-
Thyroid cancer is the most common malignancy of the endocrine system, and its incidence is increasing worldwide. In China, its incidence has increased from 1.78/104 in 1988 to 10.58/104 in 2013. Thyroid cancers are mainly classified into papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), medullary thyroid cancer (MTC), and undifferentiated or anaplastic thyroid cancer (ATC) according to the histological subtypes. Among them, PTC is the most common subtype, comprising about 80%–90% of all thyroid cancers[1].
Iodine is indispensable to humans because it is essential for thyroid hormone synthesis. There is a U-shaped relationship between iodine intake and thyroid disease, either a deficiency or excess of iodine can lead to thyroid disease[2]. However, the role of iodine intake in thyroid cancer remains uncertain. Liu et al.[3] have found that high iodine consumption may decrease the risk of thyroid cancer in iodine-deficient areas. Kim et al.[4] investigated 215 PTC patients and found that relatively low iodine intake and excess iodine intake are risk factors for the development of PTC in an iodine-replete area. Zhao et al.[5] have found that urine iodine concentration less than 100 μg/L group was inversely associated with multifocality, while excess iodine intake was independently associated with an increased risk of larger tumor size in female PTC patients. However, most of these studies used only urinary or serum iodine levels, and their results were diverse. The clinical significance of serum and urinary iodine levels and the tumorigenesis and clinicopathological features of PTC, such as whether it is associated with multifocality and lymph node metastasis, remain controversial.
In the current study, a total of 845 patients with PTC, 310 patients with benign thyroid nodules, and 374 control group were recruited. We aimed to investigate the correlation of serum and urinary iodine levels with the risk of developing PTC, and the clinicopathological characteristics of patients with PTC as per the iodine status were also compared.
Blood and urine samples of 845 PTC patients and 310 benign thyroid nodule patients were obtained from the Harbin Medical University Cancer Hospital before any medical treatment such as cytoreductive surgery, radiotherapy and chemotherapy. According to the appropriate protocols, patients with an initial diagnosis of thyroid nodule were enrolled from November 2015 to March 2018. The PTC patients and benign thyroid nodule patients were enrolled based on post-operative pathology and excluded them with abnormal liver or kidney functions. Blood and urine samples of control group were obtained from the Second Affiliated Hospital of Harbin Medical University. The control group were recruited from the health check up clinic and comprised 374 age- and sex-matched participants without thyroid diseases or evidence of other diseases, such as other cancers or other liver or kidney diseases.
Data collection from the human subjects and study protocols were approved by the Institutional Ethics Committees of the Harbin Medical University, and signed, informed consent was obtained from all the participants. All the subjects were recruited from the iodine-repleted region (the consumption rate for iodized salt in the three groups was > 95%), and had lived in their local hometown for more than 10 years, without receiving iodine-containing drugs or thyroid medications, such as amiodarone/diodone for at least 6 months. All the participants were required to fast and avoid medicine and alcohol for 12 h before sampling.
Serum and urinary iodine levels were measured using arsenic-cerium catalytic spectrophotometry. Thyroid functions were measured using chemiluminescent immunoassay. The reference intervals were determined according to WHO criteria[6].
Data processing and statistical analyses were performed using SPSS (v.13.0; IBM, Armonk, NY, USA). Continuous variables of skewed distribution are described as median. The urinary and serum iodine data were analyzed using the Mann-Whitney U test, the percentage data were compared using the chi-square test, the correlation between urine iodine and serum iodine was assessed using correlation analysis, and multiple variable analyses were performed with logistic regression analysis. P values < 0.05 were considered statistically significant.
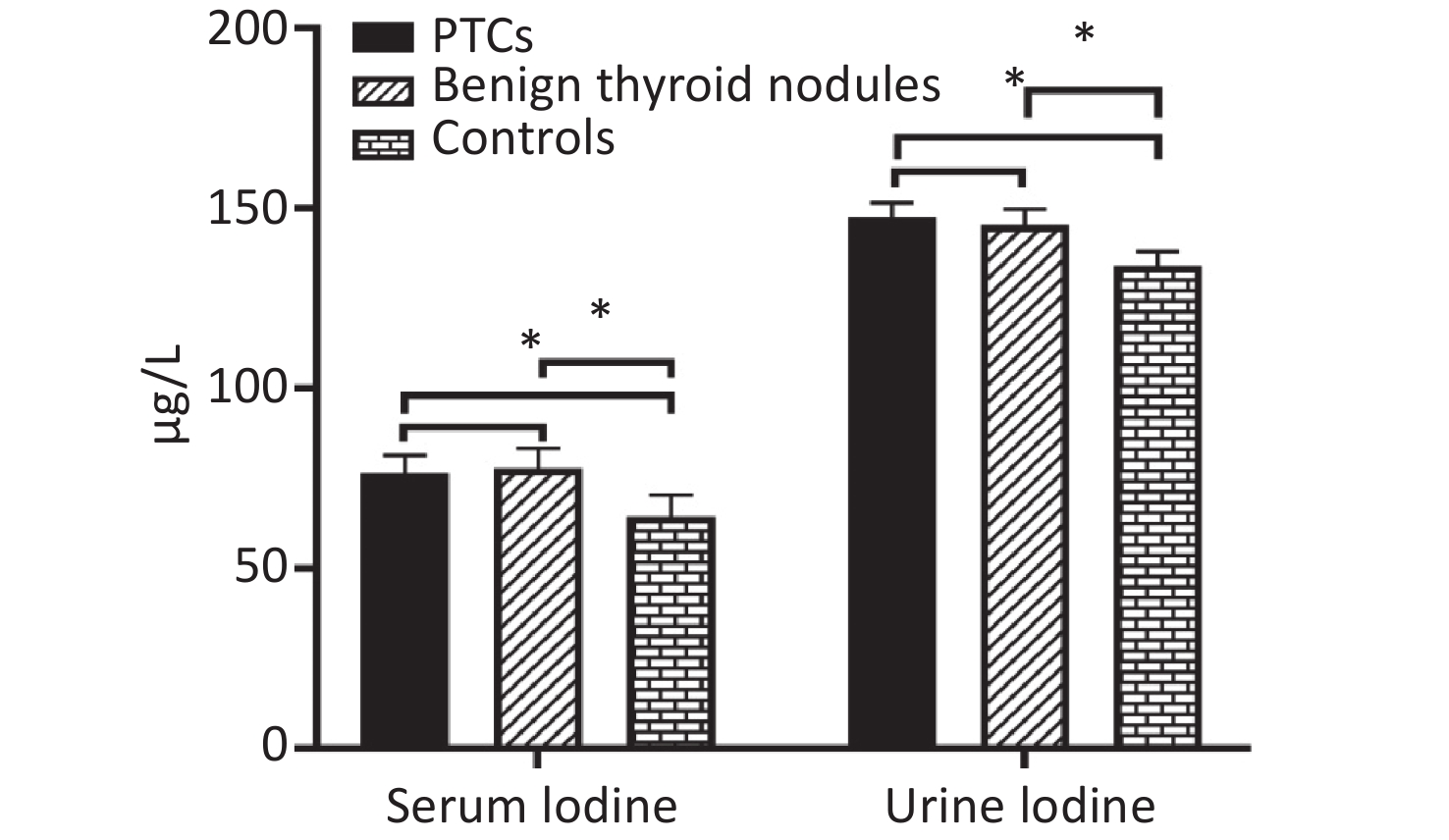
The median of serum iodine in the three groups were 76.50 μg/L, 77.94 μg/L, and 64.49 μg/L, respectively. Serum iodine level of PTC group and benign thyroid nodule group were significantly higher than in control group (P = 0.000 and P = 0.000, respectively). However, there were no significant differences in the PTC group and benign thyroid nodules patient group (P = 0.085). The median values of urinary iodine in the three groups were 147.58 μg/L, 145.27 μg/L, and 134.07 μg/L, respectively. Urinary iodine levels of PTC group and benign thyroid nodule group were significantly higher than in control group (P = 0.015 and P = 0.013, respectively). However, there were no significant differences in PTC group and benign thyroid nodule group (P = 0.491, Supplemetary Figure S1 available in www.besjournal.com).
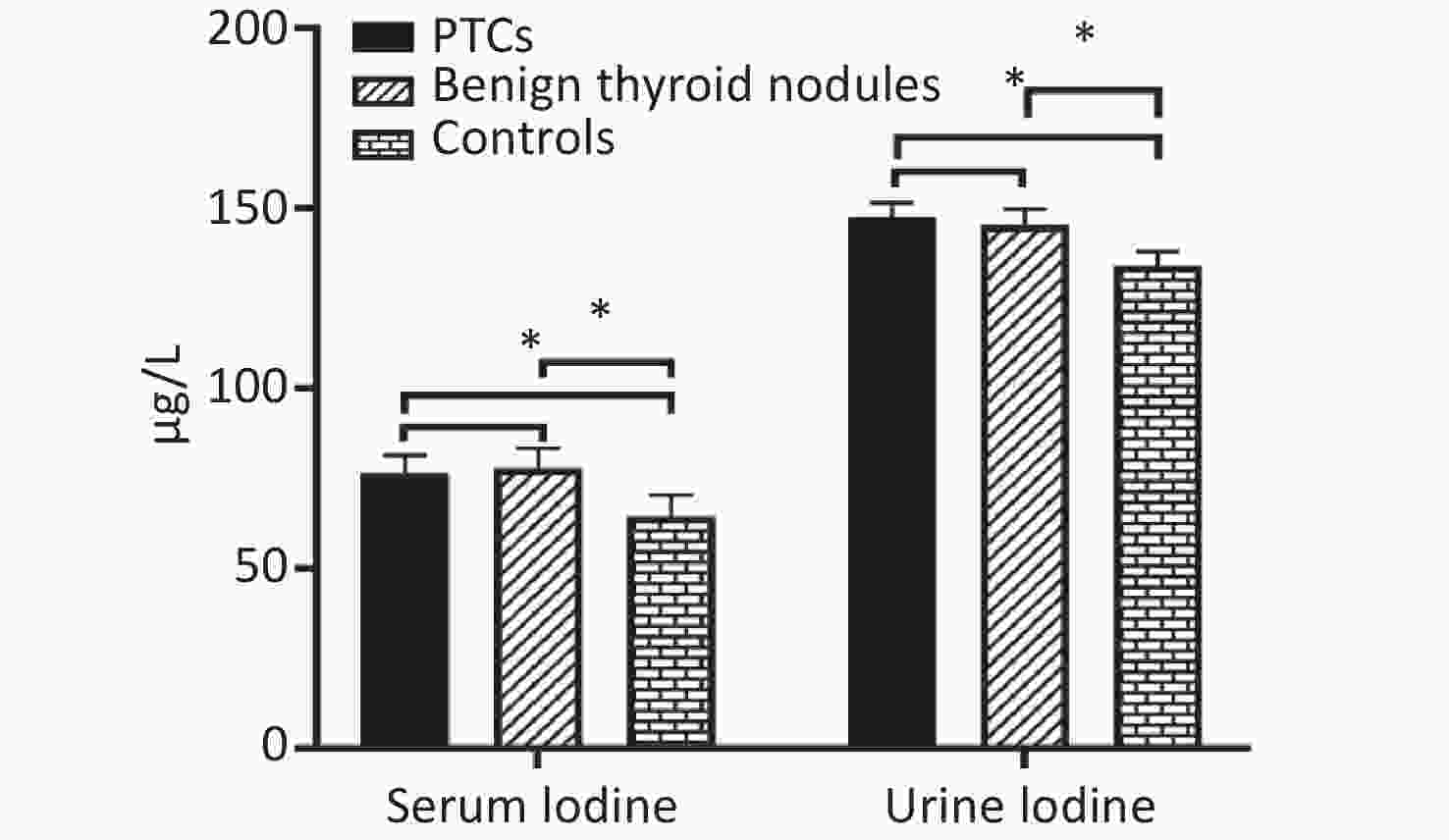
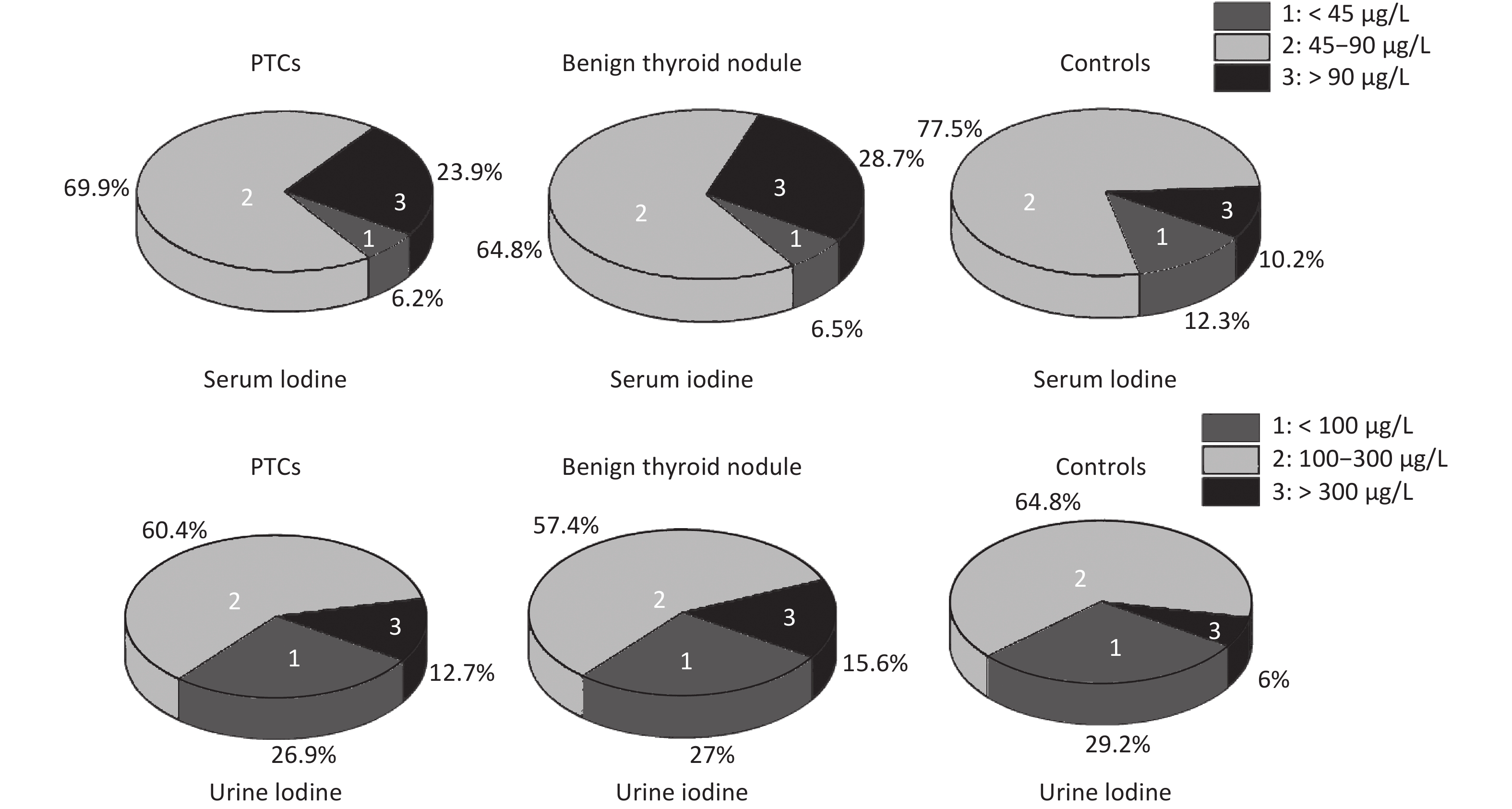
Figure S1. Distribution of median serum iodine and urinary iodine in PTCs, patients with benign thyroid nodules, and controls. Compared with the corresponding controls, there is significant difference (*P < 0.05).
The rate of insufficient iodine intake (serum iodine < 45 μg/L) in thyroid patients was lower than that in control group, and that of excess iodine intake (serum iodine > 90 μg/L) was higher than that in control group. Fewer thyroid patients had urinary iodine levels < 100 μg/L than control group, while more thyroid patients had levels > 300 μg/L. Overall, urinary iodine and serum iodine levels in PTC group were significantly higher than in the control group (Supplemetary Figure S2, available in www.besjournal.com).
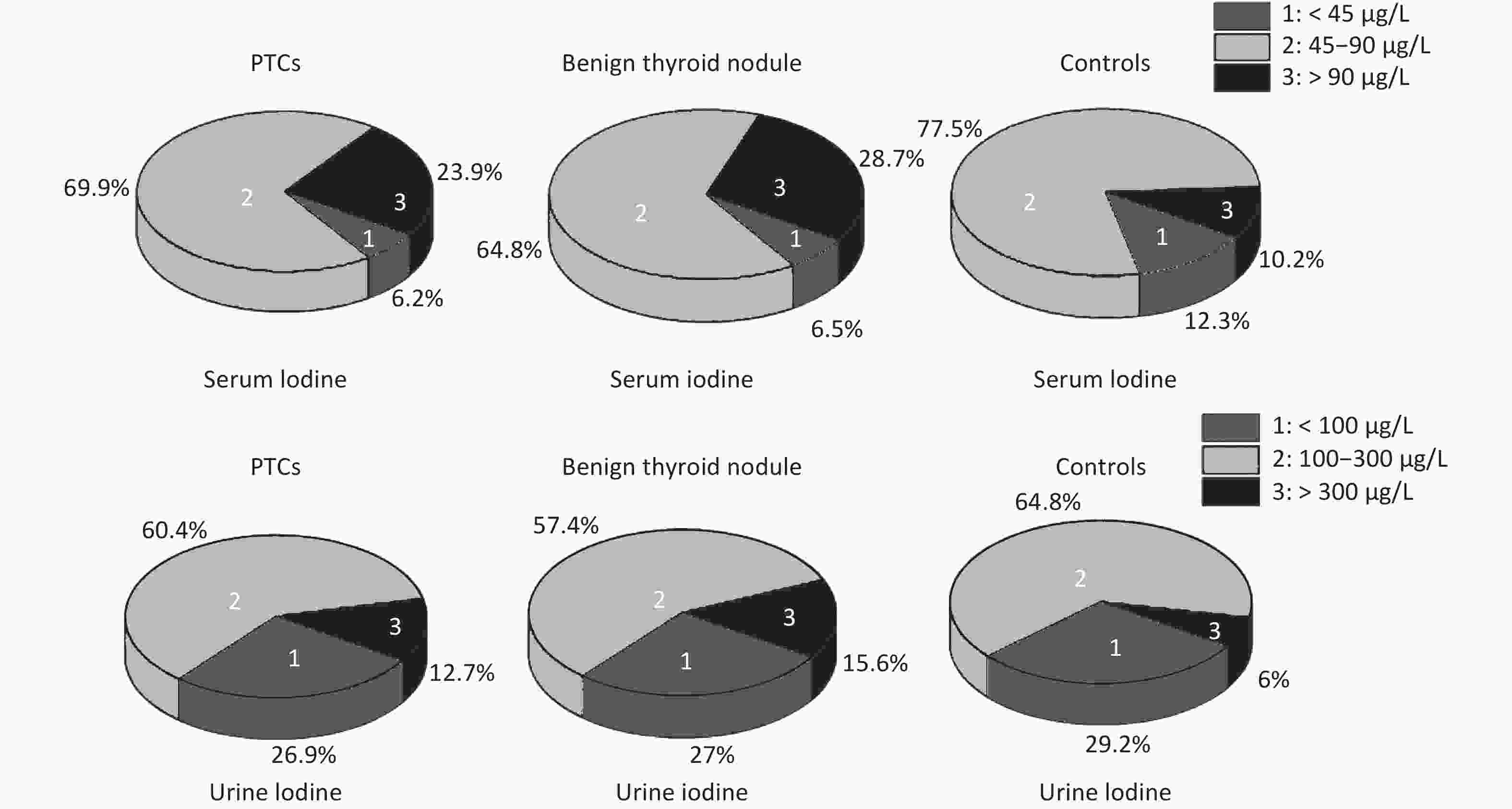
Figure S2. Serum and urinary iodine levels in PTCs and benign thyroid nodules, controls. The percentage were 23.9%, 28.7%, 10.2% in serum iodine > 90 μg/L, respectively. The percentage were 12.6%, 15.6%, 6% in urinary iodine > 300 μg/L, respectively.
As an essential trace element, iodine, functioning as a substrate for thyroid hormone synthesis, plays a crucial role in the normal development and growth during early human life and cellular and molecular metabolism throughout human life. Serum iodine reflects a relatively long-term and stable iodine level, and urine iodine reflects the transient iodine level. Thus far, most studies have mainly focused on the relationship between thyroid disease and urinary iodine levels; few studies have examined the serum iodine levels. Moreover, the relationship between iodine intake and thyroid cancer incidence remains controversial. Therefore, we compared the iodine levels both, in the serum and in the urine in thyroid diseases including PTC. We found that both serum and urinary iodine levels were higher in PTC group and benign thyroid nodule group than in healthy control group. The results were consistent with previous reports. For example, Jin et al.[7] have indicated that the median serum iodine values of adults with thyroid nodules were significantly higher than those of euthyroid adults, the research of Lee et al.[8] has shown that the median urinary iodine levels were significantly higher in the PTC group than in the control group. However, in the present study, we found no statistical difference between PTC group and benign thyroid nodule group. Therefore, we speculated that iodine intake status is a risk factor for thyroid disease. However, it may not be an independent indicator of PTC oncogenesis.
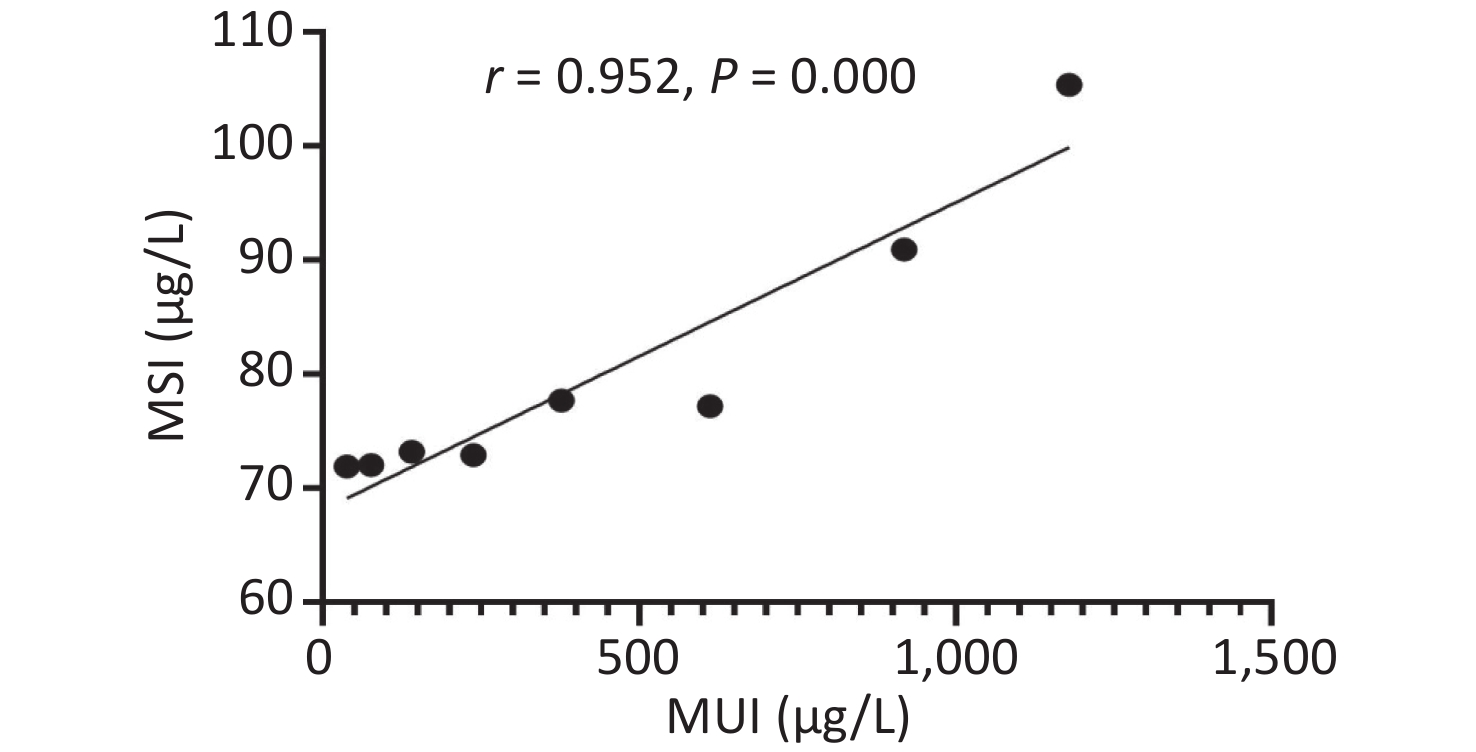
Urinary iodine levels were categorized into the following eight groups (< 50, 50–99.9, 100–199.9, 200–299.9, 300–499.9, 500–799.9, 800–999.9, and > 1,000), and we found strong non-linear correlations between serum and urinary iodine levels (Supplemetary Figure S3, available in www.besjournal.com). More recently, Jin et al.[7] also found that serum and urinary iodine levels represented a strong non-linear correlation in adults. The epidemiological results were consistent with our findings.
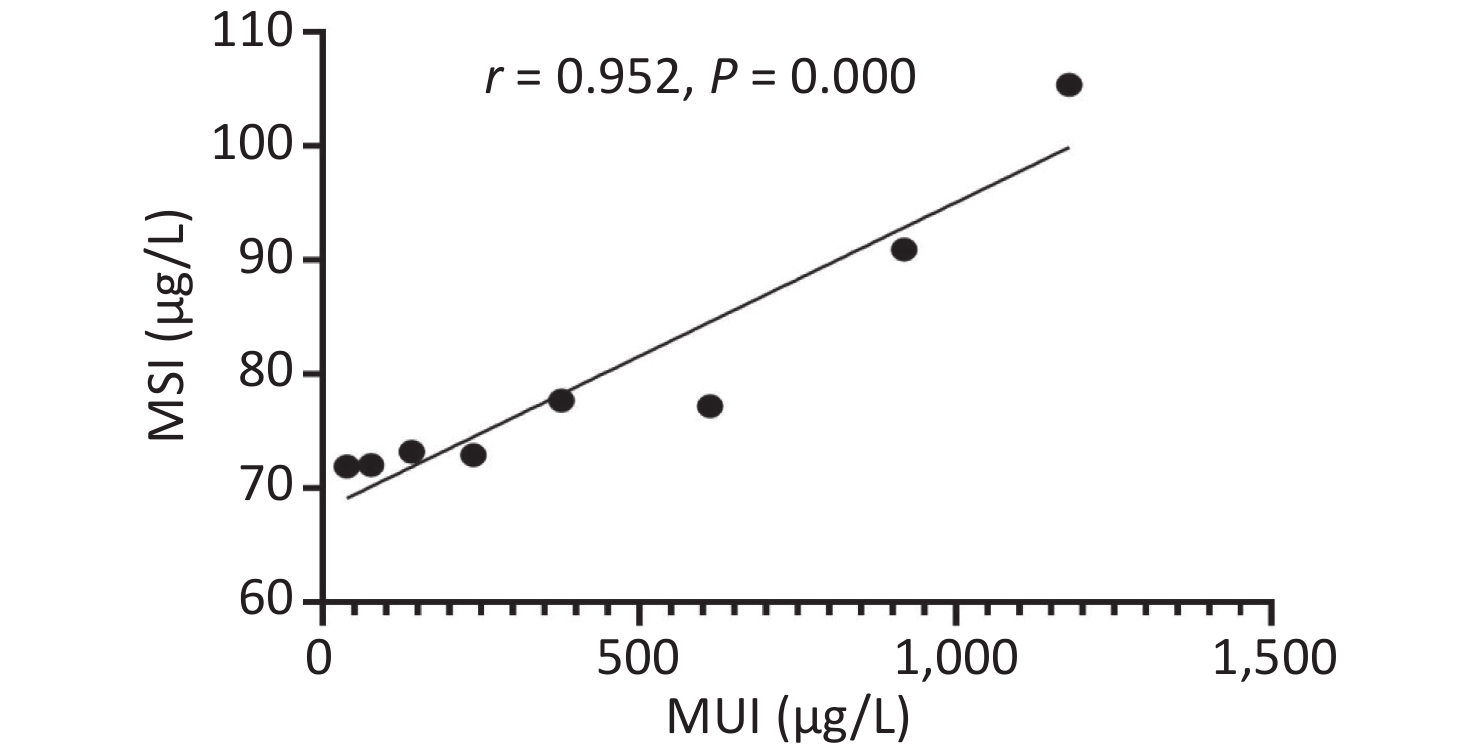
Figure S3. The relationship between serum and urinary iodine levels. MSI, median serum iodine. MUI, median urinary iodine
We performed logistic regression analyses to determine the risk in PTC group and benign thyroid nodule group. Age at diagnosis (< 45 vs. ≥ 45 years), smoking history (yes vs. no), alcohol consumption (yes vs. no), hypertension (systolic blood pressure < 120 vs. > 120), abnormal TSH levels (< 0.27 μIU/mL and > 4.2 μIU/mL), and abnormal TG levels (< 1.4 ng/mL and > 78 ng/mL) were considered in the analyses. Age < 45 years, smoking history, abnormal TSH levels, and abnormal TG were independent predictors for PTC group. Iodine status was not a risk factor in PTC group vs. benign thyroid nodule group (Table 1).
Indicators Univariate analysis Multivariate analysis OR 95% CI P value OR 95% CI P value Variable UIC (μg/L) 100–300 1.000 < 100 1.295 0.855–1.963 0.222 > 300 1.229 0.774–1.950 0.382 SIC (μg/L 45–90 1.000 < 45 1.295 0.932–1.799 0.124 > 90 1.142 0.605–2.156 0.683 Sex Male 1.000 Female 1.229 0.879–1.719 0.229 Age (years) ≥ 45 1.000 < 45 1.568 3.409–6.746 0.000* 4.970 2.990–6.854 0.000* Smoking history No 1.000 Yes 2.508 1.728–3.641 0.000* 3.287 1.359–4.259 0.003* Drinking history No 1.000 Yes 2.412 1.386–4.199 0.002* BMI (kg/m2) < 24 1.000 ≥ 24 20.904 0.000–999.000 0.991 Hypertension No 1.000 Yes 1.251 1.146–2.069 0.140 TSH Normal 1.000 Abnormal 1.777 1.080–2.922 0.024* 1.411 1.003–2.521 0.049* AITD No 1.000 Yes 1.402 0.972–2.061 0.071 TG Normal 1.000 Abnormal 1.599 1.094–2.337 0.015* 1.752 1.170–2.263 0.006* Note. UIC, urine iodine concentration. SIC, serum iodine concentration. Compared with the corresponding controls, there is significant difference (*P < 0.05). The variants with significant difference in the univariate analysis were put into the multivariate analysis. The adjusted model included age, gender, smoking history, drinking history, TSH level and TG level. Table 1. Risk factors in patients with papillary thyroid cancers
The risk of developing thyroid cancer < 45 years old was 4.970 times that of age ≥ 45 years, which is consistent with Haymart’s result, whose findings showed a higher incidence of < 45 years old component thyroid cancer[9]. Lu Lei’s study also showed that the incidence of thyroid cancer in people < 45 years old was higher than ≥ 45 years old, and the difference was statistically significant[10].
The relationship between PTC and smoking are controversial. Some scholars thought that smoking may reduce TSH levels, which can promote thyroid hyperplasia leading to thyroid cancer[11]. However, other researchers found that abnormal methylation of a smoking-related tumor suppressor gene, RARβ2, may be an important mechanism of thyroid canceration[12]. In our study, smoking was a risk factor for PTC. Further in-depth investigation are needed on their relationship in the future.
With respect to the relationship between abnormal TSH levels, especially higher TSH levels and PTC, recent studies have shown conflicting results. A meta-analysis published by Negro et al.[13] found no difference in the risk of thyroid cancer associated with TSH levels. Fighera et al.[14] found that TSH levels are associated with an increased risk of thyroid carcinoma in patients with nodular disease. More recently, a meta-analysis suggested that high serum TSH levels increased the PTC risk in a dose-dependent manner[14]. However, in the present study, we confirmed an association between TSH levels and malignancy, patients with abnormal TSH levels exhibited a significantly increased risk of malignant disease. We also found that abnormal TG levels were related with PTC, this was also proved by other epidemiological studies. Lee et al.[15] had indicated that preoperative TG levels had very high specificity in predicting thyroid cancer, it may be a useful marker for differentiating thyroid cancer from benign thyroid nodules in the cytological diagnosis of indeterminate nodules.
We compared the characteristics of PTC group according to their serum and urinary iodine level category (there were missing data in some pathological indicators due to the incomplete pathological reports before 2018 in the Third Affiliated Hospital of Harbin Medical University). According to the different serum level, there were significant differences among the patients and the corresponding control group in lymph node metastasis. According to the different urinary level, there were significant differences among the patients and the corresponding control group in lymph node metastasis, autoimmunity vs. without autoimmunity, abnormal TSH vs. normal TSH, abnormal TG vs. normal TG, bilateral vs. unbilateral, single lesion vs. multiple lesions (Tables 2-3).
Indexes N Serum iodine (μg/L) χ2 P < 45 45–90 > 90 Lymph node Metastasis 394 Yes 157 8 (5.1) 112 (71.3) 37 (23.6) No 237 22 (9.3) 180 (75.9) 35 (14.8) 6.45 0.04* Autoimmunity 652 Yes 166 25 (13.5) 118 (63.8) 42 (22.7) No 486 31 (6.4) 335 (69.2) 118 (24.4) 1.80 0.43 TSH 653 Abnormal 72 5 (6.9) 47 (65.3) 20 (27.8) Normal 581 33 (5.7) 408 (70.2) 140 (24.1) 0.76 0.69 TG 560 Abnormal 38 2 (5.3) 22 (57.9) 14 (36.8) Normal 522 31 (5.9) 368 (70.5) 123 (23.6) 3.39 0.18 Bilateral 449 Yes 128 10 (48.9) 93 (27.7) 25 (31.3) No 321 23 (51.1) 243 (72.3) 55 (68.7) 0.46 0.78 Lesions 420 Single 264 20 (64.5) 195 (62.5) 49 (63.6) Multiple 156 11 (35.5) 117 (37.5) 28 (36.4) 0.07 0.95 TNM stage 478 I/II 397 30 (88.2) 299 (84.7) 68 (74.7) III/IV 81 4 (11.8) 54 (15.3) 23 (25.3) 5.82 0.06 Note. Compared with the corresponding controls, there is significant difference (*P < 0.05). Table 2. Clinicopathological characteristics of patients with PTC according to the differences in serum iodine status
Indexes N Urine iodine (μg/L) χ2 P < 100 100–300 > 300 Lymph node metastasis 402 Yes 235 46 (39.7) 95 (67.4) 26 (50.0) No 167 70 (60.3) 46 (32.6) 26 (50.0) 6.27 0.04* Autoimmunity 653 Yes 166 57 (33.3) 84 (21.3) 25 (28.4) No 487 114 (66.7) 310 (78.7) 63 (71.6) 9.56 0.01* TSH 657 Abnormal 74 25 (33.8) 32 (43.2) 17 (23.0) Normal 583 148 (25.4) 362 (62.1) 73 (12.5) 10.89 0.00* TG 572 Abnormal 45 9 (20.0) 22 (48.9) 14 (31.1) Normal 527 138 (26.2) 318 (60.3) 71 (13.5) 10.21 0.01* Bilateral Yes 142 42 (29.6) 77 (54.2) 23 (16.2) No 327 100 (30.6) 201 (61.5) 26 (8.0) 7.35 0.02* Lesion 469 Single 268 83 (61.5) 167 (65.2) 18 (40.9) Multiple 167 52 (38.5) 89 (34.8) 26 (59.1) 9.40 0.01* TNM stage 491 I/II 416 125 (86.2) 245 (84.8) 46 (80.7) III/IV 75 20 (13.8) 44 (15.2) 11 (19.3) 0.96 0.64 Note. Compared with the corresponding controls, there is significant difference (*P < 0.05). Table 3. Clinicopathological characteristics of patients with PTC according to the differences in urinary iodine levels
In the current study, we found that the level of iodine is associated with several clinicopathological parameters of patients, such as TG-positive, lesion, abnormal TSH level, autoimmunity and lymph node. The underlying reason may be that excess iodine intake is a significant risk factor for the occurrence of BRAF mutations in the thyroid gland. Guan et al.[16] have shown that excess iodine intake might be a significant risk factor for the occurrence of BRAF mutation in the thyroid gland. Therefore, this may be a risk factor for the development of PTC. The study performed by Kim et al.[4] also indicated that iodine intake was a risk factor for BRAF mutations in papillary thyroid cancer patients in an iodine-replete area. BRAF mutation has a confirmed role in the maintenance and promotion of thyroid cancer progression, and it is associated with lymph node metastasis, advanced tumor stage, and other aggressive features. Choi et al.[17] have found that activation of the BRAF oncogene increased the expression of clinically useful predictive markers of multiple lymph node metastases in patients with BRAF V600E-positive PTC. A previous study has also confirmed the association of BRAF mutation with poorer clinicopathological outcomes of PTC. Therefore, we thought that high iodine intake may not play a role in the oncogenesis of PTC, it might be involved in PTC growth. It is noteworthy that the results regarding serum and urinary iodine levels were inconsistent. According to the different result between serum iodine and urinary iodine, the chief underlying reason may be that urinary iodine levels are a transient indicator and are relatively less stable than serum iodine levels. Furthermore, although there was a strong correlation between serum and urinary iodine levels, these two indicators may sometimes indicate different conditions.
The main limitation of this study is that it was performed in an iodine supplementation area of China. Although all the subjects consumed iodized salt and did not change their eating habits before the collection of blood and urine, multi-center studies are still needed to validate our findings.
In sum, we found that relatively high iodine intakes were significantly related with thyroid disease. However, it was not an independent risk factor for PTC patients as compared to benign thyroid nodules patients. Although iodine intakes did not directly influence the tumorigenesis of PTC, the level of iodine is associated with the development of PTC and its aggressiveness. Our data demonstrated that it is important to achieve adequate iodine intake to meet the normal nutritional need of the human body while avoiding insufficient or excess supplementation of iodine not only in control group, but also in PTC patients.
Strong Correlation of Abnormal Serum and Urinary Iodine Levels with Papillary Thyroid Cancer: A Case-control Study
doi: 10.3967/bes2020.009
- Received Date: 2019-07-04
- Accepted Date: 2019-12-23
| Citation: | XIU Cheng, HE Qian, ZHAO Hong Jian, YUAN Zhen Nan, GUO Lun Hua, WANG Feng Qian, YANG Xian Guang, TIAN Qiu Shi, SUN Qi Hao, MIAO Su Sheng, SUN Ji, FAN Li Jun, JIA Shen Shan. Strong Correlation of Abnormal Serum and Urinary Iodine Levels with Papillary Thyroid Cancer: A Case-control Study[J]. Biomedical and Environmental Sciences, 2020, 33(1): 62-67. doi: 10.3967/bes2020.009 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: