-
In 2018, the World Health Organization (WHO) reported 9.6 million cancer deaths worldwide, in accordance with the data collected by the International Agency for Research on Cancer (IARC). The fight against cancer and other non-communicable diseases is now a global priority, with cancer being a primary priority. Cancer prevention reportedly has the potential to reduce the global cancer burden[1]. This is promising as cancer is among the first two causes of premature death in 91 countries. Cancer is caused by an excessive transformation and proliferation of cells, forming a malignant tumor. These cells may then metastasize by invading neighboring tissues, and migrating through blood and lymphatic vessels, thereby forming other tumors. Numerous strategies, including surgery, radiotherapy, immunotherapy, and chemotherapy, may be used to treat cancer[2].
Chemotherapy involves the use of chemicals to destroy cancer cells or prevent their multiplication. There are many chemotherapy drugs, often combined to increase treatment effectiveness. These drugs can be administered via infusion, injection, or orally. Though chemotherapy drugs affect cancer cells, their non-specific effect results in them affecting non-cancerous cells that divide quickly, which may cause side effects such as nausea, vomiting, hair loss, and fatigue. According to the National Institute for Occupational Safety and Health (NIOSH), antineoplastic drugs used in chemotherapy are defined as hazardous drugs[3]. Since occupational exposure to antineoplastic agents is reportedly potentially risky to exposed workers, various groups, institutions, and agencies have published guidelines or recommendations for handling these agents. A limited number of studies have examined the chronic health risks associated with occupational exposure to antineoplastic agents[4]. However, the chronic effects in patients treated with these agents are well documented. Their chronic effects reportedly include infertility, mainly in female nurses, and cancer[3].
Parenteral chemotherapy requires preparation in aseptic conditions to yield a final sterile product. To minimize exposure risk, specific policies and procedures are implemented in all departments involved in antineoplastic drug use such as the pharmacy, clinical services, and during their transportation, storage, and distribution[5].
Regarding the risk associated with cytotoxic drug handling, we decided to investigate environmental cytotoxic contamination and evaluate the healthcare worker risk associated with the chemotherapy drug administration process, based on Failure Modes, and Effects and Critical Analysis (FMECA) for identifying chemotherapy process failures before incident occurrence[6]. To compare the exposure risk of healthcare workers, the situations in two teaching hospitals in Tunisia and France were compared.
The study was performed in 2018 in the pharmacy and oncology departments of two teaching hospitals in Tunisia and France.
In Tunisia, the University Hospital Centre of Habib Bourguiba of Sfax, a teaching hospital with 520 treatment beds (including chemotherapy treatment), 20 inpatient beds, and 14 outpatient beds or armchairs, was involved. In public hospitals, drug treatments are dispensed by the hospital pharmacy for needy patients, and by the social insurance before hospitalization for other patients. Drugs are provided directly to the patient or their family, then stored in their premises and brought to them on the treatment day. All treatments are extemporaneously prepared by nurses in a laminar flow cabinet in the oncology department, using syringes and needles.
In France, the European Georges Pompidou Hospital (814 beds, 43 clinical units), a part of the Paris West Hospitals Group of AP-HP (Public Assistance-Paris Hospitals) in which the oncology activity is distributed amongst nine oncology units with 91% of the production done in the outpatient care unit, including 39 beds or armchairs, was involved. Antineoplastic drugs are stored and handled by pharmacy staff in a centralized chemotherapy production unit in the hospital pharmacy, using secure preparation and administration transfer devices. After reception of a computerized prescription, it is then processed by the pharmacy technicians, and the final chemotherapy products are dispensed and transferred to the patient care unit for administration by nurses.
The number of preparations were estimated to be 20,000 and 32,000 per year for the Tunisian and French hospitals, respectively.
Briefly, platinum was used as a tracer of residual chemical contamination. Samples were collected from workplace surfaces of the different areas involved in the chemotherapy process (medical prescription reception to preparation administration), using a representative and standardized sampling protocol (100 cm² per sample). Areas were delimited as preparation and administration areas. We then defined three preparation (handling area, drug storage, and preparation room) and four administration (patient reception room, nurse workstation, beds/armchairs, and toilets) areas.
Sampling was performed at the end of the working day, after the daily cleaning, in accordance with the sampling protocol described by Chappuy et al.[7], consisting of the wiping of a defined 10 cm × 10 cm surface with a moistened swab (the paddle of the swab is moistened with 200 μL of water). If it is impossible to collect the sample from a 10 cm ×10 cm surface, the whole surface is sampled. The head paddle is then placed into a conical tube, desorption is performed by adding 10 mL of water, the tube is vortexed for 30 s, and the swab is removed. After pre-concentration by the cloud point method, samples were analyzed by graphite furnace atomic absorption spectrophotometry using a validated method with a low limit of detection (LLOD) and low limit of quantification (LLOQ) of 0.02 and 0.06 ng/cm2, respectively[7].
The mean contaminations in the drug preparation and administration areas of both hospitals were compared using the Welch two sample t-tests. Contaminated sample rates were compared using the Pearson's Chi-squared test with Yates' continuity correction and Fisher's exact test. Statistical analyses were performed online[8].
The potential exposure risk of healthcare workers during the chemotherapy process was assessed for both hospitals using a previously published risk analysis method, based on Failure Modes and Effects and Critical Analysis (FMECA)[6]. According to this approach, one situation at risk called ‘potential failure modes’ was considered for each healthcare worker at each step of the process. The risk of each failure mode was quantified by the risk priority number (RPN) obtained by multiplying the four factors (G × O × E × A: where, G is the gravity of the failure, O its potential occurrence, E the exposure of each worker to this failure mode, and A the possibility of avoidance before failure occurrence).
A total of 297 samples (116, Tunisia; 181, France) were collected from both hospitals with significant proportions of contaminated samples between the two hospitals (P < 0.0001) and mean contamination (P = 0.0003).
The mean contamination were 3.74 ng/cm2 and 0.2 ng/cm2 for the Tunisian and French hospitals, respectively, both higher than 0.1 ng/cm2, the limit fixed by Kiffmeyer et al.[9].
The proportion of contaminated samples and mean contamination in each area are detailed in the Table 1.
Item Tunisian hospital French hospital Test No. samples Samples >
LLOD (%)Mean ± SD
Samples >
LLODNo. samples Samples >
LLOD (%)Mean ± SD
Samples > LLODSamples >
LLOD (%)Mean ± SD
Samples > LLODPatient reception room 14 29 < LLOQ 18 0 < LLOD NA NA Drug storage 5 20 < LLOQ 8 0 < LLOD NA NA Preparation area 23 83 0.56 ± 1.37 13 15 0.20 ± 0.10 P = 0.0003**
P < 0.05* Handling area 34 94 7.63 ± 10.94 12 25 0.38 ± 0.07 P = 0.0001*** P = 0.0007* Nurse workstation 12 67 2.92 ± 7.58 72 14 0.20 ± 0.12 P = 0.0005*** P > 0.05* Beds/armchairs 20 60 1.09 ± 2.69 42 14 < LLOQ P = 0.0007** P > 0.05* Toilet 8 25 0.13 ± 0.05 16 63 < LLOQ P > 0.05*** P > 0.05* Total 116 67 3.74 ± 8.12 181 10 0.20 ± 0.14 P < 0.0001** P = 0.0003* Note. LLOD: low limit of detection, LLOQ: low limit of quantification, NA: not applicable, *Welch two sample t-test, **Pearson's Chi-squared test with Yates’ continuity correction, ***Fisher’s exact test. Table 1. Mean contamination (ng/cm2) of samples with residual contamination higher than LLOD and percent of samples (%) with residual contamination higher than LLOD on workplace surfaces in the different area involved in the chemotherapy process from the reception of drugs to the administration of the final product
In Tunisia, the maximal contamination was found inside the laminar flow cabinet used to prepare chemotherapy treatments (37.77 ng/cm2), while in France, it was found on nurse workstation surfaces (0.46 ng/cm2).
Environmental cytotoxic contamination investigations showed differences between the practices in the Tunisian and French hospitals. In France, chemotherapy preparation is centralized in the pharmaceutical unit and produced in isolators by qualified pharmacy technicians under the pharmaceutical responsibility and aseptic conditions verified regularly by operational qualification. Meanwhile, in Tunisia, drugs are prepared directly in the oncology unit by nurses independently of the pharmaceutical department, using limited resources such unsuitable or insufficient material and dysfunctional cytotoxicity protection equipment, which impact final product quality and patient safety, by increasing healthcare worker occupational exposure risk.
The potential occupational exposure risk was assessed for each potential failure mode by the RPN (Figure 1) and classed as ‘acceptable’ situations (RPN ≤ 74), ‘tolerable under control situations’ (74 < RPN ≤ 194), or ‘unacceptable’ situations (RPN > 194).
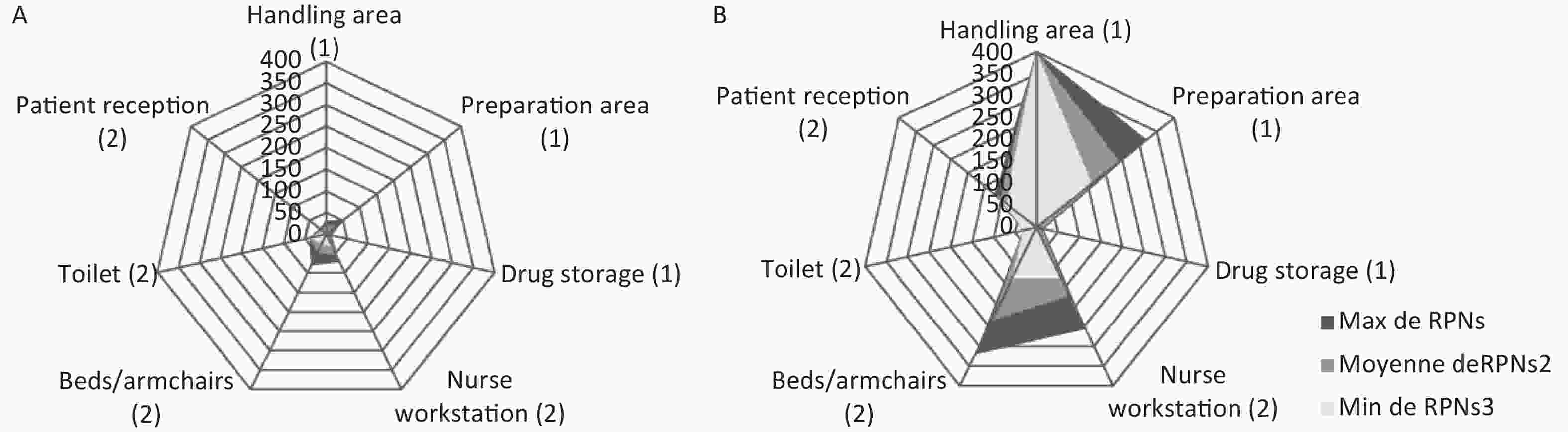
Figure 1. Kiviat diagrams representing the minimal (RPN min), mean (RPN mean) and maximal (RPN max) for failure modes identified in the chemotherapy process in a French (A) and a Tunisian (B) teaching hospitals (1 for preparation steps and 2 for administration steps)
Mean RPNs (30 in France versus 150 in Tunisia) were significantly different (P < 0.0001), with mean RPNs of 20 in France and 152 in Tunisia for the three steps of chemotherapy preparation, and mean RPNs of 40 in France and 149 in Tunisia for the four administration steps.
Despite a maximal RPN of 80 for family members during administration, all areas of preparation and administration were associated with acceptable risk of exposure. On the contrary, in the Tunisian hospital, results highlighted four areas associated with tolerable under control situations: two areas of preparation (handling and preparation areas) and two areas of administration (patient reception and nurse workstation). Only one area was associated with unacceptable risk: bed and armchairs.
The acceptable calculated risk of exposure for family members in the Tunisian hospital is underestimated because we did not include the steps of health insurance drug delivery, risk could be highly risky; family members could be directly exposed to cytotoxic drugs in cases of drug damage.
Economic and health development have considerably increased in Tunisia lately. Tunisia is in its third cancer control plan; the first and second were in the 2006–2010 and 2010–2014 periods, respectively. Despite the previous plans and trained cancer prevention and management personnel (top medical skills and paramedical services), oncological care remains suboptimal.
In France and over the last twenty years, chemotherapy drug preparation has gradually become centralization under pharmaceutical supervision in specific controlled areas to ensure better protection of healthcare workers handling cytotoxic drugs and improve patient safety against drug toxicity[5]. Chemotherapy treatments are prepared in collective and protective equipment such as vertical laminar flow cabinets or isolators. Despite the large awareness in high-income countries, the progress in others in low, with most patients being unable to access essential cancer services. In Tunisia, patients benefit from financial coverage for most cancer treatment protocols. However, the quality of care remains limited, with a significant exposure risk for healthcare workers, families, and the environment.
Furthermore, numerous studies have explored contamination, and have contributed to the sensitization of pharmaceutical and medical staffs in France to the occupational risk associated with cytotoxic drug preparation and administration[7,10,11]. In Tunisia, practices are more heterogeneous and only two Tunisian teaching hospitals have centralized cytotoxic drug preparation to the pharmaceutical unit. At present, no regulatory text details specifications for anticancer drug handling and preparation.
Our study is the first to investigate cytotoxic drug contamination in developing countries, elucidating the high exposure risk for healthcare workers. In this context, the availability of cancer treatments in Tunisia must be accompanied by measures to ensure optimal patient care and staff safety, and this study should encourage the establishment of regulatory texts to improve practices by public health authorities.
Acknowledgements This original article is part of a cooperative project between French and Tunisian hospitals financed by the French Ministry of Health, and is aimed at promoting international hospital cooperation projects for strengthening the working partnerships of their medical teams (administrative or technical) with foreign counterparts. The manuscript was written, revised, and approved by all authors.
Antineoplastic Drug Handling: Higher Risk for Healthcare Workers in Tunisia than in France?
doi: 10.3967/bes2020.108
- Received Date: 2019-10-26
- Accepted Date: 2020-07-22
| Citation: | Kaouther Zribi, Laetitia Minh Mai Le, Asok Rajkumar, Hail Aboudagga, Mounir Frikha, Amandine Dietrich, Emna Zribi, Fathi Safta, Eric Caudron. Antineoplastic Drug Handling: Higher Risk for Healthcare Workers in Tunisia than in France?[J]. Biomedical and Environmental Sciences, 2020, 33(10): 803-806. doi: 10.3967/bes2020.108 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: