-
Since the bioeffects of ultrasound were discovered, its use has become widespread in the field of therapeutics [1]. Two classes of ultrasound bioeffects on tissues and cells are known: thermal and non-thermal [2]. Ultrasound exhibiting thermal bioeffects is primarily applied to cancer and tumor ablation for cell necrosis [3]. Ultrasound displaying non-thermal bioeffects is generally adapted for tendon healing, bone regeneration, and angiogenesis via cell proliferation and differentiation [4]. These bioeffects of ultrasound are closely related to ultrasound parameters. Among these influential factors, the ultrasound frequency, energy emission, and stimulation duration play important roles [5].
The frequency used in therapeutic ultrasound was reported to be generally no more than 10 MHz because of the limit of cell absorption and ultrasound attenuation[6]. An ultrasound transducer of 2.5 MHz, which is well tolerated and accessible was applied in this study. At 2.5 MHz, therapeutic ultrasound is considered as high-frequency ultrasound. High-frequency ultrasound has higher energy concentration, directivity, and resolution. A linear-focused ultrasound field can make up in attenuation for its positive concentration characteristics. Ultrasound with high energy emission is broadly reported to kill cells via thermal bioeffects. To prevent cells from burning to death, low-energy emission was applied in this study. With the attenuation of high-frequency ultrasound, continuous stimulation ultrasound was imposed on cells.
Human umbilical vein endothelial cells (HUVECs) are widely used in research for their accessible and robust activity. Moreover, protocol and materials for HUVEC culture are well established. Quantities of HUVECs are distributed to the important parts of human organs and tissues. Therefore, this study makes use of cultured HUVECs.
Ultrasound stimulation can influence the proliferation and morphology of HUVECs via complex mechanisms. However, it is unclear whether the heat or the mechanical mechanism plays the dominant role in these effects. In addition, a paucity of research has addressed the effects of high-frequency ultrasound with variable emission energies on cells. Research about the effect distribution on cells by linear-focused ultrasound remains lacking. As a supplement, this study explored the modification effect of high-frequency, low-energy, linear-focused ultrasound (HFLELFUS) at 2.5 MHz with different emission energies on the proliferation and morphology of HUVECs and the relationship between the distribution of these effects and the linear-focused ultrasound field.
Cells were isolated from the original human umbilical vein endothelial cell strain within 4 decades via 0.25% Trypsin (Gibco, New York, USA). The original cell strain was initially labeled with Green fluorescent protein GFP488. Cells were seed in sterile Petri dishes (Jet, Shanghai, China). Cells were cultured with sterile Culture medium DMEM/High glucose (HyClone, Utah, USA) consisting of 4.00 mL glutamine, 4,500 mg/L glucose, and sodium pyruvate. The cell density calculated using a hemocytometer (Jiangsu, China) was 8 × 104 cells/mL.
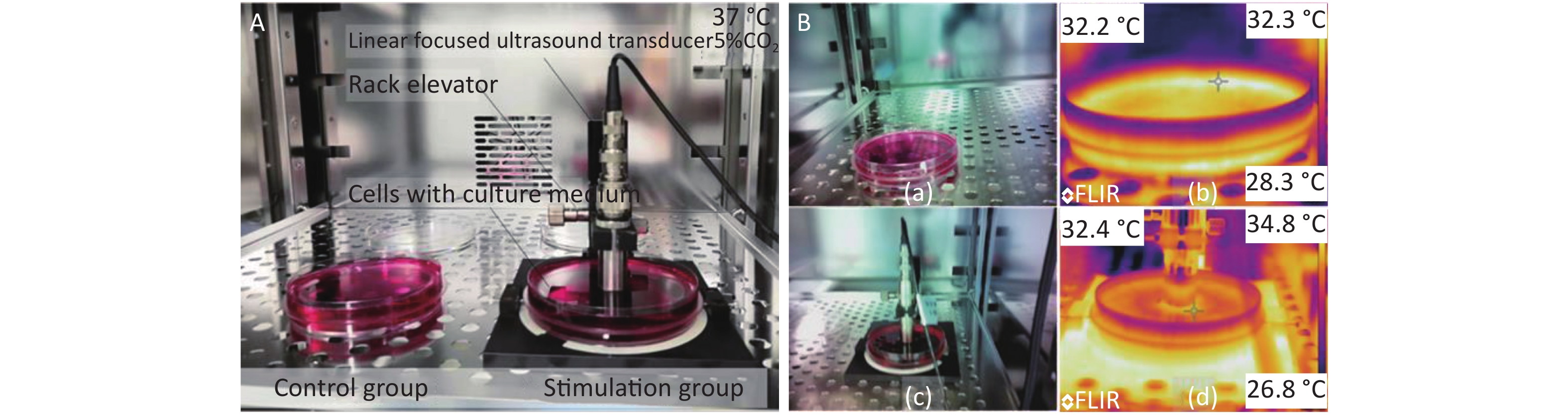
The ultrasound transducer with a frequency of 2.5 MHz was mounted on a self-made fixture to impose HFLELFUS on cells (Figure 1A). A pulsed ultrasound transducer and receiver setting of 1 kHz repeat frequency and 50 Ohm coupled resistance was used to drive the ultrasound transducer to generate an ultrasonic field. The ultrasound field produced from the ultrasound transducer was linear-focused. The distance between the ultrasound transducer head and the cells was adjusted via this fixture to ensure that the cells remained in the focal plane. An oscilloscope was used to display whether the cells were in the focal plane though the first echo. Cell proliferation and morphology were regulated by changing the emission energy.
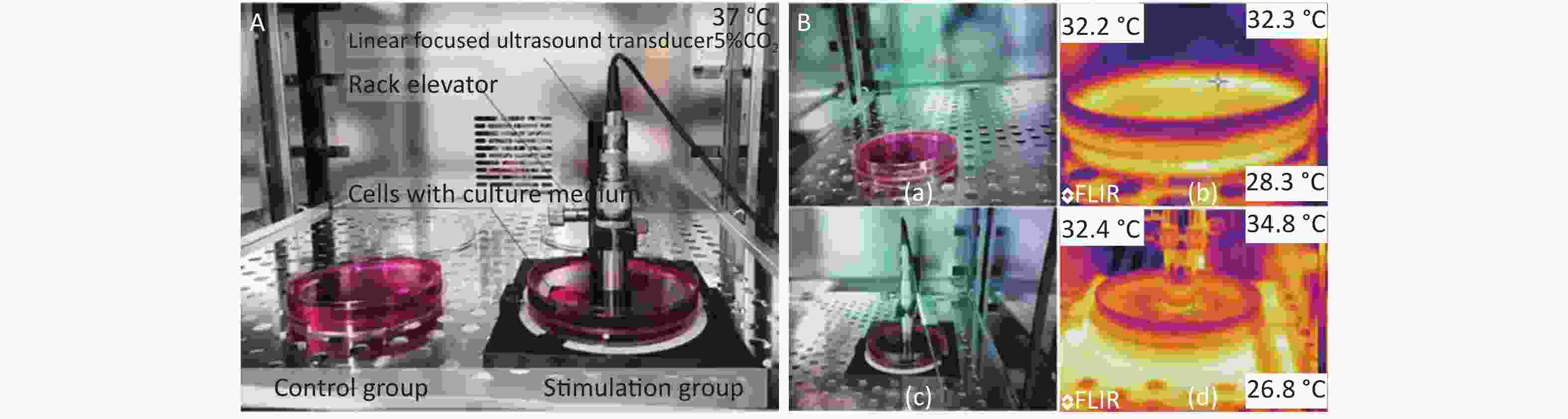
Figure 1. Experimental modification of cells with high-frequency, low-energy, linear-focused ultrasound (HFLELFUS). (A) Cells in the stimulation groups were continuously exposed to HFLELFUS while cells in the control groups were open to air. All cells were cultured in incubator at 37 °C with 5% CO2. (B) After 48 h in culture, the largest difference in temperature between the stimulated and control groups was observed. The temperature of the control group is shown in figure 1 (Bb). The highest temperature of the stimulation group is displayed in figure 1 (Bb).
Cells were divided into control groups and stimulation groups (1, 2, 4, 8, 16, and 32 μJ). The quantity of cells in each petri dish was 4 × 105 cells. Each group was replicated three times. Cells were cultured in an incubator (Olympus Japan) at 37 ℃ in 5% CO2. In the stimulation groups, cells were continuously exposed to linear-focused ultrasound for 48 h. Meanwhile, cells in the control groups were exposed to air.
In the initial state, the quantity of cells was detected by an inverted light microscope (Leica, USA). After 48 h in culture, cells fixed in 4% polyformaldehyde (BioSharp, Anhui, China) were detected by confocal fluorescence microscopy (Olympus, Japan). The temperature of cell culture in groups was detected with an infrared thermal imager (FLIR 600, USA). The resolution power of this infrared thermal imager was 0.1 °C. Images that recorded the quantity and morphology of cells were analyzed in the software ImageJ to calculate the proliferation rate and identify changes in cell morphology.
The results were expressed as mean ± SEM (standard error of the mean). Differences between the stimulation group and control group were assessed with the Student’s t-test. Statistical significance was established at a value of P < 0.01[7].
The initial real temperature in all groups was 25.4 ℃. At the end of the experiment, the difference in temperature between groups was no more than 0.2 ℃ The highest temperature was 32.4 ℃, observed in the group stimulated with 32 μJ, which was 0.2 ℃ higher than the temperature of the control groups (Figure 1B). No significant difference was demonstrated in the temperature of the groups. We therefore concluded that the influence of ultrasound on the cells was via a mechanical mechanism.
All culture media were from the same source. At the end of the experiment, the difference in temperature between groups was narrow. The highest temperature was 32.4 ℃, observed in the group stimulated with 32 uJ, which was 0.2 ℃ higher than that in the control groups (Figure 1B).
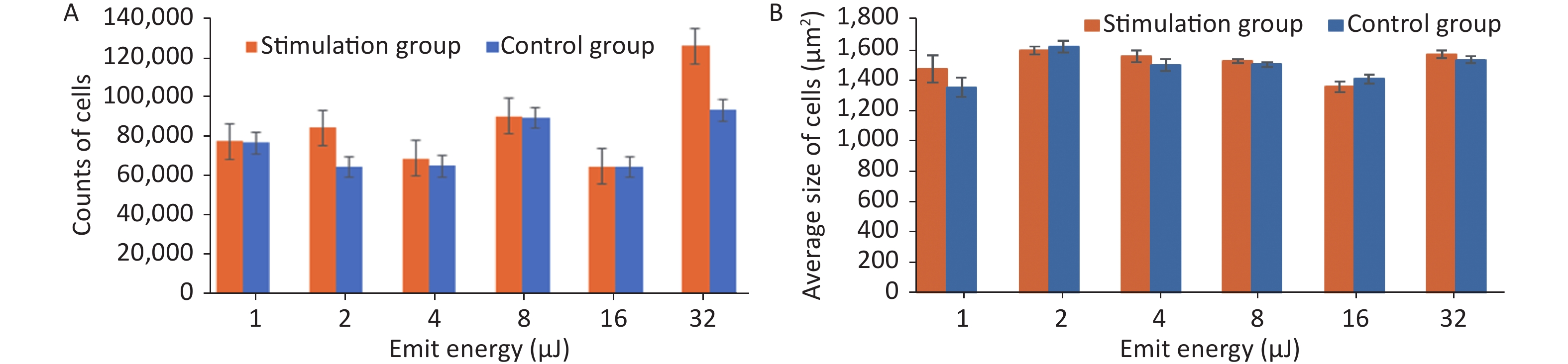
Stimulation groups generally contained higher cell numbers than the corresponding control groups as shown in Table 1. The largest proliferation rate of 35.08% ± 0.35% was achieved when cells were exposed to HFLELFUS with an emission energy of 32 μJ (Figure 2A). Morphological changes in HUVECs were implied by average size. The largest difference between the two groups appeared in the experiment with 1 μJ (Figure 2B). The cells in groups stimulated with 32 μJ emission energy were not only more numerous but also larger than those in control groups.
Emit energy (μJ) Proliferation in stimulation group (cells) Proliferation in control group (cells) Proliferation rate (%) 1 74,424 ± 26 73,588 ± 20 1.14 ± 0.30 2 81,163 ± 20 62,043 ± 28 30.82 ± 0.40 4 66,122 ± 28 62,253 ± 20 6.21 ± 0.40 8 86,880 ± 31 85,938 ± 26 1.14 ± 0.19 16 62,000 ± 26 61,980 ± 18 0.03 ± 0.44 32 121,148 ± 35 89,684 ± 26 35.08 ± 0.35 Table 1. Proliferation in both groups and subsequent proliferation rate
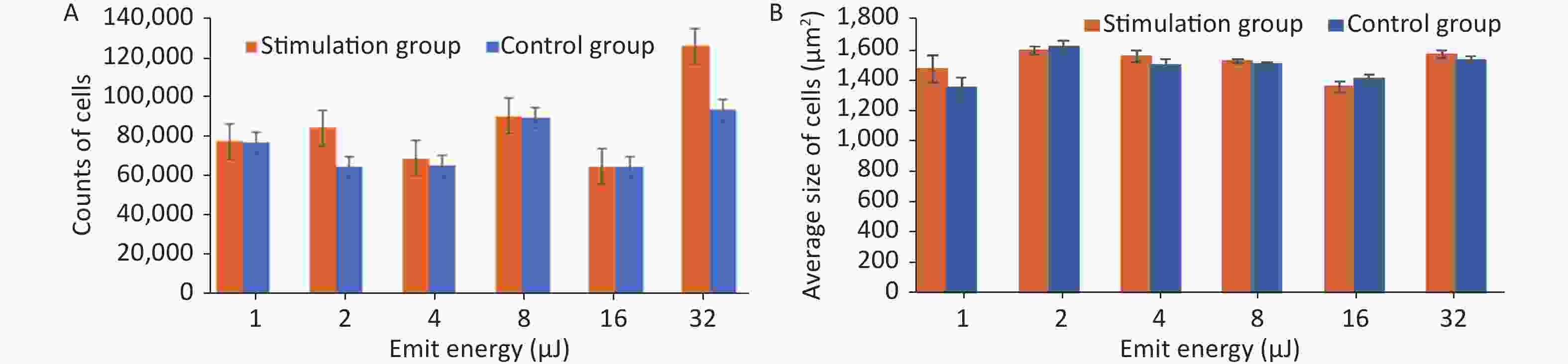
Figure 2. The quantity and average size of cells in the stimulation and control groups were calculated and compared. (A) Average number of cells in the experiment with emission energy ranging from 1 to 32 uJ. (B) Average size of cells in the experiment with emission energy ranging from 1 to 32 uJ. Statistical significance was established at a value of P < 0.01.
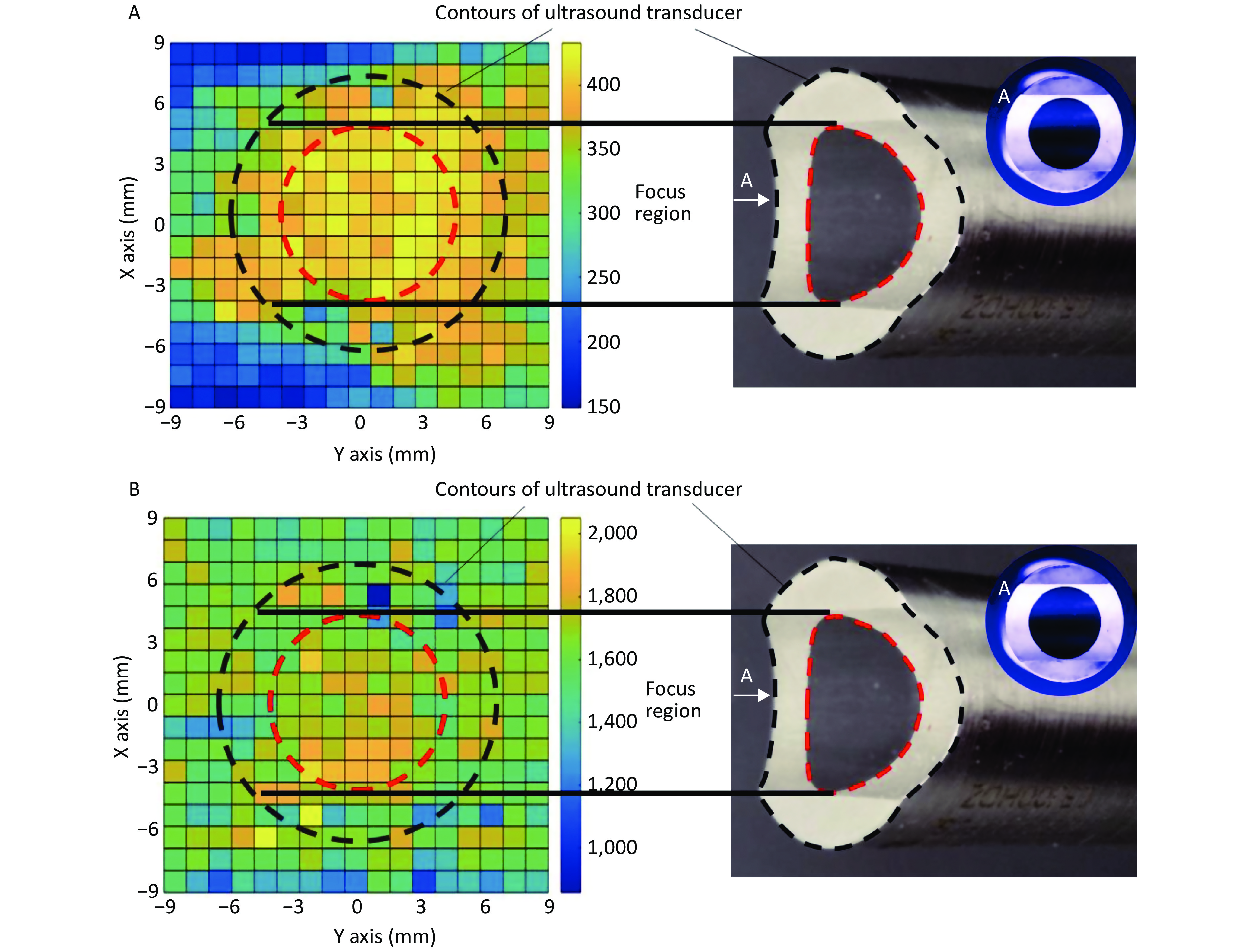
In the focal plane, the power of the linear-focused ultrasound field decreased with increasing distance from the focused line. Cells in the focused region of HFLELFUS are obviously more numerous than in other locations (Figure 3A). Cells with a large average size were mostly distributed in the focused region under the ultrasound transducer (Figure 3B). A general region standing for high proliferation was displayed in the proliferation distribution figures. Our results verify that the modifying effect of HFLELFUS on cell proliferation and morphology is positively related to the distribution of the ultrasound field.
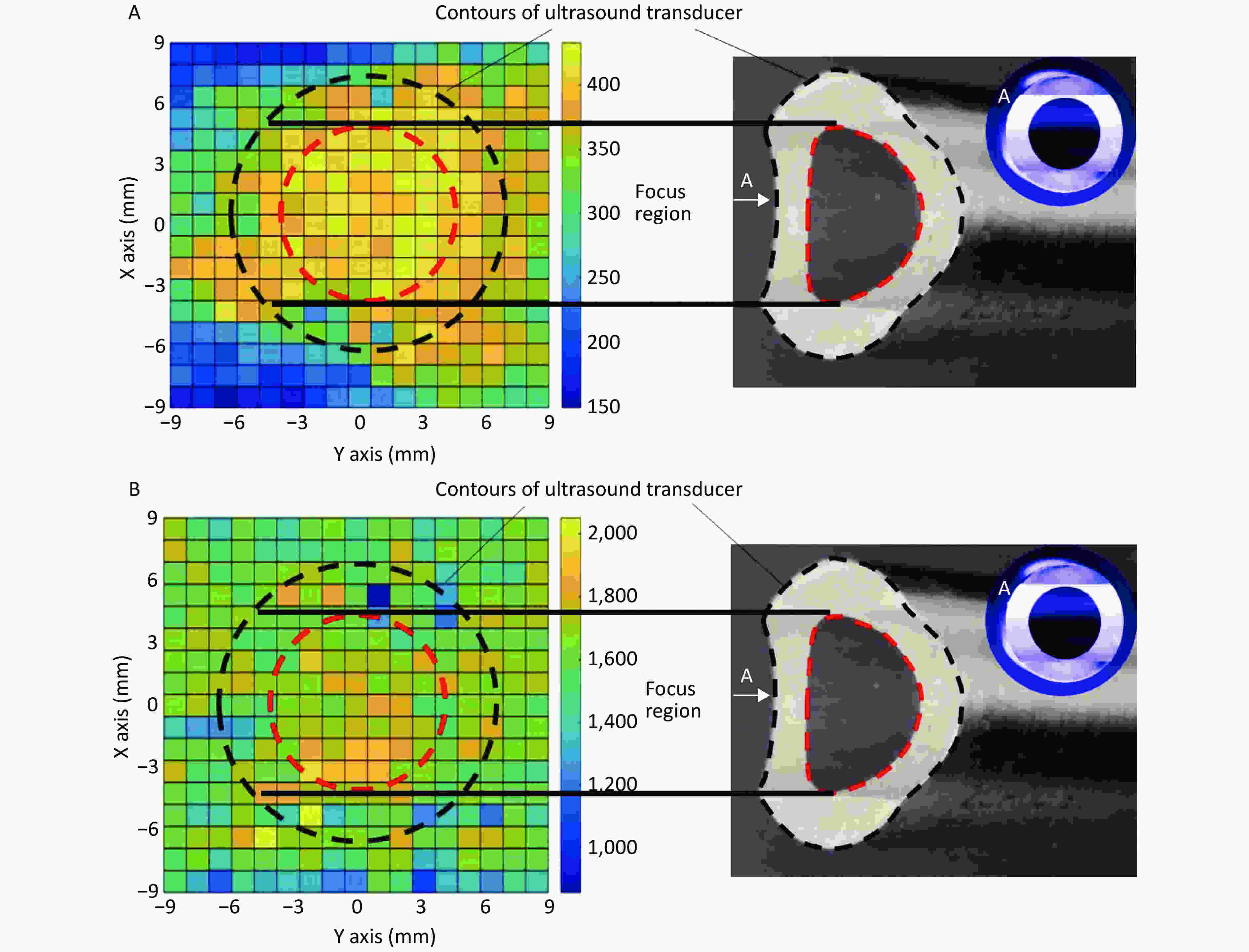
Figure 3. Relationship between modifying effects of high-frequency, low-energy, linear-focused ultrasound (HFLELFUS) on proliferation and cell morphology with a linear-focused ultrasound field. (A) Distribution of modifying effects on cell proliferation. (B) Distribution of modifying effects on cell morphology.
Previous studies reported that cell culture played an important role in tissue engineering via 3D bioprinting [8]. Ultrasound can change the proliferation, differentiation, and morphology of cells via bioeffects. The mechanical mechanism behind these bioeffects is complex. In our work, the mechanical mechanism is primarily the bioeffects of HFLELFUS in this study on HUVECs. HFLELFUS promotes not only proliferation but also increased cell size in HUVECs. Proliferation reached its highest rate in the focused line of HFLELFUS, indicating that the effect of ultrasound on cells can be adjusted via structure.
In previous studies, the frequency of ultrasound applied in therapy mainly ranged from 0.1 to 1.5 MHz [9]. In this study, HFLELFUS at 2.5 MHz was applied for better directivity and resolution. In previous studies, cells or tissues were stimulated discontinuously for a couple of weeks. In this study, the modifying effects of HFLELFUS on cells were observed in 48 h with continuous irradiation. Significant effects appeared when the emission energy was 32 μJ. In our works, the results manifest that the modifying effects of HFLELFUS on cells can be optimized by adjusting the emission energy. Linear-focused ultrasound stimulates the target position of objects more precisely. HFLELFUS in this study enhanced the proliferation of HUVECs, indicating that HFLELFUS could be applied for cell culture in tissue repair via 3D bioprinting. Instructive usage existed in achieving the anisotropy of tissues.
In summary, this study demonstrated that HFLELFUS at 2.5 MHz with continuous stimulation can modify cell proliferation and morphology in vitro. The effects can be optimized by adjusting the emission energy. More direct and precise target stimulation can be achieved through linear-focused ultrasound. These results imply the potential utility of HFLELFUS in 3D bioprinting and in achieving anisotropy in tissue engineering. Further research on the modifying effects of HFLELFUS on cell culture process in 3D bioprinting will be required to explore the potential of HFLELFUS as a means to accelerate tissue transplant. The authors would like to acknowledge the Testing Center in Suzhou Intelligent Manufacturing Research Institute for providing laser scanning confocal microscopy and mechanical testing
Modification of Proliferation and Morphology in Human Umbilical Vein Endothelial Cells via in vitro High-Frequency, Low-Energy, Linear-Focused, Continuous Ultrasound Stimulation
doi: 10.3967/bes2020.096
- Received Date: 2019-09-17
- Accepted Date: 2020-03-30
| Citation: | SUN An Yu, LAI Jiao Jiao, DU Hui Lin. Modification of Proliferation and Morphology in Human Umbilical Vein Endothelial Cells via in vitro High-Frequency, Low-Energy, Linear-Focused, Continuous Ultrasound Stimulation[J]. Biomedical and Environmental Sciences, 2020, 33(9): 727-730. doi: 10.3967/bes2020.096 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: