selectin (%) 6.42 15.61 < 0.001 IR + elevated angiotensin Ⅱ (%) 6.07 15.12 < 0.001 Note. SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; WC, waist circumference; FPG, fasting plasma glucose; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; TC, total cholesterol; TG, triglyceride; HOMA-IR, homeostatic model assessment-insulin resistance; CRP, C-reaction protein; sICAM-1, soluble intercellular cell adhesion molecule-1; sE-selectin, soluble sE-selectin. Data are presented as mean ± SD or n (%) for normally distributed variables and median (interquartile range) for non-normally distributed variables, respectively. Table 1. Baseline Characteristics of Individuals with or without T2DM during Follow-up
Figure 1 presents the incidence of T2DM according to IR, inflammatory biomarkers, and endothelial dysfunction. Compared with patients without IR or any elevated inflammatory biomarkers, subjects with IR, or elevated CRP, OR sICAM-1, or sE-selectin had higher T2DM rates (all P < 0.05). Furthermore, subjects with the coexistence of IR and elevated CRP, sICAM-1, sE-selectin or angiotensin Ⅱ (any) had higher T2DM rates (all P < 0.05).
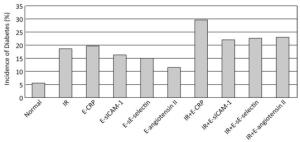
Figure 1. The T2DM incidence according to IR and inflammatory biomarkers. Normal, normal level of insulin resistance without any elevated inflammatory biomarkers; IR, high level of insulin resistance; E-CRP, elevated C-reactive protein; E-sICAM-1, elevated soluble intercellular cell adhesion molecule-1; E-sE-selectin, elevated soluble sE-selectin; E-angiotensin Ⅱ, elevated angiotensin Ⅱ.
Compared with subjects without IR or any elevated inflammatory biomarkers, those with IR, elevated CRP, elevated sICAM-1 and elevated sE-selectin were associated with an increased risk of T2DM before adjustments. Furthermore, the coexistence of IR with elevated CRP, elevated sICAM-1, elevated sE-selectin or elevated angiotensin Ⅱ increased the risk of T2DM. After adjusting for age, gender, smoking, drinking, blood pressure, waistline, TC, TG and HDL-C, the associations remained significant, odds ratios [OR, 95% confidence interval (CI)] of T2DM associated with IR, elevated CRP, elevated sICAM-1, or elevated sE-selectin and the coexistence of IR and elevated CRP, elevated sICAM-1, elevated sE-selectin, or elevated angiotensin Ⅱ were 1.944 (1.405-2.691), 2.003 (1.449-2.767), 1.706 (1.232-2.362), 1.560 (1.123-2.165), 2.708 (1.809-4.054), 1.885 (1.155-3.078), 2.101 (1.340-3.295), and 2.260 (1.426-3.582), respectively (Table 2). Elevated angiotensin Ⅱ was not significantly associated with T2DM.
Variables Unadjusted OR (95% CI) P Value Multivariate-adjusted OR (95% CI) P Value Non-IR + low biomarkers 1.000 (reference) 1.000 (reference) IR 2.590 (1.922, 3.492) < 0.001 1.944 (1.405, 2.691) < 0.001 Elevated CRP 2.880 (2.139, 3.879) < 0.001 2.003 (1.449, 2.767) < 0.001 Elevated sICAM-1 1.923 (1.412, 2.619) < 0.001 1.706 (1.232, 2.362) 0.002 Elevated sE-selectin 1.659 (1.239, 2.320) < 0.001 1.560 (1.123, 2.165) 0.014 Elevated angiotensin Ⅱ 1.119 (0.806, 1.554) 0.525 1.081 (0.767, 1.523) 0.843 IR + elevated CRP 4.210 (2.895, 6.123) < 0.001 2.708 (1.809, 4.054) < 0.001 IR + elevated sICAM-1 2.536 (1.598, 4.025) < 0.001 1.885 (1.155, 3.078) 0.014 IR + elevated sE-selectin 2.697 (1.765, 4.122) < 0.001 2.101 (1.340, 3.295) 0.013 IR + elevated angiotensin Ⅱ 2.788 (1.811, 4.292) < 0.001 2.260 (1.426, 3.582) 0.004 Note. 95% CI, 95% confidence interval; OR, odds ratio; IR, insulin resistance; CRP, C-reactive protein; sICAM-1, soluble intercellular cell adhesion molecule-1; sE-selectin, soluble E-selectin. Odds ratios were adjusted for age, gender, drinking, smoking, blood pressure, waistline, total cholesterol, triglyceride and high-density lipoprotein-cholesterol. Table 2. Associations between T2DM, IR, and Elevated Inflammatory Markers
This cohort study examined the relationships between IR, inflammatory biomarkers and endothelial dysfunction with T2DM, as well as the combined effects of IR, inflammatory biomarkers and endothelial dysfunction on T2DM among an Inner Mongolian population. Our results demonstrated that IR, elevated inflammatory biomarkers (CRP, sICAM-1, sE-selectin and their coexistence with IR at baseline), and endothelial dysfunction were significantly associated with T2DM incidence. Subjects with higher CRP, sICAM-1, sE-selectin levels and those with the coexistence of IR were all at a significantly higher risk of T2DM than those without IR or any elevated inflammatory biomarkers. Additionally, an increasing trend for the risk of T2DM was found among patients with the coexistence of IR and increased inflammatory biomarkers and endothelial dysfunction. These findings provide further support that IR, inflammation and endothelial dysfunction (as well their coexistence) are associated with T2DM incidence.
Compelling evidence has demonstrated that IR is associated with coronary heart disease, hypertension and dyslipidemia in T2DM patients[2, 4]. A recent study conducted in an Iranian population with normal glucose regulation at baseline found that IR was a strong risk factor for T2DM [hazard ratio (HR) 2.50, 95% CI 1.27-4.90 for men; HR 2.13, 95% CI 1.29-3.52 for women]. Moreover, short-term intensive insulin therapy can ameliorate the pathophysiology in early T2DM. These findings led to the suggestion that IR was the predominant pathogenetic factor for T2DM development.
Some studies have indicated that CRP is associated with T2DM. Abbas Dehghan et al.[5] reported that high serum CRP (> 1 mg/L) was one a major contributor to T2DM risk in the general population ≥ 55-years-old (HR 1.67, 95% CI 1.34-2.09), and that women had a greater hazard ratio than men (1.77 vs. 1.42). Another study showed that elevated ICAM-1 and E-selectin were independently associated with T2DM. A prospective nested case-control study[6] within the Nurses' Health Study found that T2DM subjects had significantly higher baseline median ICAM-1 and E-selectin levels than controls (all P ≤ 0.004), and that patients with elevated ICAM-1 and E-selectin (the top quintile) exhibited an increased adjusted risk for incident T2DM (HR 3.56, 95% CI 2.88-5.58; HR 5.43, 95% CI 3.47-8.50, respectively). A limited number of studies have reported conflicting data on the relationship between angiotensin Ⅱ and T2DM. Several cross-sectional studies have suggested that angiotensin Ⅱ values increase with increasing glucose intolerance severity among T2DM subjects[7]; however, another study showed that angiotensin Ⅱ was not elevated in insulin-resistant obese individuals with impaired glucose tolerance. In our study, elevated angiotensin Ⅱ levels were not associated with T2DM. To our knowledge, no other prospective studies have examined this association.
CRP, sICAM-1 and sE-selectin are biomarkers of inflammation and endothelial dysfunction that are independently associated with T2DM in Inner Mongolians. Moreover, our findings indicated that the coexistence of IR and elevated inflammatory biomarkers conferred a greater risk for T2DM than either alone. Similarly, Matsumoto et al. [8] found that patients with the combination of increased inflammation and IR were at a 5-fold higher relative risk than those without inflammation or IR among 350 Japanese T2DM patients.
Although IR has been thought to be fundamental to T2DM development, the mechanism of this additive effect with inflammatory and endothelial dysfunction biomarkers remains unknown. Recent studies have suggested that CRP has a strong relationship with IR, as HOMA-IR levels consistently increased with increasing CRP concentrations. Alternatively, endothelial dysfunction is an early abnormality of the IR state that might contribute to T2DM. Ingelsson et al.[9] demonstrated that VCAM-1, E-selectin and CRP were strongly associated with IR and metabolic syndrome. Chu et al.[7] also showed that angiotensin Ⅱ played an integral role in regulating insulin secretion and insulin sensitivity, indicating a possible link between angiotensin Ⅱ and IR. While endothelial dysfunction and inflammation might be important players in IR pathogenesis[2], IR also induces oxidative stress, which promotes inflammatory cytokines and activates the renin-angiotensin system. Furthermore, increased glucose concentrations under hyperglycemic conditions cause excessive mitochondrial reactive oxygen species production, which in turn results in oxidative damage and inflammation.
Our findings support the hypothesis that IR and elevated inflammatory biomarkers are independently associated with T2DM incidence; however, when present together, there is an increased T2DM risk. Both inflammation and IR are important links in the T2DM mechanism; either systemic inflammation promotes T2DM development by introducing IR, or IR promotes T2DM development by exacerbating systemic inflammation.
These results provide clues for preventing T2DM. Previous studies have indicated that lifestyle interventions may decrease inflammatory cytokine levels. Additionally, glucose-lowering medications, such as thiazolidinediones, not only have lower tissue and serum inflammation, but also have beneficial effects on numerous markers of endothelial function[3, 10]. Therefore, dietary guidance and drug therapies may be of considerable significance in preventing T2DM and related complications.
This study has several strengths that deserve mention. First, oral glucose-tolerance and fasting plasma glucose tests were all performed to define T2DM at follow-up, as the combination of increasing fasting and 2-h glucose levels has been shown to identify more T2DM cases than either alone. Second, the follow-up period was relatively long, which enabled us to determine the long-term effects of IR, inflammatory biomarkers and endothelial dysfunction, as well as their coexistence on T2DM. However, there were also some limitations to this study. First, we measured insulin and inflammatory and endothelial dysfunction biomarkers only once at baseline, and therefore, could not evaluate the effects of changes in these biomarkers over time on outcomes. Furthermore, several important inflammatory and endothelial dysfunction biomarkers, such as tumor necrosis factor-alpha and vascular cell adhesion molecule-1, were not measured in this study. Finally, data regarding endothelium-dependent or endothelium-independent flow-mediated vasodilation were not collected.
Combined Influence of Insulin Resistance and Inflammatory Biomarkers on Type 2 Diabetes: A Population-based Prospective Cohort Study of Inner Mongolians in China
doi: 10.3967/bes2018.038
the National Natural Science Foundation of China 81102190
the National Natural Science Foundation of China 81773509
- Received Date: 2017-09-08
- Accepted Date: 2018-02-06
| Citation: | QIU Qiao Yan, ZHANG Bei Lei, ZHANG Ming Zhi, WU Jia Hui, ZHOU Jing Wen, LIANG Zhu, ZHANG Yong Hong, ZHANG Shao Yan. Combined Influence of Insulin Resistance and Inflammatory Biomarkers on Type 2 Diabetes: A Population-based Prospective Cohort Study of Inner Mongolians in China[J]. Biomedical and Environmental Sciences, 2018, 31(4): 300-305. doi: 10.3967/bes2018.038 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: