-
Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF) procedure was first introduced by Foley et al. to reduce approach-related muscle damage [1,2]. Since its introduction, many investigators have reported significant advantages of open posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF), including, but not limited to, less intraoperative blood loss, less postoperative pain, decreased postoperative narcotic usage, early ambulation, and decreased length of hospital stay [3-7].
Midline lumbar fusion (MIDLF) is a novel minimally invasive fusion technique that comprises the posterior midline approach, microsurgical laminectomy, and cortical bone trajectory (CBT) screw fixation in combination with PLIF or TLIF. It was first introduced by Minuno et al. in 2014[8]. The most prominent feature of MIDLF is the use of CBT screws. The CBT screw is a promising alternative to traditional pedicle-trajectory screws for use in a posterior fixation construct. The advantage associated with this technique is the increased cortical bone contact, providing enhanced screw grip and interface strength independent of trabecular bone mineral density; however, the screw is smaller and shorter than the traditional pedicle screw [8,9]. Biomechanical studies demonstrate not only superior pullout force and insertion torque for the CBT screw compared with the pedicle screw [10,11], but also the more medial entry point of the CBT screw which permits less soft tissue disruption. The medial-to-lateral trajectory is theoretically safer because the screw is directed away from the neural elements, while the open midline approach permits direct decompression of the neural elements [12,13].
Many studies compared the effectiveness of MIDLF, open TLIF [14,15] and PLIF [16,17] in the treatment of degenerative lumbar spinal pathology. The results were associated with less blood loss, fewer transfusions, reduced operation time, and shorter length of hospital stay, with no difference in complications, providing comparable improvement in clinical symptoms. However, only two published studies have compared MIDLF with MI-TLIF so far [18,19]. Kasukawa et al. compared TLIF with percutaneous pedicle screw insertion (P-TLIF, n = 6) and TLIF with pedicle screw insertion with CBT (CBT-TLIF, n = 10). The clinical outcomes showed CBT-TLIF resulted in less blood loss and a shorter operative duration than P-TLIF [18]. Thirty-seven patients (MI-TLIF, n = 15, MIDLF, n = 22) were enrolled in Elmekaty et al.’s study with a minimum follow-up period of 1 year. They reported MIDLF was less invasive, and no significant differences were observed regarding fusion, screw loosening, and clinical outcomes between the two groups [19].
This study aimed to elucidate the potential advantages of MIDLF over MI-TLIF by comparing the clinical outcomes, radiographical fusion rates, and complications of both approaches in larger sample size and with longer follow-ups.
-
We conducted a retrospective study of consecutive patients undergoing posterior lumbar fusion for the treatment of lumbar pathology and compared two cohorts: MIDLF and MI-TLIF. Consecutively treated patients who underwent L4-L5 fusion for degenerative lumbar spondylolisthesis or stenosis with symptoms consistent with typical pathologies, such as radiculopathy and/or neurogenic claudication with or without back pain, were included and considered for surgery because of the unresponsiveness to conservative treatment, such as medication and/or epidural block.
Patients who underwent decompression and fusion for infection, tumor, trauma, or other pathological fractures were excluded. Similarly, patients who had previous lumbar instrumentation (except for simple discectomy or laminectomy alone), underwent both anterior and posterior fusion, or had a spinal cord stimulator were excluded. Sixteen consecutive patients who had undergone L4-L5 MIDLF for lumbar degenerative etiology since June 2017 were followed up for at least 2 years (MIDLF group, mean follow-up period: 26 months). As a historical control group, 34 consecutive patients who had undergone L4-L5 MI-TLIF before June 2017 were followed up for at least 2 years after surgery (MI-TLIF group, mean follow-up period: 30 months). The study was approved by the Ethics Committee of the Peking University Third Hospital, and all participating patients provided written informed consent.
-
Basic demographic details, including age at surgery, sex, and body mass index (BMI), and the diagnosis were collected. Comorbidities included diabetes, ischemic heart disease and hypertension, smoking status, and osteoporosis. The diagnosis of osteoporosis was established exclusively based on bone mineral density T-scores that are ≤ −2.5 at the spine or hip. Procedure data included procedure performed, operation time, estimated blood loss (EBL), transfusion record, including any units of packed red blood cells or from cell saver autotransfusion, total drainage volume, the hemoglobin (HGB), and hematocrit (HCT) values before and on the first day after surgery and hospitalization. All patients had preoperative evaluations with static (anterior-posterior and lateral) and dynamic (flexion/extension) plain lumbar spine radiography, magnetic resonance imaging, and computed tomography (CT). Clinical assessments in terms of Oswestry Disability Index (ODI), the 36-Item Short-Form Health Survey (SF-36), and visual analog scale scores (VAS) for back and leg pain were evaluated before surgery and at 3-, 6-, 12-, and 24-month follow-up after surgery by independent researchers who were not blinded to the type of surgery performed. Fusion rates were assessed using the Bridwell classification [20] at 6-, 12-, and 24-month follow-up postoperatively by two independent researchers.
Perioperative complications were recorded; the follow-up was calculated through the most recent clinic visit, and any subsequent lumbar revision surgeries were recorded.
-
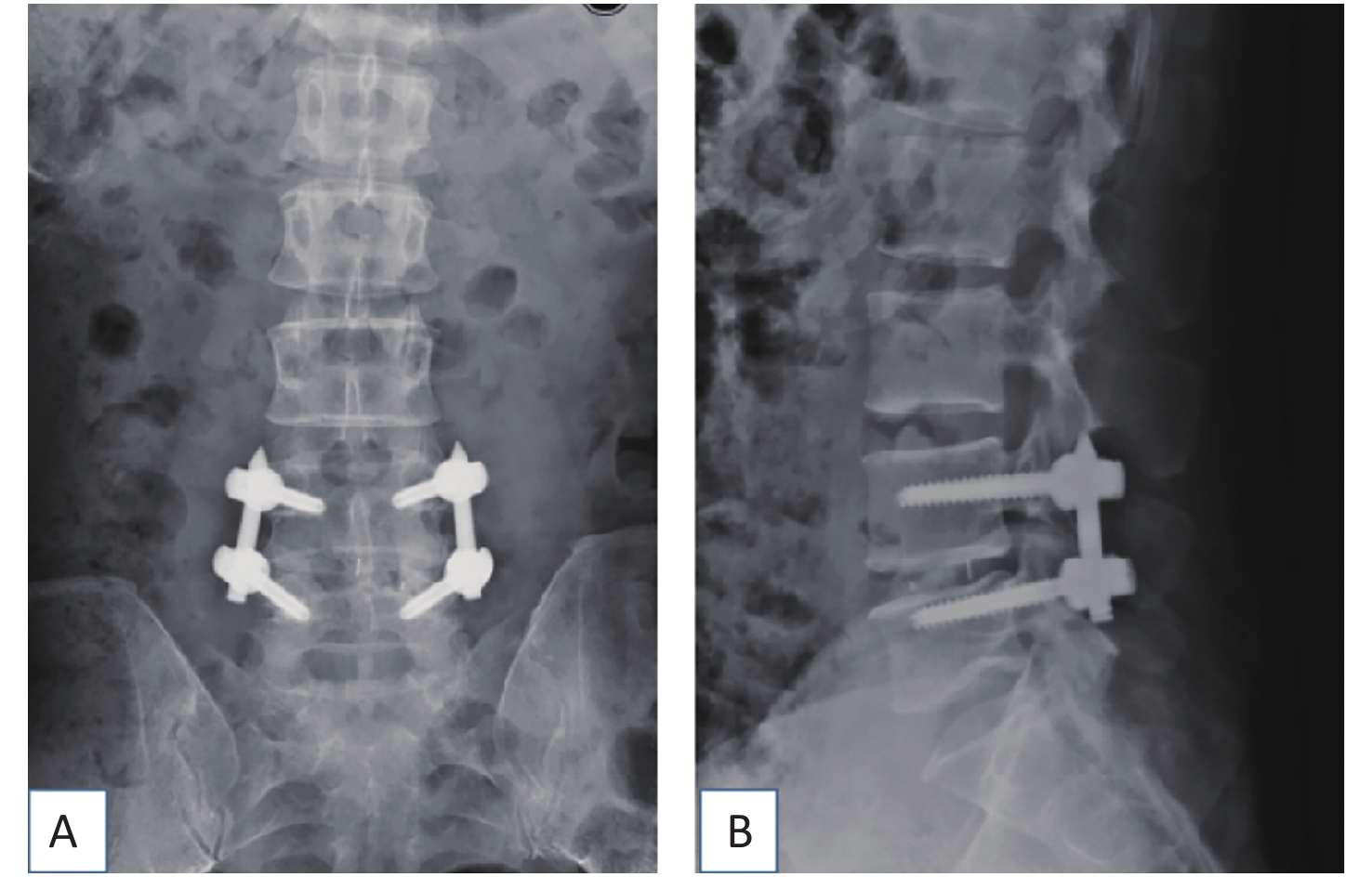
The TLIF surgical approach was decided based on the symptomatic side. If both sides were symptomatic, the incision was made on the side with severer pathology. The mobile C-arm x-ray machine was used to confirm the desired operative level. A parasagittal incision was made 3 to 5 cm lateral to the midline, and sequential soft tissue dilators were inserted down to the facet complex. Subsequently, facetectomy was performed using ultrasound scalpel from lateral to medial to expose the posterior lateral aspect of the disc. Discectomy was performed, and endplates were prepared. Disc space distraction was similarly performed with intradiscal spreaders before placing bone graft anterior and contralateral to the annulotomy followed by an interbody cage (Medtronic, Opal, length 28 mm, width 10 mm, height 7–13 mm). Fluoroscopy was used to ensure satisfactory placement of the interbody cage. Subsequently, decompression was performed by removing the rest of the ipsilateral facet and lamina and resecting the lateral margin of the ligamentum flavum to expose the ipsilateral exiting and transversing roots. The tubular retractor was angled medially, and the patient was tilted to visualize the contralateral side in cases where symptoms and magnetic resonance images showed bilateral disease. Subsequently, decompression was performed on the contralateral side where indicated. After adequate decompression, the percutaneous pedicle screw-rod construct was placed through the same incision and another similar construct through a contralateral incision (Diameter 6.0 mm, length 45–50 mm, Viper, Medos Int SARL, Raynham, USA) (Figure 1). Compression was applied before the final tightening of the construct to attempt restoring lordosis. Hemostasis and wound irrigation were performed before closure by layers.
-
Positioning: Standard prone positioning can be achieved with any choice of an appropriate table. Prior to preparing the patient, we adjusted the patient’s position in a way that rotates the vertebrae of interest neutrally on the anterior-posterior and lateral fluoroscopic views. Standard sterilization and draping were performed.
Exposure: At a minimum, the incision should extend from the pars of the cranial level to be fused to the inferior aspect of the spinous process of the caudal level to be fused. A standard posterior midline approach was taken. Typically, an incision of 3 to 5 cm is sufficient for a single-level fusion. Subperiosteal dissection of the paraspinal muscles aids in identifying the pars and ascertaining an appropriate starting point. The lateral exposure of the deep muscle compartment should proceed to, but not beyond, the lateral edge of the facet and pars regions of interest. In L4-L5 instrumentation, we exposed the inferior aspect of the L3-L4 facet, the L4 pars region, the L4-L5 facet, and the L5 pars region. Care was taken to preserve the multifidus attachments to the facets. Two angled cerebellar retractors adequately exposed the surgical field, obviating the need for larger or more aggressive retractors on the paraspinal muscles.
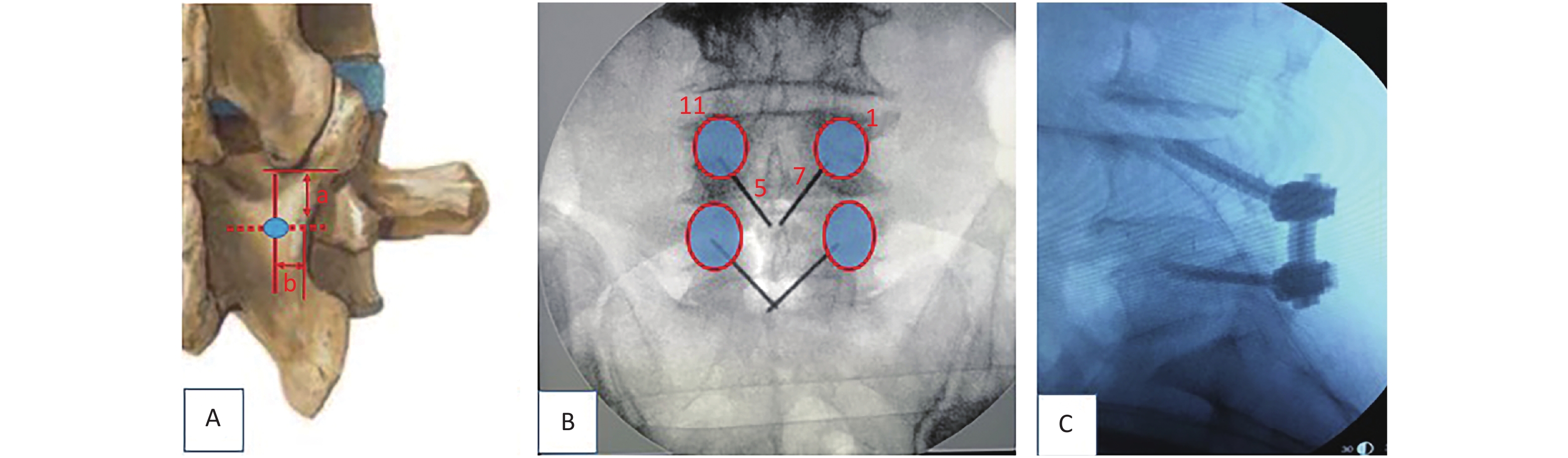
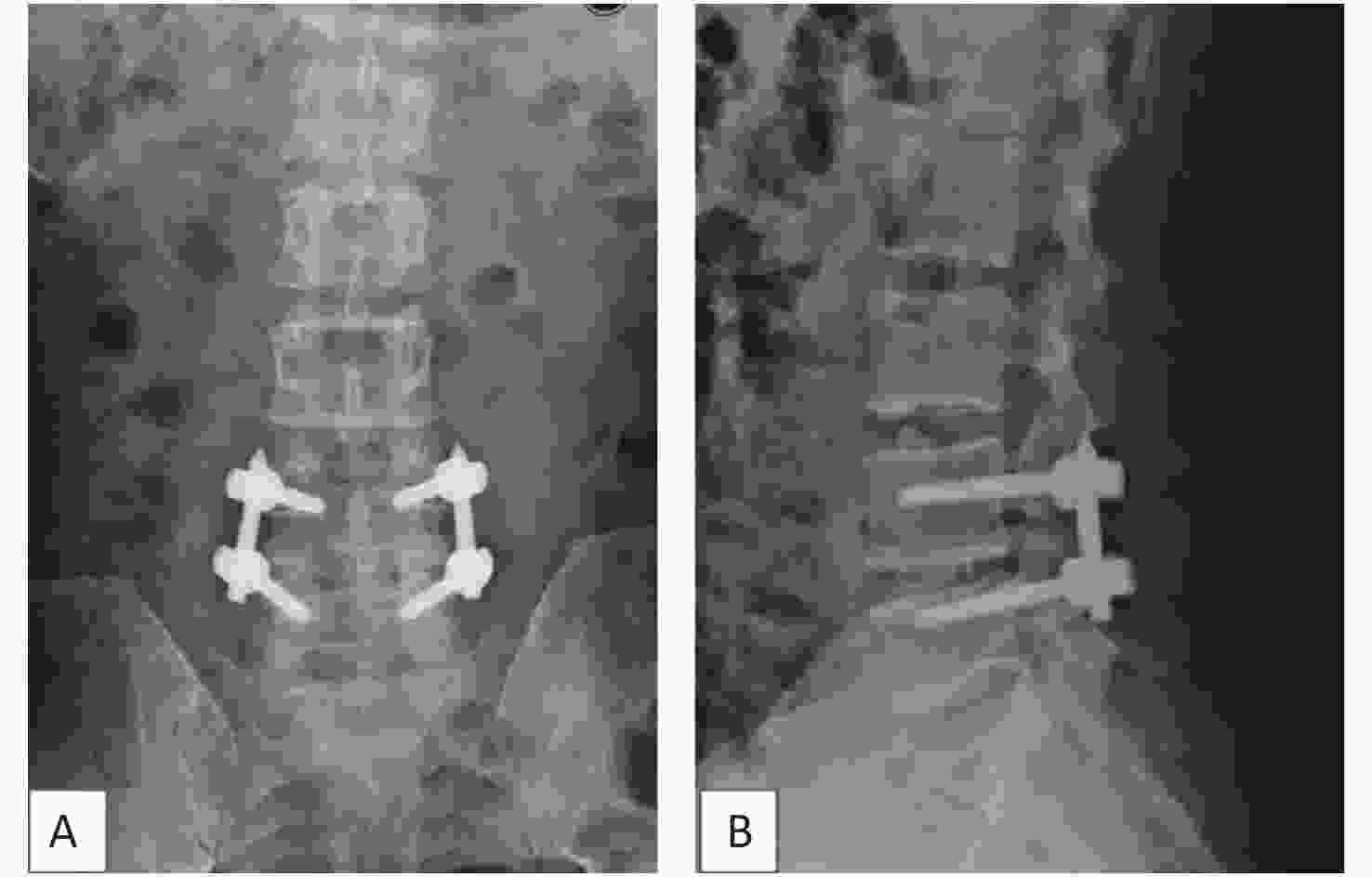
Pilot hole preparation: A trajectory from medial inferior to lateral superior was planned for screw placement. In our experience, the insertion point to be selected should be an intersection of approximately 5 mm (a value) below the lowest tip of the inferior articular process of the upper segment and about 4 mm (b value) inward from the narrowest point of the isthmus (Figure 2A). Another way to choose the starting points of CBT, as described by Delgado-Fernández J [21], is by locating an entry point 1 mm inferior to the inferior border of the transverse process and 3 mm medial to the lateral margin of the isthmus. Kirschner wires of 1-inch were inserted through the cortex using needle drivers after a small pilot hole was created with a 2-mm burr tip. The intended starting hole and trajectories were estimated using these wires. In the sagittal plane, the caudal-to-cranial trajectory was aimed toward the superior endplate at the midpoint of the vertebral body (Figure 2B, C), and fluoroscopy was used to confirm the starting point and trajectories.
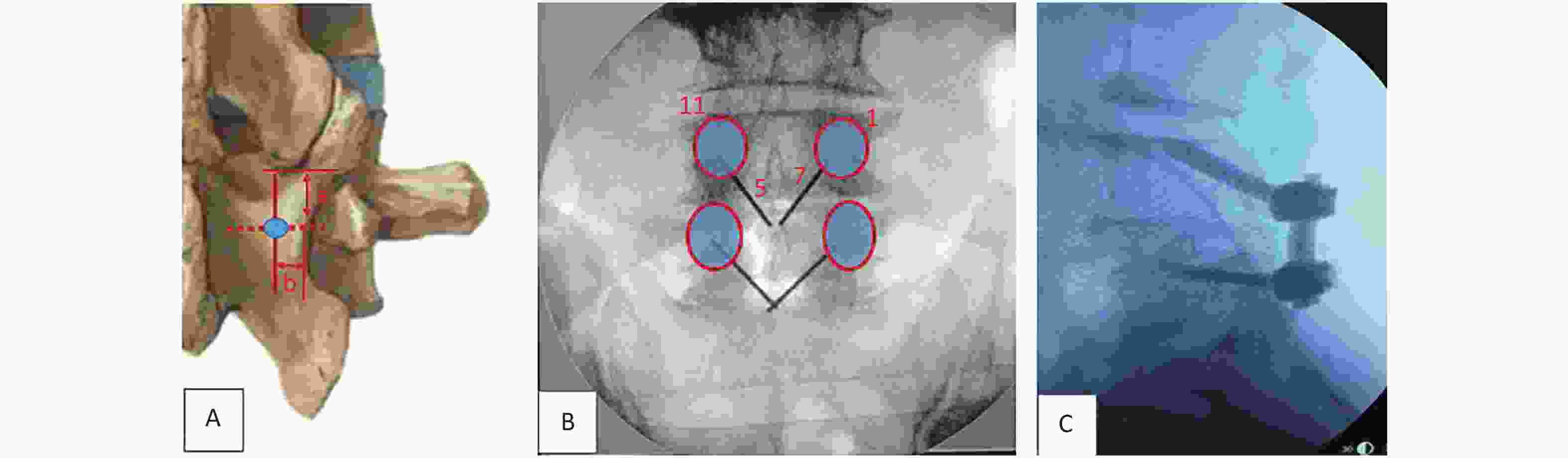
Figure 2. The starting point of CBT (cortical bone trajectory). The red circle illustrates the project of pedicles. (A) The insertion point to be selected is an intersection of approximately 5 mm (a value) below the lowest tip of the inferior articular process of the upper segment and about 4 mm (b value) inward from the narrowest point of the isthmus. (B) In the left pedicle, the CBT starts at the 5 o’clock orientation and moves to the 11 o’clock orientation using the clock face. In the right pedicle, CBT starts at the 7 o’clock orientation and moves to the 1 o’clock orientation. (C) The trajectory is approximately 20° from the plane parallel to the superior endplate, aiming toward the superior endplate at the midpoint of the vertebral body.
Tapping: Prior to tapping, a fine ball-tipped probe was used to palpate the pathway for breaches and measure the screw length. We preferably used 4.35 mm and 5.0 mm taps in turn, proceeding in a slow methodical manner, because the resistance through the dense cortical region is substantial.
Decompression and PLIF: Necessary decompression of the central canal, lateral recess, and foramen were to proceed as required by the observed pathology. We preferably used the ultrasound scalpel to complete decompression. As the decompression proceeded laterally, the surgeon had to remain cognizant of the importance of preserving the bone stock in the region of the pars. A safe distance of at least 5 mm between the created screw hole and margin of bony resection is mandatory to avoid pars fracture. To increase the visibility for the PLIF, we generally tap and seal the tunnels by bone wax. Autologous bone graft from the spinous processes and laminae was milled and combined with blood from the surgical field. After sufficient intervertebral disc material was removed, the morcellized graft was packed into the interbody space before a polyetheretherketone cage (Medtronic, Opal, length 28 mm, width 10 mm, height 7–13 mm) filled with local bone graft insertion was created to establish the largest possible surface area for the bony union.
Placing screws and rods: After the desired screw length and diameter were ascertained, the screw was inserted manually after probing each screw tunnel for breaches (Diameter 5.0 mm, length 35–40 mm, Expedium, DePuy, Raynham, USA). The tactile sensation of traversing different bone densities may be more apparent in healthy bone than in osteoporotic bone. The final screw position should be checked with perfect anteroposterior and lateral projections that are parallel the endplates. Compression was applied before the final tightening of the construct to attempt to restore lordosis.
Closure: The closure was achieved in a layered fashion over a negative pressure drain.
-
The drainage tube was removed 24 to 48 hours later. All patients in both groups were allowed and encouraged to ambulate from the first day after the operation. Strict orders were given to physiotherapists in the wards to attempt ambulating the patient the day after the operation. Patients were deemed fit for discharge after successfully ambulating with the aid of a walking stick or without assistance for at least 50 m. The duration of hospitalization stay was the number of days the patient took to be adequately independent for home discharge. All patients were administered non-steroidal analgesics for 2 weeks.
Each patient in both groups wore a lumbosacral orthosis for 3 months after surgery. The activities of waist torsion and bending were forbidden.
-
Statistical analysis was performed with SPSS version 19.0 (IBM, New York, NY). The Pearson χ2 test was used to compare categorical data (fusion rates, etc.), whereas the independent t-test was used to compare continuous variables (operative time, etc.). Analysis of variance was used to evaluate the differences in ODI, VAS back and leg pain, and SF-36 physical component score/mental component score (PCS/MCS) within each group. When the assumption of normal distribution was not reasonable, we used Mann-Whitney U two-sample tests for comparing the means. Values of P < 0.05 were considered statistically significant.
-
All patients had a single-level (L4-L5) surgery. There were statistical differences in age and the percentage of patients who experienced major comorbidities and osteoporosis between the two groups. The mean ages for the MIDLF and MI-TLIF groups were 68.7 ± 7.7 years and 57.7 ± 7.8 years, respectively (P < 0.001). The percentage of major comorbidities and osteoporosis for the MIDLF and MI-TLIF groups were 56.2% vs. 20.5% (P < 0.001) and 37.5% vs. 11.8% (P = 0.034), respectively. In the MIDLF group, 4 patients had spondylolisthesis, and 12 had degenerative disc disease with spinal stenosis, whereas in the MI-TLIF group, 8 patients had spondylolisthesis, and 22 had degenerative disc disease with spinal stenosis. The distribution of the disease etiology was not significantly different between the two groups (P > 0.05). No statistical differences regarding sex ratio, percentage of smokers, and BMI were observed between the groups (Table 1). Preoperative medical history, ODI, VAS back and leg pain, SF-36 PCS and MCS, HGB and HCT were similar between the groups. (Table 1).
Total Cases (n = 50) MIDLF (n = 16) MI-TLIF (n = 34) P-value Age (mean ± SD) 68.7 ± 7.7 57.7 ± 7.8 < 0.001 Sex ratio (F:M) 8:8 16:18 0.452 BMI (kg/m2, mean ± SD) 23.8 ± 2.5 24.7 ± 2.9 0.336 Smokers (n, %) 2, 13% 6, 17% 0.127 Major comorbidities (ischemic heart disease, diabetes, and hypertension) (n, %) 9, 56.2% 7, 20.50% < 0.001 Disease etiology Spondylolisthesis (n) 4 8 0.508 Degenerative disc disease with spinal stenosis (n) 12 22 0.461 Medical history (month) Back pain (mean ± SD) 41.3 ± 43.1 48.8 ± 73.7 0.708 Leg pain (mean ± SD) 49.3 ± 63.0 16.6 ± 24.4 0.061 Objective clinical outcome ODI (%) 65.1 ± 7.4 64.5 ± 11.1 0.862 VAS back pain (mean ± SD) 4.5 ± 1.7 5.5 ± 1.8 0.070 VAS leg pain (mean ± SD) 5.6 ± 1.0 6.0 ± 2.2 0.367 HGB-Pre (mean ± SD) 142.6 ± 10.3 144.0 ± 15.8 0.764 HCT-Pre (mean ± SD) 0.4 ± 0.03 0.4 ± 0.04 0.315 BMD (T < -2.5) (%) 37.50% 11.80% 0.034 SF-36 PCS (mean ± SD) 38.6 ± 7.8 36.4 ± 8.9 0.407 SF-36 MCS (mean ± SD) 47.8 ± 15.1 44.8 ± 15.7 0.534 Note. ODI, Oswestry Disability Index; VAS, visual analog scale; BMD, bone mineral density; MIDLF, midline lumbar fusion; MI-TLIF, minimally invasive transforaminal lumbar interbody fusion; SF-36, 36-Item Short-Form Health Survey; PCS, physical component score; MCS, mental component score. HGB, hemoglobin; HCT, hematocrit. Bold numbers indicate statistically significant differences (P < 0.05). Table 1. Preoperative comparison of the MIDLF and MI-TLIF groups
Table 2 reflects the perioperative comparison between the two groups. Operative time was significantly shorter for the MIDLF cases (MIDLF: 174 min, MI-TLIF: 229 min, P < 0.001). No statistical differences in blood loss, HGB of the first day after surgery [HGB(D1)], and total drainage volume were observed between both groups (P > 0.05). However, there was a lower HCT on the first day after surgery [HCT(D1)] in the MIDLF group (0.34 ± 0.02) than in the MI-TLIF (0.36 ± 0.04) group (P = 0.037). Hospitalization was shorter for the MIDLF cases (MIDLF: 2.5 d, MI-TLIF: 3.2 d, P = 0.056); however, the difference was not statistically significant. One patient in the MI-TLIF group (2.9%, 1 of 34 patients) required blood transfusion postoperatively, whereas none of the patients in the MIDLF group required transfusion (P = 0.331).
Variables MIDLF (n = 16) MI-TLIF (n = 34) P-value Operative time (min) 174.3 ± 27.9 229.4 ± 50.0 < 0.001 Blood loss (mL) 69.3 ± 36.7 82.5 ± 59.2 0.343 HGB (D1) 118.1 ± 8.1 122.7 ± 16.6 0.202 HCT (D1) 0.34 ± 0.02 0.36 ± 0.04 0.037 Total drainage volume (mL) 156.0 ± 92.0 223.3 ± 238.3 0.161 Hospitalization (days) 2.5 ± 0.8 3.2 ± 1.9 0.056 No. of transfusion (n) 0 1 0.331 Note. MIDLF, midline lumbar fusion; MI-TLIF, minimally invasive transforaminal lumbar interbody fusion; HGB, hemoglobin; HCT, hematocrit. Bold numbers indicate statistically significant values (P < 0.05). Table 2. Perioperative comparison of the MIDLF and MI-TLIF groups
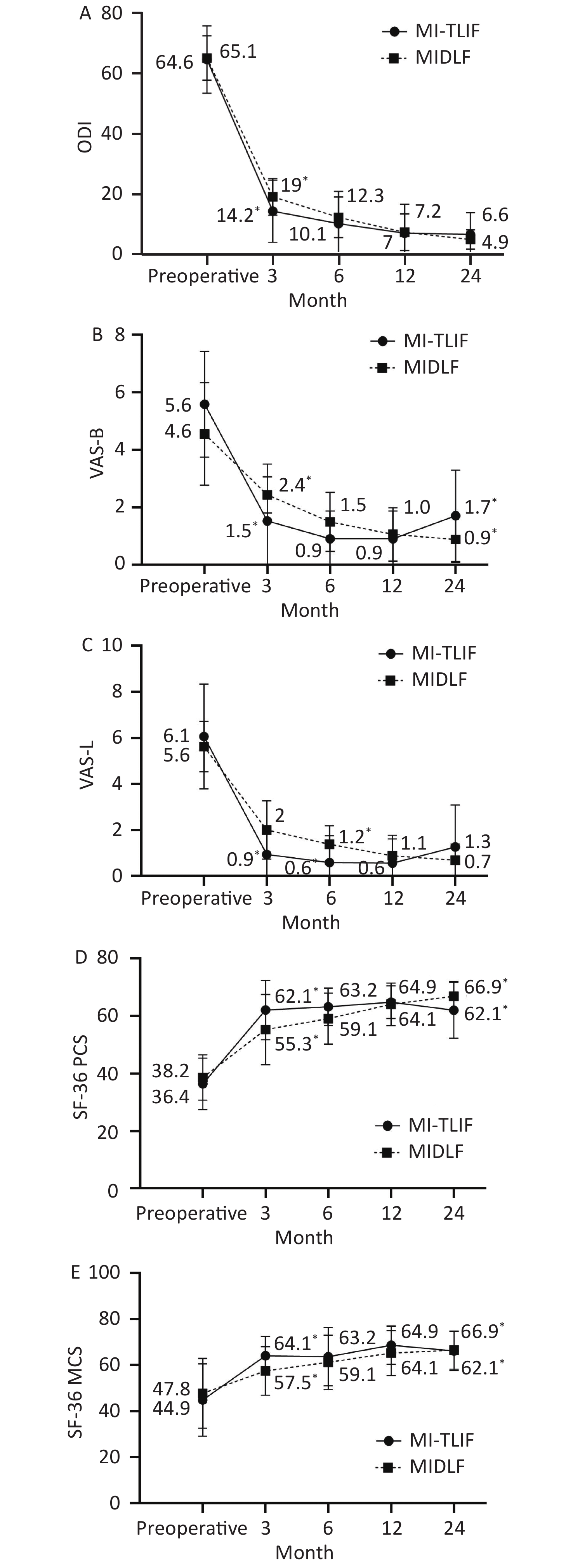
Table 3 reflects the comparison of postoperative recovery between the groups. Postoperatively, both MIDLF and MI-TLIF groups showed significant improvement in ODI, VAS back and leg pain, and SF-36 PCS and MCS. However, significant differences were observed between the two groups in the abovementioned five parameters at the 3 months follow-up (P < 0.05). At the 6 months follow-up, VAS leg pain scores (1.3 ± 0.8 vs. 0.5 ± 1.1, P = 0.018) and SF-36 PCS (59.1 ± 8.8 vs. 63.2 ± 6.5, P = 0.007) were worse in the MIDLF group than in the MI-TLIF group. No statistical differences were observed between the groups at the 12 months follow-up (P > 0.05). At the 24 months follow-up, VAS back pain scores and SF-36 PCS were worse in the MI-TLIF group than in the MIDLF group (0.8 ± 0.8 vs. 1.7 ± 1.1, P = 0.018 and 66.8 ± 5.1 vs. 62.0 ± 9.7, P = 0.027, respectively). Clinical results at each time point between the groups are shown in Figure 3.
Clinical Outcomes MIDLF MI-TLIF P-value 3 month ODI 19.0 ± 6.1 14.2 ± 10.3 0.047 VAS back pain 2.4 ± 0.6 1.5 ± 1.0 0.001 VAS leg pain 2.0 ±1.2 0.9 ± 1.1 0.005 SF-36 PCS 55.3 ± 12.2 62.1 ± 10.3 0.046 SF-36 MCS 57.5 ± 10.6 64.1 ± 8.3 0.021 6 month ODI 12.2 ± 6.7 10.1 ± 10.7 0.469 VAS back pain 1.5 ± 1.0 0.9 ± 0.9 0.055 VAS leg pain 1.3 ± 0.8 0.5 ± 1.1 0.018 SF-36 PCS 59.1 ± 8.8 63.2 ± 6.5 0.007 SF-36 MCS 61.2 ± 11.7 63.6 ± 12.6 0.522 12 month ODI 7.2 ± 6.1 6.9 ± 9.5 0.915 VAS back pain 1.0 ± 0.9 0.9 ± 0.9 0.605 VAS leg pain 0.8 ± 0.8 0.5 ± 1.0 0.303 SF-36 PCS 64.1 ± 7.4 64.8 ± 5.7 0.704 SF-36 MCS 65.3 ± 9.7 68.6 ± 8.3 0.215 24 month ODI 4.8 ± 3.2 6.5 ± 7.1 0.26 VAS back pain 0.8 ± 0.8 1.7 ± 1.5 0.018 VAS leg pain 0.6 ± 0.7 1.2 ± 1.8 0.116 SF-36 PCS 66.8 ± 5.1 62.0 ± 9.7 0.027 SF-36 MCS 66.5 ± 8.3 66.1 ± 8.5 0.881 Note. ODI, Oswestry Disability Index; VAS, visual analog scale; MIDLF, midline lumbar fusion; MI-TLIF, minimally invasive transforaminal lumbar interbody fusion; SF-36, 36-Item Short-Form Health Survey; PCS, physical component score; MCS, mental component score; mo, months. Bold values indicate statistically significant values (P < 0.05). Table 3. Comparison of clinical outcomes between the MIDLF and MI-TLIF groups
The majority in the MIDLF and MI-TLIF groups (94% vs. 88%) managed to achieve grade 1 fusion at minimal 2 years of follow-up. The MIDLF group had slightly better fusion grades at 6, 12, and 24 months follow-up; however, this was not statistically significant (Table 4).
Bridwell Grade 1 Fusion MIDLF (%) MI-TLIF (%) P 6 month 62 53 0.525 12 month 94 85 0.391 24 month 94 88 0.544 Note. MIDLF, midline lumbar fusion; MI-TLIF, minimally invasive transforaminal lumbar interbody fusion. Table 4. Fusion rates of the MIDLF and MI-TLIF groups (%)
-
Eleven perioperative complications were recorded in the MI-TLIF group. Approach-related complications (interbody migration and neurologic deficit in one patient; dura tear and adjacent segmental disease in one patient, respectively; nonunion, four patients) were evident in eight patients. Other minor complications, including superficial wound infection, urinary tract infection, and anemia (one each), were transient (less than1 week) in 10 of 34 patients, representing a complication rate of 29.4%. There was one case of symptomatic cage migration with neurologic deficit (6 months postoperatively) that was surgically revised. There was one case of durotomy which did not need repair. There were four cases of nonunion (Grade 2, Bridwell classification) with low back pain during the last follow-up (average, 25 months), aggravated by the weight-bearing or a significant level of activity; however, they did not experience sciatic radiating pain, and their daily activity and work were not affected. All four cases were managed conservatively.
On the contrary, five perioperative complications were recorded in the MIDLF group. Approach-related complications (screw malposition, neurologic deficit, epidural hematoma, one patient; wound infection and nonunion in one patient, respectively) were evident in 3 of 16 patients, representing a complication rate of 18.75% (3/16). Revision surgery was performed for one case. The right foot dropped immediately after the operation, and the malposition of the right L4 screw and epidural hematoma were confirmed during revision exploration. The CBT screws on the right sides were replaced by the pedicle screws. There was one case of nonunion (Grade 2, Bridwell classification) with mild low back pain at 12 months follow-up. The clinical manifestation was similar to those cases in the MI-TLIF group and was managed conservatively.
The overall complication rate was not significantly different between the groups (18.75% vs. 29.4%, P = 0.423).
-
MI-TLIF procedure has been a worldwide authorized technique in the past two decades, pushing the advances in minimally invasive spine technology with recognized clinical efficacy [3-5,7]. However, to the best of our knowledge, only two studies compared the effectiveness of MIDLF and MI-TLIF [18,19]. There are several limitations to their studies, including the small number of patients (n = 16 (10:6) and n = 37 (22:15) in each group and the short mean follow-up period (11 months and 14 months, respectively). More importantly, there are problems associated with the indication of MIDLF.
The first problem is the suitability of the S1 for CBT fixation. The ideal trajectory of CBT screws should fully utilize the vertebral/pedicle complex, obtaining a 4-point fit between the dorsal cortex at the site of insertion, medially-oriented posterior pedicle wall, laterally oriented anterior pedicle wall, and curvature of the vertebral body wall[22]. Anatomically, the sacrum does not contain a true pedicle of the cortical bone ring, rather a confluence of the cancellous bone. Peretz et al. referred to sacral osteoporotic changes, stating that the sacral ala atrophies first, resulting in a concentrated, dense area centrally at the sacral [23]. If the CBT screw is inserted in the cephalic and divergent orientation, it is extremely likely to decrease the biomechanical stability and pullout strength of the CBT screws and increase the incidence of screw loosening. Sakaura et al. reported the astonishing results by CT examination 6 months after surgery [24], which verified our concerns. The results showed that 44 of 386 caudal screws (11.4%) in 29 of 193 patients (15.0%) loosened. The incidence of caudal screw loosening was 6.0% (20/334) of the screws in 9.6% of the patients after floating PLIF (L2-L6) and 46.2% (24/52) of the screws in 50.0% of the patients after lumbosacral PLIF (S1) by CBT fixation. Therefore, MIDLF is suitable for spine fusion above L5-S1[25].
The second problem is whether the spondylolysis is a appropriate indication for MIDLF. A finite element study by Matsukawa et al. demonstrated that the CBT construct showed lower vertebral fixation strength in flexion (39.0%, P < 0.01), extension (35.6%, P < 0.01), lateral bending (50.7%, P < 0.01), and axial rotation (59.3%, P < 0.01) than the convention pedicle screws construct for spondylolytic vertebra[2]. They suggested that CBT screws are less optimal for stabilizing the spondylolytic vertebra due to their low fixation strength compared with pedicle screws.
However, the percentages of L5-S1(22/59) and isthmic spondylolisthesis (16/59) were too high in Elmekaty’s study [19]. For the aforementioned reasons, the most appropriate indication for MIDLF is a short segmental fusion above L5-S1 for lower lumbar pathologies [13]. Isthmic spondylolisthesis is a contraindication [26]. This is the reason we only chose cases that underwent MIDLF at L4-L5 without spondylolysis.
Significant differences in age were observed between the two groups (68.7 ± 7.7 vs. 57.7 ± 7.8). We attempted selecting a group of older cases in the MI-TLIF group and compared with the MIDLF group; however, significant differences were still observed in age. Similarly, the age selection difference was observed in Elmekaty’s study [19], the average age of patients in the MIDLF and MI-TLIF groups were 62.7 and 49.3 years, respectively. MIDLF was a less invasive technique regarding fusion, screw loosening, and clinical outcomes than MI-TLIF. Additionally, Lee [27] reported minimally invasive TLIF using a unilateral approach and single cage (at a single-level) in patients over 65 years; thirty-eight patients were enrolled. The mean age of these patients at operation was 71.82 ± 4.71 years (range, 65–82 years). Compared with our study, the fusion rate (94.0% vs. 100%), complication rate (12.5% vs. 15.8%) and clinical outcomes were not significantly different. Hence, owing to the CBT screw having more advantage on an osteoporotic bone, MIDLF tends to be used for degenerative lumbar disease, especially in older patients with osteoporosis.
Both MIDLF and MI-TLIF have the characteristic of reducing approach-related trauma during surgery. However, the MI-TLIF needs other approaches for percutaneous pedicle screws insertion. The narrow field of view, the use of only a two-dimensional image, and a significant learning curve complicate the procedure. In contrast, MIDLF can be performed via a less invasive and familiar posterior midline approach. In this study, the mean operative time was significantly shorter in the MIDLF group than in the MI-TLIF group. The mean blood loss, total drainage volume, and hospitalization were less or shorter in the MIDLF group, although no significant difference was observed between the groups. Given these results, it seems that MIDLF could be less invasive than MI-TLIF. Regarding clinical outcomes, both groups achieved improved efficacy. However, the process of recovery in each group was obviously different. Clinically, the MI-TLIF group showed better and faster recovery than the MIDLF group because there was a statistically significant difference at 3 months follow-up in the improvement of pain intensity (VAS back pain and leg pain), ODI, and SF-36 PCS/MCS scores. At 6 months follow-up, the gap between the two groups gradually narrowed. However, the MI-TLIF group still had significant advantages in VAS leg pain and SF-36 PCS. A similar efficacy was observed at 12 months follow-up between the two groups. However, as the follow-up progressed over time, the clinical efficacy of the MI-TLIF group showed a few signs of rebound (Figure 3). Combining the complications, such as adjacent segmental disease and nonunion, the results may be associated with the adverse rebound of VAS back pain and SF-36 PCS in the MI-TLIF group.
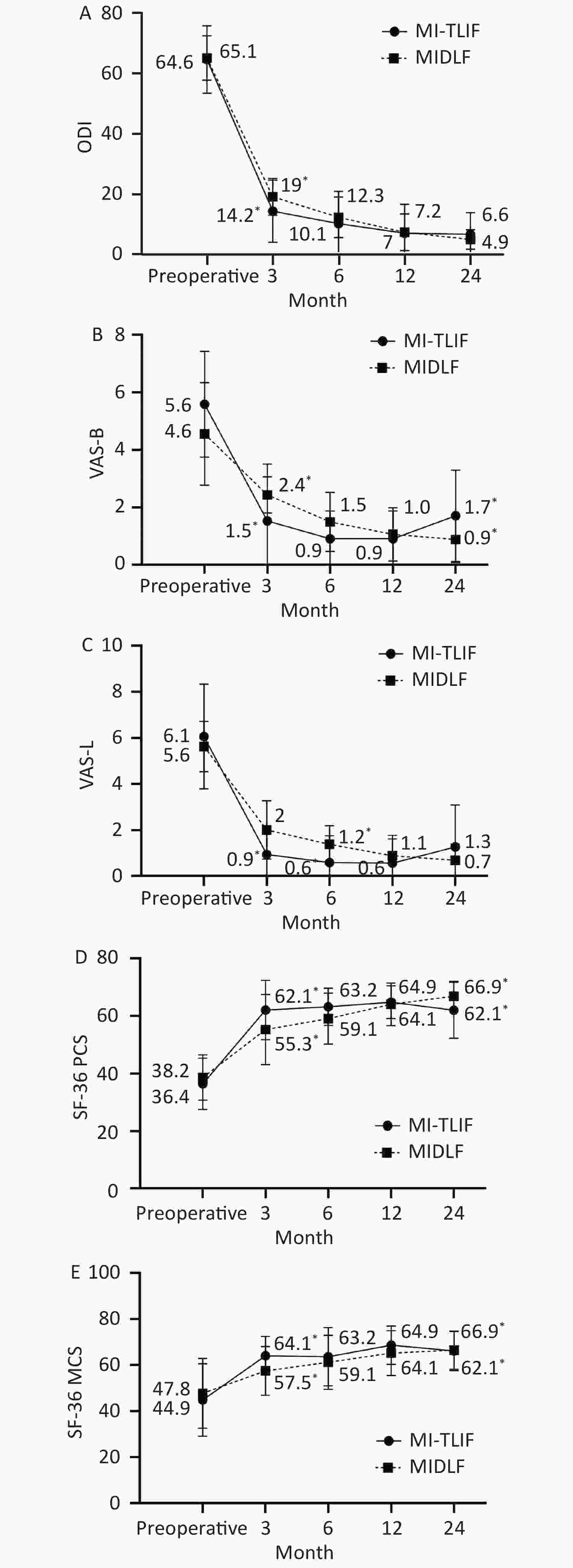
Figure 3. The graphs show the changes over time between the MIDLF and MI-TLIF groups in disability (A), back and leg pain (B, C), physical (D), and mental (E) quality of life.
The MIDLF group had slightly better fusion rates (albeit statistically not significant) at 6, 12, and 24 months follow-up than the MI-TLIF group (Table 4). Thus, MIDLF is ultimately as effective as MI-TLIF in fusion.
The perioperative complication rate was 12.5% in the MIDLF group and 11.7% in the MI-TILF. No statistically significant difference in the overall complication rate was observed between the two groups at 2-year follow-up. Sclafani et al. conducted a systematic review of complications associated with the minimally invasive spine surgery [28]. For fusion procedures, the most common complications were implant malposition, neural injury, and nonunion. The overall postoperative complication rate was 11.0% (109 of 966 cases).
This study has some limitations. First, although a retrospective study was performed, we believe that a randomized controlled study is ideal. Second, the sample size was not large enough to confirm the results; thus, further investigations with many patients and long-term follow-up are necessary to verify the effectiveness of MIDLF. Further, the proportions of the elderly, comorbidities, and osteoporosis were higher in the MIDLF group than in the MI-TLIF group. Despite these unfavorable factors, MIDLF was comparable with MI-TLIF regarding clinical outcomes and fusion rates. These results verified a meaningful advantage of using MIDLF for the elderly, especially those with osteoporosis; however, these heterogeneous factors could potentially skew the functional outcome scores and perioperative parameters, such as pain and rehabilitation, resulting in outcome biases.
In conclusion, despite different mechanisms of action, MIDLF is comparable to MI-TLIF at L4-5 in clinical outcomes and fusion rates, and the results verified the meaningful advantage of using MIDLF for the elderly, especially those with osteoporosis.
Two-Year Outcomes of Midline lumbar Fusion Versus Minimally Invasive Transforaminal Lumbar Interbody Fusion in the Treatment of L4-L5 Degenerative Disease
doi: 10.3967/bes2020.114
- Received Date: 2020-06-29
- Accepted Date: 2020-10-10
-
Key words:
- Minimally invasive techniques /
- Cortical bone trajectory /
- Clinical outcomes /
- Midline lumbar fusion /
- Transforaminal lumbar interbody fusion
Abstract:
| Citation: | WU Feng Liang, DANG Lei, ZHOU Hua, YU Miao, WEI Feng, JIANG Liang, LIU Zhong Jun, LIU Xiao Guang. Two-Year Outcomes of Midline lumbar Fusion Versus Minimally Invasive Transforaminal Lumbar Interbody Fusion in the Treatment of L4-L5 Degenerative Disease[J]. Biomedical and Environmental Sciences, 2020, 33(11): 839-848. doi: 10.3967/bes2020.114 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: