-
The dynamic assembly of microtubules plays a key role in maintaining structural and functional integrity of eukaryotic cells, especially with regard to neuronal differentiation or neurite outgrowth and synaptic plasticity, which contribute to the development of the nervous system and memory formation. Phosphorylation and dephosphorylation of microtubule-associated proteins (MAPs) are processes known to control the dynamic organization of the microtubule cytoskeleton. Accumulated evidence on hyperphosphorylation of tau protein, a MAP, indicates that it results in tau detachment from microtubules. This in turn impairs neurite outgrowth and induces synaptic atrophy through aggregation and formation of paired helical filaments, which are major components of neurofibrillary tangles (NFTs), the pathological hallmark features of Alzheimer’s disease (AD)[1]. A serine residue at position 262 (Ser262) of the tau protein is particularly noteworthy, since its phosphorylation can stabilize free tau and initiate phosphorylation of other sites, further mediating Aβ 42-induced tau toxicity and neurodegeneration[2, 3]. The phosphoprotein phosphatase PP2A is responsible for the dephosphorylation of tau, and a decrease in its expression and activity might contribute to tau hyperphosphorylation, and thus to the impairment of neurite outgrowth in the neurons of AD patients[4]. This phenomenon can also be reproduced in vitro using a PP2A inhibitor okadaic acid (OA)[5]. Previous studies have demonstrated that safflower yellow, a phenolic chalcone constituent of safflower (Carthamus tinctorius L.) mainly containing hydroxy safflower yellow A (HSYA) and safflower yellow A (SYA), markedly alleviates cognition impairment by reducing tau hyperphosphorylation in AD mice[6]. However, the active compound of safflower yellow responsible for neurite injury alleviation is still unknown. In this study, we investigated the effects of HSYA and SYA on neurite outgrowth impairment induced by OA in all-trans retinoic acid (ATRA)-differentiated SH-SY5Y cells, as well as the underlying mechanisms.
We used preparative HPLC to obtain HSYA and SYA with high purities of > 98.5%. OA, ATRA, methyl thiazolyl tetrazolium (MTT), 2’,7’-dicloro fluorescente amarelo-bis-acetato (DCFH-DA), MitoSOX, and Mito-TEMPO were purchased from J&K Scientific Ltd. (Beijing, China). Neurite outgrowth impairment induced by OA in differentiated SH-SY5Y neuroblastoma cells is an ideal in vitro model that mimics early pathological changes in cholinergic neurons of individuals with AD[7]. In this study, we investigated the effects of HSYA and SYA on OA-induced neurite atrophy and hyperphosphorylation of tau at Ser262 (p-Tau262) in ATRA-differentiated SH-SY5Y cells. Additionally, intracellular reactive oxygen species (ROS) levels and the mitochondrial membrane potential (MMP) were determined to reveal the underlying mechanisms of action.
The neuroblastoma SH-SY5Y cell line was obtained from the China Center for Type Culture Collection (Wuhan, China) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 100 IU/mL penicillin, 100 μg/mL streptomycin (basic medium, BM), and 10% fetal bovine serum (FBS; Gibco) at 37 °C in an atmosphere of 5% CO2 and 95% air. Cell differentiation was induced by incubation with 5.0 μmol/L ATRA in DMEM containing 1% FBS for 4 days[8]. Neurite outgrowth impairment was induced in differentiated SH-SY5Y cells by addition of fresh medium containing 1% FBS and 40 nmol/L OA for 6 hours, and subsequently observed using an inverted microscope (Nikon Eclipse TE300, Japan) after Giemsa staining. Cell body diameter and neurite length were analyzed using Image Pro Plus 6.0 software. Neurite outgrowth was analyzed by determining the ratio of total neurite length to cell body diameter for 300 cells, each indicated by a single data point[9].
Cell cycle distribution analysis was performed using flow cytometry (ACEA NovoCyte, China) after staining with 50 μg/mL propidium iodide. The effects of HSYA and SYA on cell viability were measured using the MTT assay 24 hours after exposure. Total tau protein (Tau-5) and p-Tau262 levels were evaluated by western blotting with the respective primary antibodies (anti-Tau-5 and anti-p-Tau262, Abcam). Total intracellular ROS (tROS) and mitochondrial ROS (mtROS) levels were monitored using specific fluorescent probes, DCFH-DA (10.0 μmol/L) and Mito-SOX (5.0 μmol/L), respectively, followed by flow cytometry. The mitochondria-targeted antioxidant Mito-TEMPO (1.0 μmol/L) was used as a positive control. The MMP was monitored using flow cytometry after staining with JC-1.
All data are expressed as mean ± standard deviation (SD) from three repetitions, with three parallel treatments per experiment. Data were analyzed using one-way ANOVA or non-paired Student’s t-test using SPSS 18.0 software (SPSS Inc., Chicago, IL., USA). Differences were considered statistically significant at a P < 0.05.
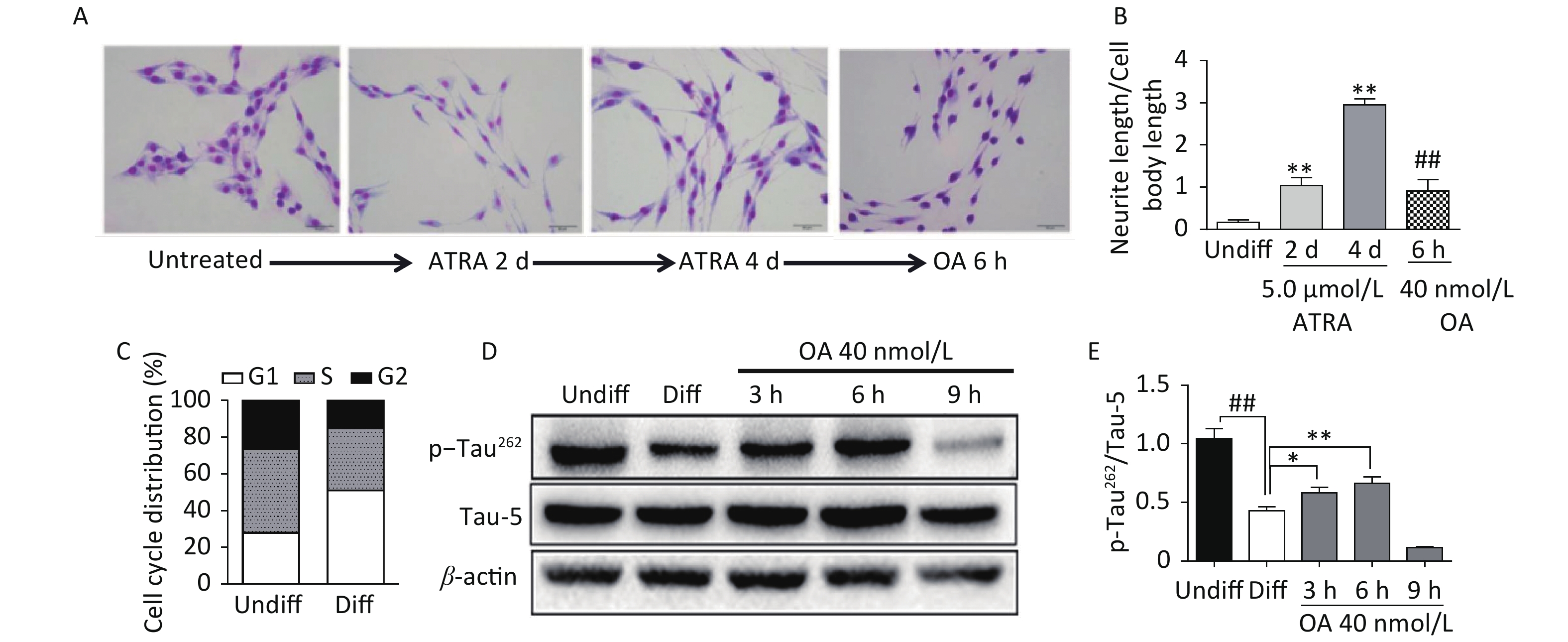
Exposure to ATRA (5.0 μmol/L) for 4 days resulted in significant differentiation of SH-SY5Y cells, which was characterized by neurite outgrowth and elongation (Figure 1A and B), and G1 phase arrest (Figure 1C). Further exposure to 40 nmol/L OA significantly induced neurite atrophy in differentiated SH-SY5Y cells (Figure 1A and B). No significant change in Tau-5 expression was found during SH-SY5Y cell differentiation, while p-Tau262 levels were markedly decreased (Figure 1D and E). Conversely, OA exposure for 6 hours led to significantly upregulated p-Tau262 levels, which were markedly decreased after 9 hours of exposure (Figure 1D and E).
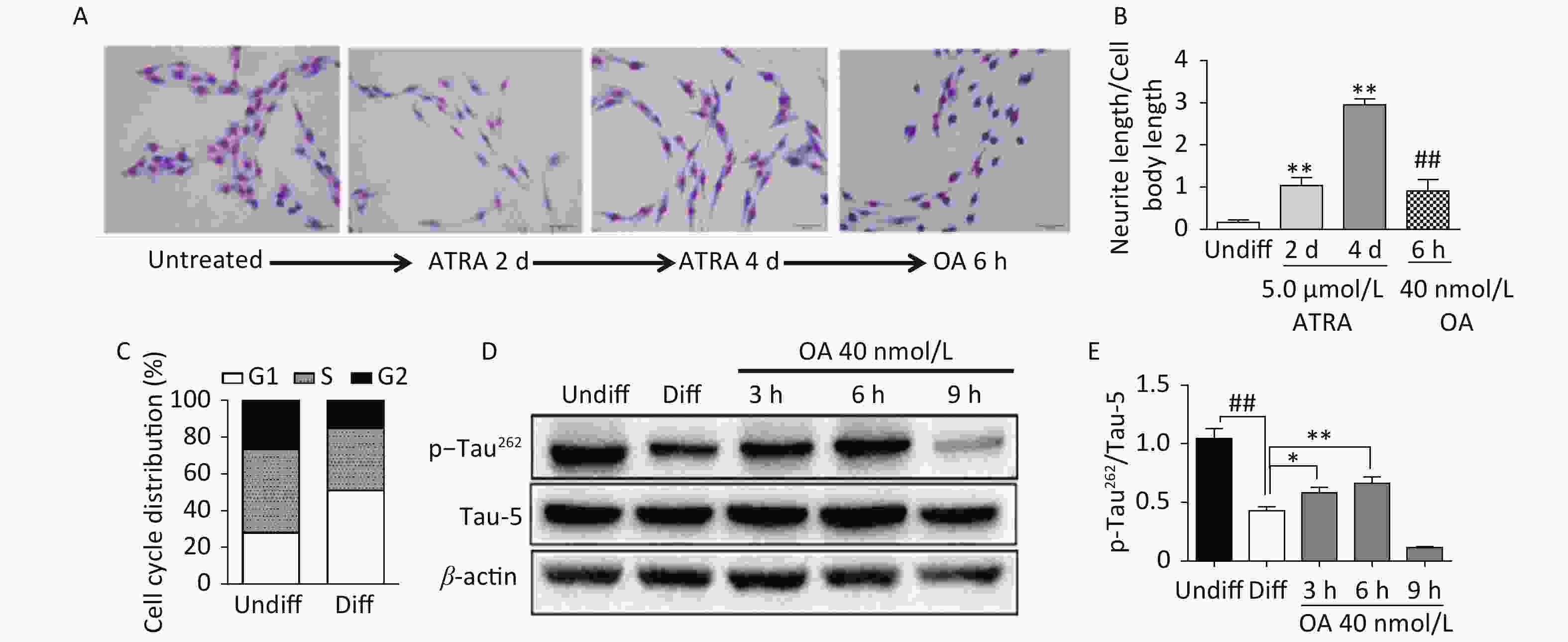
Figure 1. Neurite outgrowth impairment induced by okadaic acid (OA) in all-trans retinoic acid (ATRA)-differentiated SH-SY5Y cells. The latter were treated with 5.0 μmol/L ATRA for 4 days to induce cholinergic differentiation. Differentiated cells were incubated with 40 nmol/L OA for another 6 hours. (A) Cell morphology changes and neurite outgrowth were visualized using Giemsa staining (200× magnification). (B) Neurite outgrowth was quantitatively analyzed by counting the ratio of neurite and cell body lengths using Image Pro Plus 6.0. (C) Cell cycle distribution analysis was performed using flow cytometry. (D) Levels of total tau protein (Tau-5) and tau phosphorylated at Ser262 (p-Tau262) were assessed using western blotting. (E) Quantitative analysis of p-Tau262 and Tau-5 in differentiated SH-SY5Y cells. **P < 0.01 vs. undifferentiated cells (Undiff); ##P < 0.01 vs. differentiated SH-SY5Y cells (Diff). Data from three independent experiments are shown.
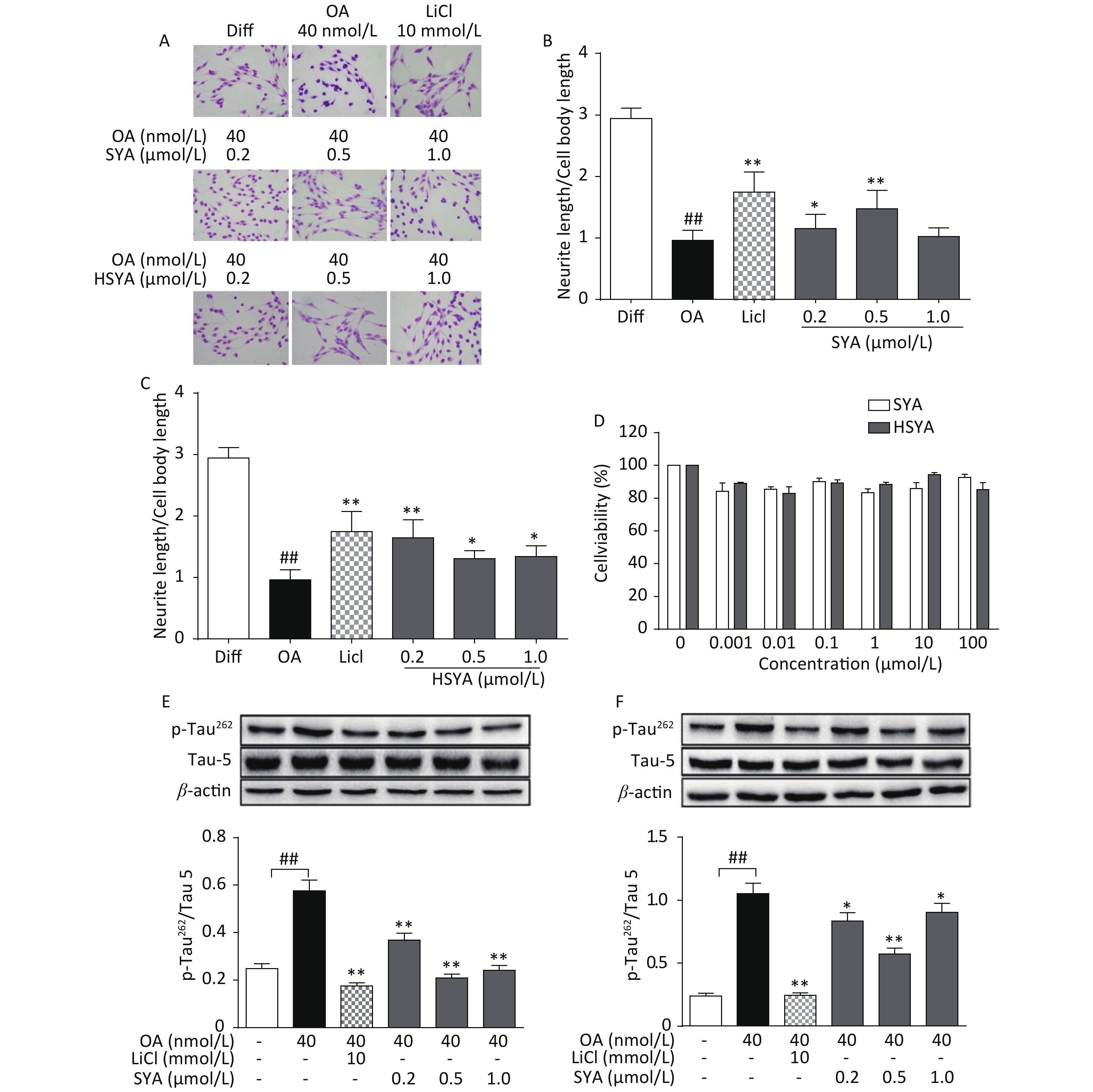
The MTT assay revealed no significant cytotoxicity being induced by HSYA and SYA in SH-SY5Y cells at concentrations of up to 100 μmol/L (Figure 2D). Co-treatment with HSYA and SYA significantly antagonized neurite outgrowth impairment induced by OA (Figure 2A-C). Lithium chloride (LiCl, 10 mmol/L) served as a positive control, and elicited similar effects (Figure 2A-C). Results from our in vitro study demonstrated that co-treatment with SYA and HSYA reversed the increase in p-Tau262 induced by OA in differentiated SH-SY5Y cells.
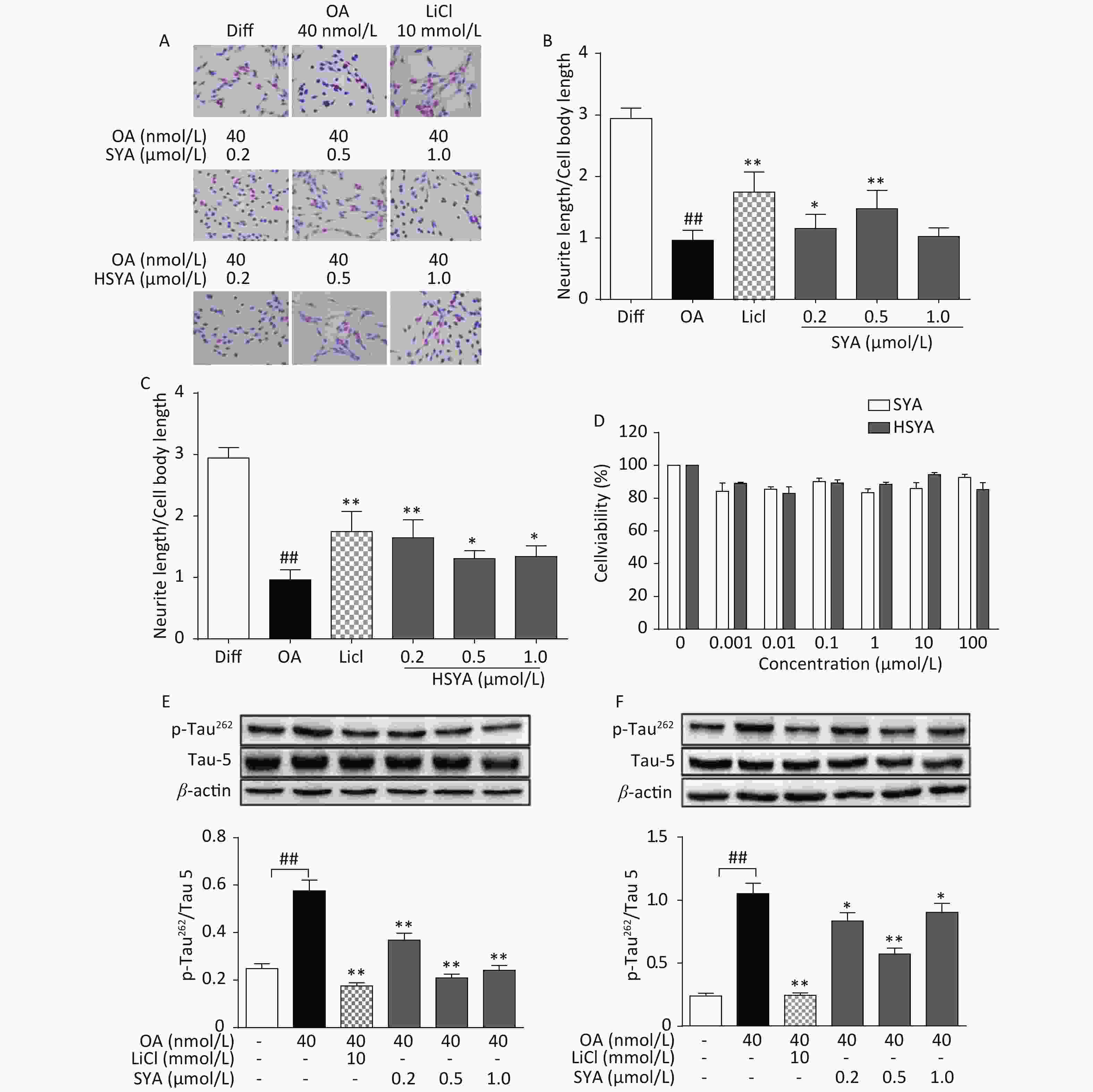
Figure 2. Safflower yellow A (SYA) and hydroxy safflower yellow A (HSYA) alleviate neurite outgrowth impairment induced by okadaic acid (OA) in differentiated SH-SY5Y cells. All-trans retinoic acid (ATRA)-differentiated SH-SY5Y cells were treated with OA (40 nmol/L) alone or in combination with LiCl (10 mmol/L), SYA, or HSYA for 6 hours. Cellular morphological changes were observed using Giemsa staining (200× magnification) (A). Neurite outgrowth affected by SYA (B) and HSYA (C) was quantitatively analyzed by measuring the ratio of total neurite length and cell body diameter using Image Pro Plus 6.0 software. Effects of SYA and HSYA on SH-SY5Y cell proliferation were measured using the MTT assay after 24 hours of exposure (D). Total tau protein (Tau-5) and Ser262-phosphorylated tau (p-Tau262) levels were evaluated using western blotting after incubation with OA (40 nmol/L) alone or in combination with SYA (E) or HSYA (F) for another 6 hours. LiCl treatment (10 mmol/L) was used as a positive control. ##P < 0.01, OA-treated differentiated cells vs. ATRA-differentiated cells (Diff); *P < 0.05, **P < 0.01 vs. OA-treated differentiated cells. Data from three independent experiments are shown.
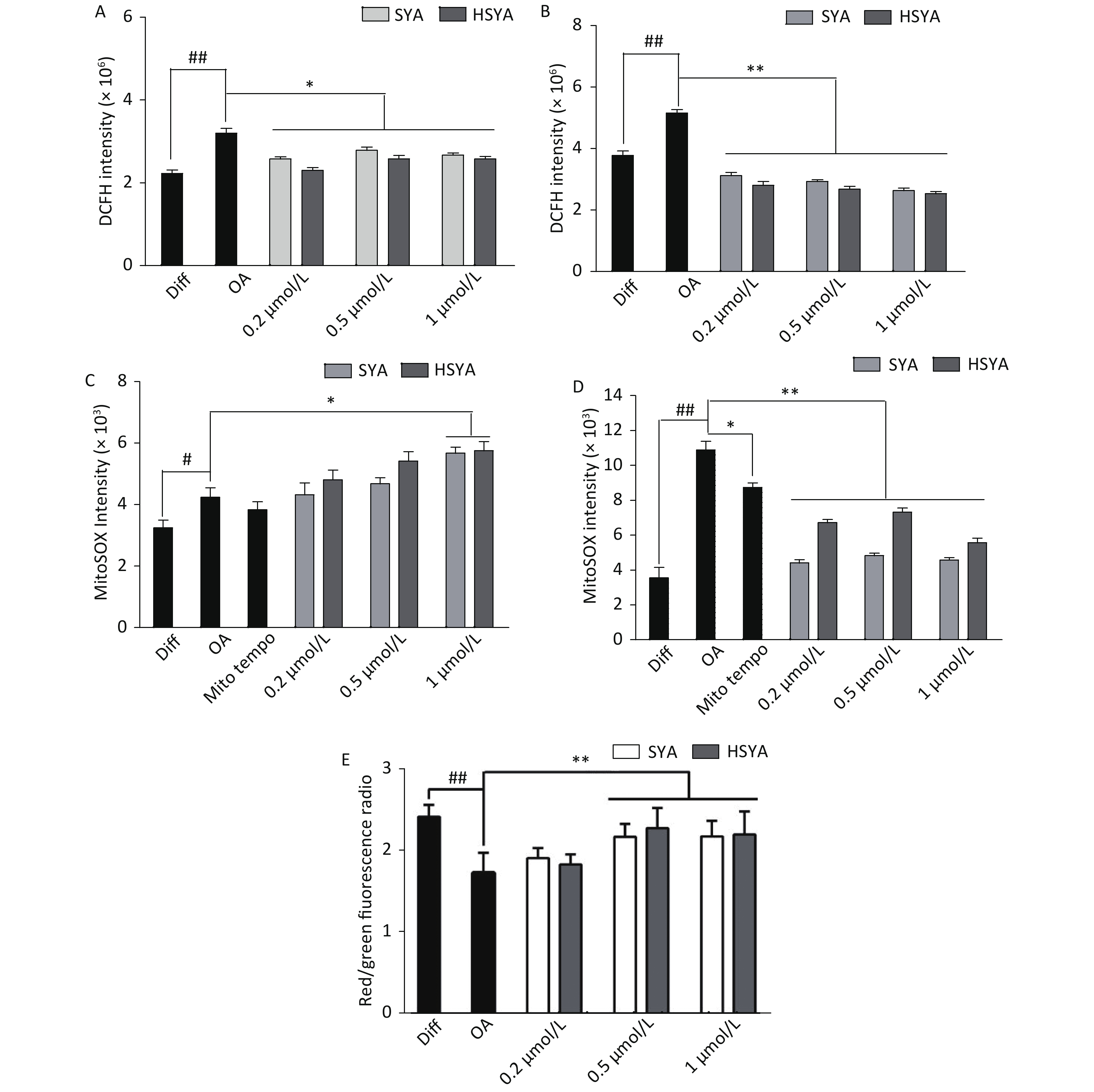
Energy metabolism disorders and oxidative stress associated with mitochondrial dysfunction are involved in the progression of AD [10]. Intracellular tROS and mtROS levels were investigated during early (30 min) and late exposures (6 h). OA exposure significantly elevated tROS and mtROS levels during early and later stages (Figure 3A-D). HSYA and SYA co-treatment significantly mitigated the increase in tROS at both stages (Figure 3A and B). Both HSYA and SYA markedly decreased mtROS levels at later stages, similar to Mito-TEMPO, while both upregulated mtROS formation during early stages (Figure 3C and D). OA exposure for 30 min induced a marked drop in the MMP in differentiated SH-SY5Y cells, while co-treatment with both SYA and HSYA significantly reversed the OA-induced MMP reduction (Figure 3E).
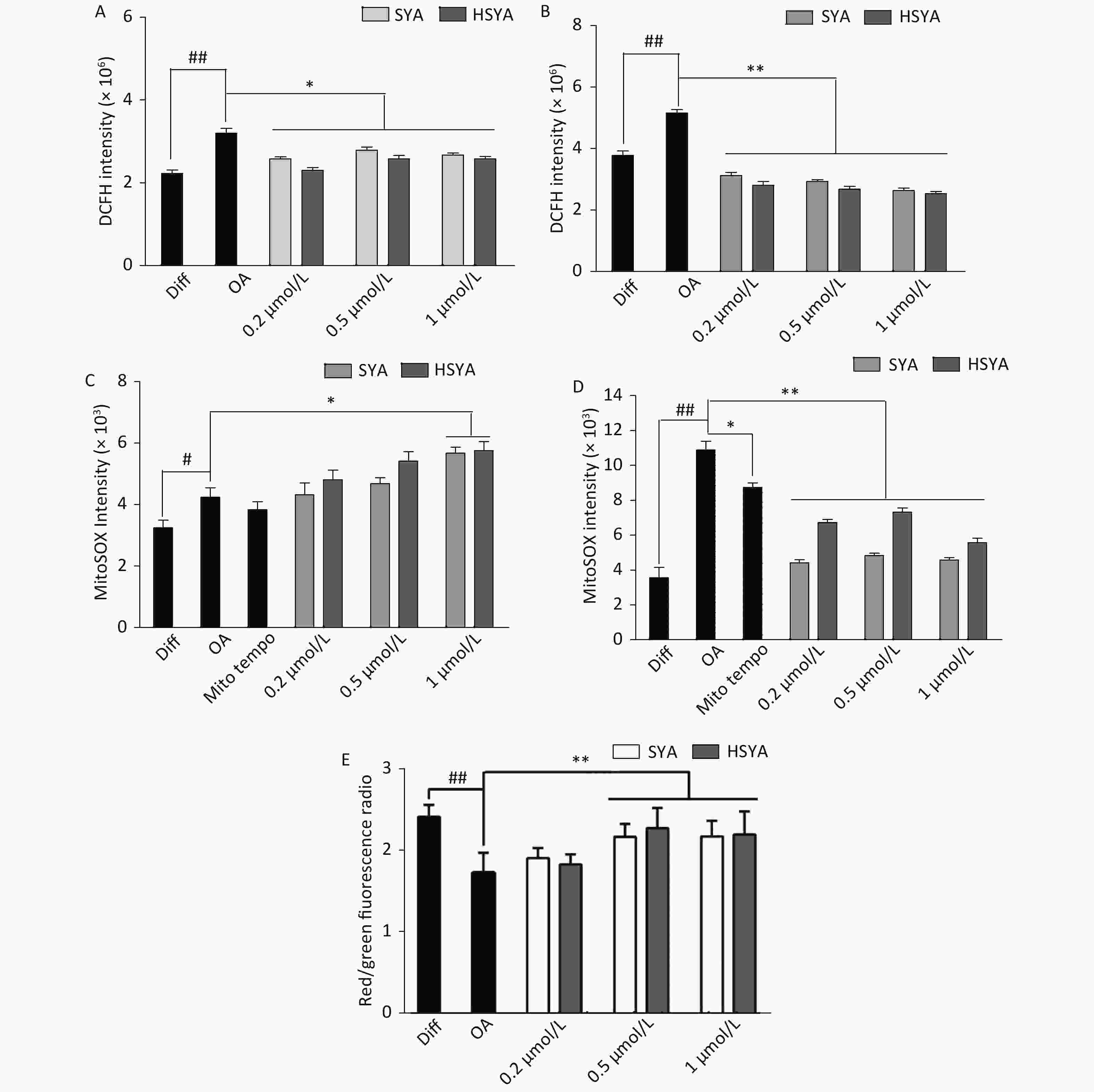
Figure 3. Effects of safflower yellow A (SYA) and hydroxy safflower yellow A (HSYA) on intracellular reactive oxygen species (ROS) formation and mitochondrial membrane potential (MMP) decline induced by okadaic acid (OA) in differentiated SH-SY5Y cells. All-trans retinoic acid (ATRA)-differentiated SH-SY5Y cells were treated with OA (40 nmol/L) alone or in combination with Mito-TEMPO (1.0 μmol/L), SYA, or HSYA for 30 min (A, C) and 6 hours (B, D), respectively. Total intracellular ROS (tROS) (A, B) and mitochondrial ROS (mtROS) (C, D) levels were measured using flow cytometry after DCFH-DA (10 μmol/L) or Mito-SOX (5 μmol/L) staining for 30 min. The MMP (E) was measured using flow cytometry after staining with JC-1 (10 μmol/L) for 30 min. #P < 0.05, ##P < 0.01 vs. ATRA-differentiated SH-SY5Y cells (Diff); *P < 0.05, **P < 0.01 vs. OA treated cells (OA). Data are representative of three independent experiments.
In conclusion, our present findings demonstrate that both HSYA and SYA alleviated OA-induced impairment of neurite outgrowth and p-Tau262 hyperphosphorylation in differentiated SH-SY5Y cells. HSYA and SYA antagonized the OA-induced MMP reduction and oxidative stress during early and later stages. Overall, we provide evidence that both HSYA and SYA could protect neurite outgrowth by modulating tau phosphorylation, although the underlying mechanisms and roles of the two time phases regulating mtROS need further investigation.
The authors have no conflicts of interests to declare.
Safflower Yellow Compounds Alleviate Okadaic Acid-Induced Impairment of Neurite Outgrowth in Differentiated SH-SY5Y Cells
doi: 10.3967/bes2020.110
- Received Date: 2019-12-22
- Accepted Date: 2020-06-30
| Citation: | WANG Zhen Hua, SHI Xiao Bing, LI Gang, HAO Xue Yan, YUAN Zhen Zhen, CAO Xiao Hai, WANG Hong Lun, LI Ji, MA Cheng Jun. Safflower Yellow Compounds Alleviate Okadaic Acid-Induced Impairment of Neurite Outgrowth in Differentiated SH-SY5Y Cells[J]. Biomedical and Environmental Sciences, 2020, 33(10): 812-816. doi: 10.3967/bes2020.110 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: