-
The exploitation of nanotechnology has increased in all ramifications due to the spectacular potential of nano-products (NPs). Among the nano-products, iron oxide (Fe2O3) NPs has attracted more scientific attentions due its superior characteristics. Hematite is chemically and thermodynamically more stable than other iron NPs, with immense applications in all fields. Iron NPs are also used as nano-carriers for targeted drug delivery and for destruction of cancer cells via magnetic hyperthermia[1]. These metallic NPs are license by US Food and Drug Administration for clinical applications[2]. However, despite of the commercial momentousness, the risks associated with Fe2O3 NPs to humans have attracted attention of toxicologists.
To establish the biocompatibility of Fe2O3 NPs against toxicity associated with conventional NPs, this study aimed to evaluate the biocompatibility of green synthesized hematite (α-Fe2O3) NPs in male and female rats. For the in vivo assays, Fe2O3 NPs was orally administered to rats in order to access its effect on nephro, cardiac, and hepatic tissues. Biochemical and histological studies were conducted to trace the morphological changes in vital organs.
Iron oxide NPs synthesized in Rhus punjabensis leaves were used in this study[3]. Scanning electron microscopy and X-ray diffraction analysis confirmed the presence of (42 ± 5) nm sized NPs with rhombohedral geometry. In vivo toxicological assessment was done following the rectified methodology (Bch#0265) established by Ethical Committee of Quaid-i-Azam University, Islamabad, Pakistan. Male and female rats (Sprague-Dawley) of 230–280 g were used in this study. Animals were grouped into six of six as follows: group 1 and 2 includes control for male and female rats, respectively; group 3, 4, 5, and 6 comprises male and female rats treated with 100 (low dose) and 200 (high dose) mg Fe2O3 NPs/kg, respectively. In each group rats were orally administered NPs (one mL suspension in water) using oral gauge. All animals were kept under controlled conditions (water, feed, light, and temperature) in an aluminum cage for 14 d, and their physical activities were monitored daily. Thereafter, animals were sacrificed, blood and organs were collected for biochemical and histological investigations.
For the biochemical analysis, serum obtained was subjected to biochemical analysis using commercially available diagnostic kit from Roche Basel, Switzerland. The biochemical tests performed include assays for alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotrans ferase (AST), total bilirubin (T.B.), albumin (ALB), total proteins (T.PR), creatinine (CRET), body urea nitrogen (BUN), electrolytes (Na, K, and Cl ions), total cholesterol (TCHO), triglycerides (TG), lactate dehydrogenase (LDH), and creatinine phosphokinase (CPK). Furthermore, antioxidant enzymes status of the rats was also assayed in the serum. Serum electrolytes were measured using 9180 Electrolyte Analyzer Roche diagnostics. Enzyme assays such as catalase (CAT), peroxidase (POD), reduced glutathione (GSH), and superoxide dismutase (SOD), as well as, protein content, lipid peroxidation (TBARS), and nitrite content were assayed following previously described methods[4].
The vital organs (liver, kidney, heart, brain, spleens, ovary, and testes) were excised and fixed with 10% formalin, and histological slides were prepared by following standard protocol for embedding tissue on paraffin. The process comprised various steps including fixation, dehydration (with 50%, 70%, 90%, and 100% alcohol), embedding of tissues on paraffin, cutting (3–4 μm thin sections), and staining with Eosin and Haematoxylin. Slides were examined under Nikon Microscope Eclipse 80i, and photomicrographs were captured.
Acute toxicity in Sprague-Dawley rats of plant-synthesized Fe2O3 NPs was observed, however, rats showed no sign or symptom of sneezing, diarrhea, lacrimation, salivation, piloerection, lesions, and weight loss during the experiment. The rats showed normal food intake, urination, purgation, and were stable and active. All rats were alive and no death occurred till the end of the experiment.
Liver function tests of the rats revealed that the male rats were more effected than the female rats. In male rats, ALT, ALP, and AST activities were significantly increased at high and low dose of administration of NPs. Total bilirubin was non-significant increase (7%) at high dose, while it declined (29%) at low dose relative to control (Table 1). On the other hand, in female rats, ALT decreased non-significantly, while ALP and AST increased significantly. These results are in consonant with those of previous studies where male rats were more affected than female rats[4, 5].
Biochemistry Control High dose % Inc/Dec Low dose % Inc/Dec Male ALT 46 63 37 54 17 ALP 121 280 131 227 88 AST 147 330 124 261 78 T.B. 0.14 0.15 7 0.1 −29 ALB 3.5 3.2 −9 3.4 −3 T.PR 5.3 4.4 −17 4.7 −11 BUN 19 22.4 18 18.7 −2 CRET 0.5 0.6 20 0.6 20 Na 139 132 −5 128 −8 Cl 103 98 −5 98 −5 K 4.8 5 4 5.2 8 CHOL 57 104 82 101 77 TG 95 127 34 87 −8 LDH 1,525 2,354 54 2,014 32 CPK 327 462 41 392 20 Female ALT 41 34 −17 29 −29 ALP 60.33 91 51 72 19 AST 140.33 200 43 144 3 T.B. 0.11 0.1 −9 0.1 −9 ALB 3.65 3.6 −1 3.2 −12 T.PR 5.47 5.1 −7 4.8 −12 BUN 18.47 16.6 −10 17.1 −7 CRET 0.45 0.5 11 0.4 −11 Na 139.33 138 −1 142 2 Cl 104 104 0 104 0 K 4.2 4.3 2 4.4 5 CHOL 44.33 49 11 48 8 TG 86 93 8 80 −7 LDH 2,218 2,856 29 2,574 16 CPK 208 264 27 190 −9 Note. Inc/Dec, increase/decrease; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; T.B. total bilirubin; ALB, albumin; T.RP, total proteins; CRET, creatinine; BUN, body urea nitrogen; Na, sodium; K, potassium; Cl, chlorine; TCHO, total cholesterol; TG, triglycerides; LDH, lactate dehydrogenase; CPK, creatinine phosphokinase. Table 1. Serum biochemistry of male and female rats treated with high (200 mg/kg) and low (100 mg/kg) dose of Fe2O3 biosynthesized NPs
To assess nephrotoxicity, Renal Function Tests were performed along with electrolytes assays. Results reveal that the male rats exhibited a non-significant rise in creatinine level (20%), while BUN increased by 18% at high dose compared to low dose (−2%). In female rats, the opposite trend was observed. Non-significant effects of Fe2O3 NPs on serum electrolytes was observed in both male and female rats and their values were almost similar to their respective control (Table 2). Metals that are considered biocompatible to living system such as titanium oxide and iron oxide NPs, among others, do not show any form of toxicity to the renal system.
Tests Control High dose % Inc/Dec Low dose % Inc/Dec Male CAT (U/min) 2.33 0.60 −74 1.06 −54 POD (U/min) 0.23 0.13 −43 0.13 −43 SOD (U/mg protein) 16.72 2.72 −84 4.91 −71 GSH (nmol·L−1·min·mg protein) 184.34 172.19 −7 190.52 3 Protein (g/dL) 5.6 4.40 −21 4.66 −17 TBARS (nmol·L−1·min·mg protein) 27.15 18.56 −32 16.91 −38 Nitrite (μmol·L−1·mL) 30.52 40.38 32 33.71 10 Female CAT (U/min) 3.63 0.60 −83 2.46 −32 POD (U/min) 0.23 0.05 −84 0.13 −60 SOD (U/mg protein) 1.98 2.82 43 3.32 68 GSH (nmol·L−1·min·mg protein) 223.54 189.97 −15 196.51 −12 Protein (g/dL) 5.36 4.83 −10 5.10 −5 TBARS (nmol·L−1·min·mg protein) 22.18 22.01 −1 19.97 −10 Nitrite (μmol·L−1·mL) 21.22 23.97 20 21.78 3 Note. Inc/Dec, increase/decrease; CAT, catalase; PD peroxidase; SOD, superoxide dismutase; GSH, reduced glutathione; TBARS, Thiobarbituric acid reactive substances. Table 2. Status of antioxidant enzymes, protein, TBARS and nitrites of male and female rats treated with high (200 mg/kg) and low (100 mg/kg) dose of Fe2O3 biosynthesized NPs
For the heart function tests, total cholesterol, and triglycerides were significantly higher in male rats at high and low and dose administrations. LDH and CPK were significantly higher in male rats at high dose, while non-significant increases were observed at high dose followed by low dose administration in the female rats. Similar to our findings, Wistar rats upon oral exposure of TiO2 NPs, showed an increase in TC but decrease in TG levels[6].
Results demonstrate that CAT and POD were significantly suppressed in both male and female rats, while SOD was significantly reduced in male rats (84% and 71%) but elevated at low (68%) and high (43%) dose in female rats, respectively (Table 2). The GSH did not show any significant change in both male and female rats related to their respective control. Results also reveal that the protein content reduced in both male and female groups, although in females, protein level reduction was less when compared to other groups. The level of TBARs reduced in male and female rats as compared with their respective control (Table 2). Nitrite was significantly increased in male (78%) and female (44%) rats at high dose followed by low dose administration. The most common mechanism behind toxicity of nanoparticles is oxidative stress, which results in production of reactive oxygen species which, in turn, overwhelms the antioxidant system[7]. These findings are in contrast to those of other reports where CAT, SOD, and GSH was reduced significantly even at very low dose administration of chemically synthesized NPs.
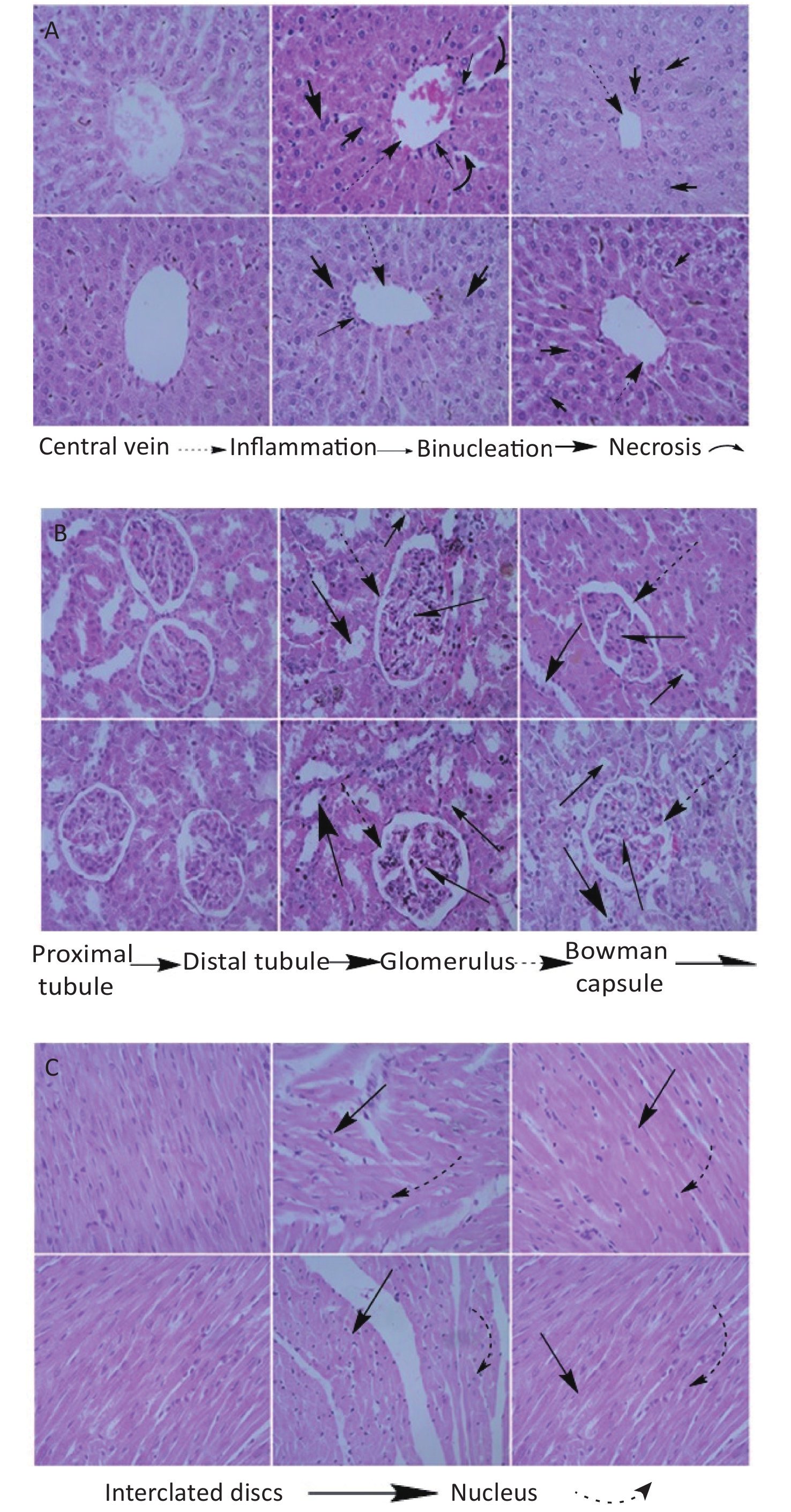
High dose Fe2O3 NPs exposure caused liver necrosis in male (Figure 1A). Minor inflammation in liver was also observed in female rats, however, it was not as obvious as in the male rats. The central vein was intact in all groups. These findings are corroborated by serum biochemical indices, where ALT, AST, and ALP were significantly elevated at high dose in male as compared to the other groups. Morphological changes in renal tissues showed dilation of tubules in male at high dose administration. Overall, no severe renal pathology was seen. Bowman’s capsule and glomerulus were intact in all the groups. At high dose, male heart had minor alteration in intercalated disks, however, this alteration was lesser in female at high dose administration. The pancreas histology showed almost normal architecture in all groups (Figure 2A). The most obvious change was the prominence of the brown crystals in all groups, except the control groups. Suggestively, this might be as a result of the Fe2O3 NPs administered to the rats. No pathological signs in the brain histology was seen in any group (Figure 2B). However, the testis of male rats treated with low dose showed comparable number of spermatids relative to control, which reduced at high dose administration, although the reduction was not significant (Figure 2C). Basement membrane in all groups were intact. Ovary images showed developing oocytes and similar pattern of nucleus as seen in control. Histological images of rat’s lungs show intact bronchioles in all groups, except in male rats treated with high dose. Focal tubular impairment in kidney and remarkable white pulp and congestion or stuffing of red pulp in spleen have been observed following exposure of rats to Fe2O3 NPs[8]. Intratracheal administration of Fe2O3 NPs causes hyperplasia characterized by follicular lymphoid, with aggregation of inflammatory cells around bronchia in rats[9]. Results from this study are contradictory to majority of studies, and one of the possible reasons might be the involvement of phytochemicals or antioxidants that caps the NPs during fabrication, hence the lessened toxicity of the naked NPs.
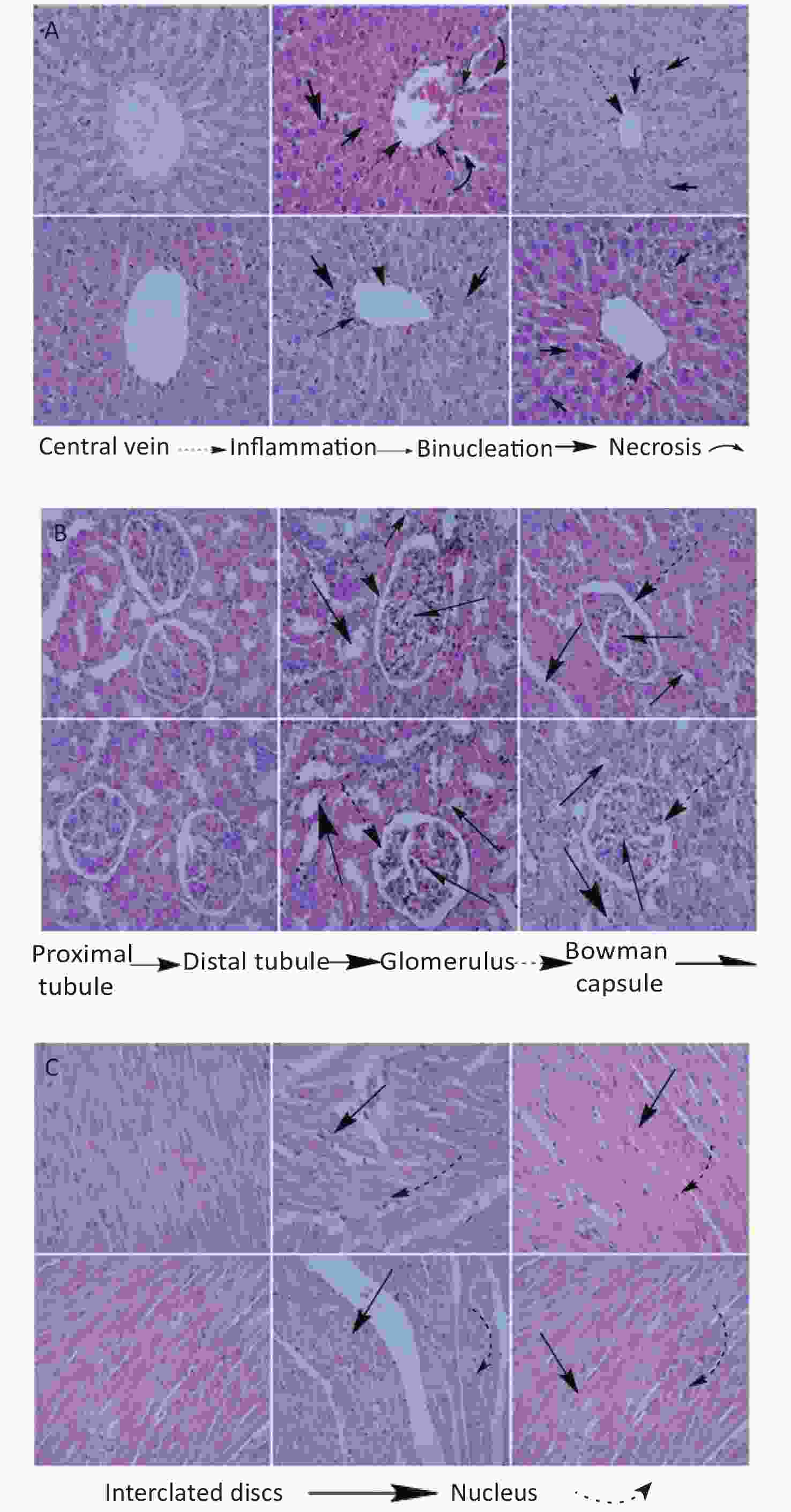
Figure 1. Histology of organs of rats treated with high (200 mg/kg) and low (100 mg/kg) dose of Fe2O3 biosynthesized NPs. (A) Histology of liver, (B) Kidney, (C) Heart. From left to right control, high and low dose male in the upper row and control, high and low dose female in lower row. Slides were examined under Nikon Microscope Eclipse 80i and photographed were captured at 40X.
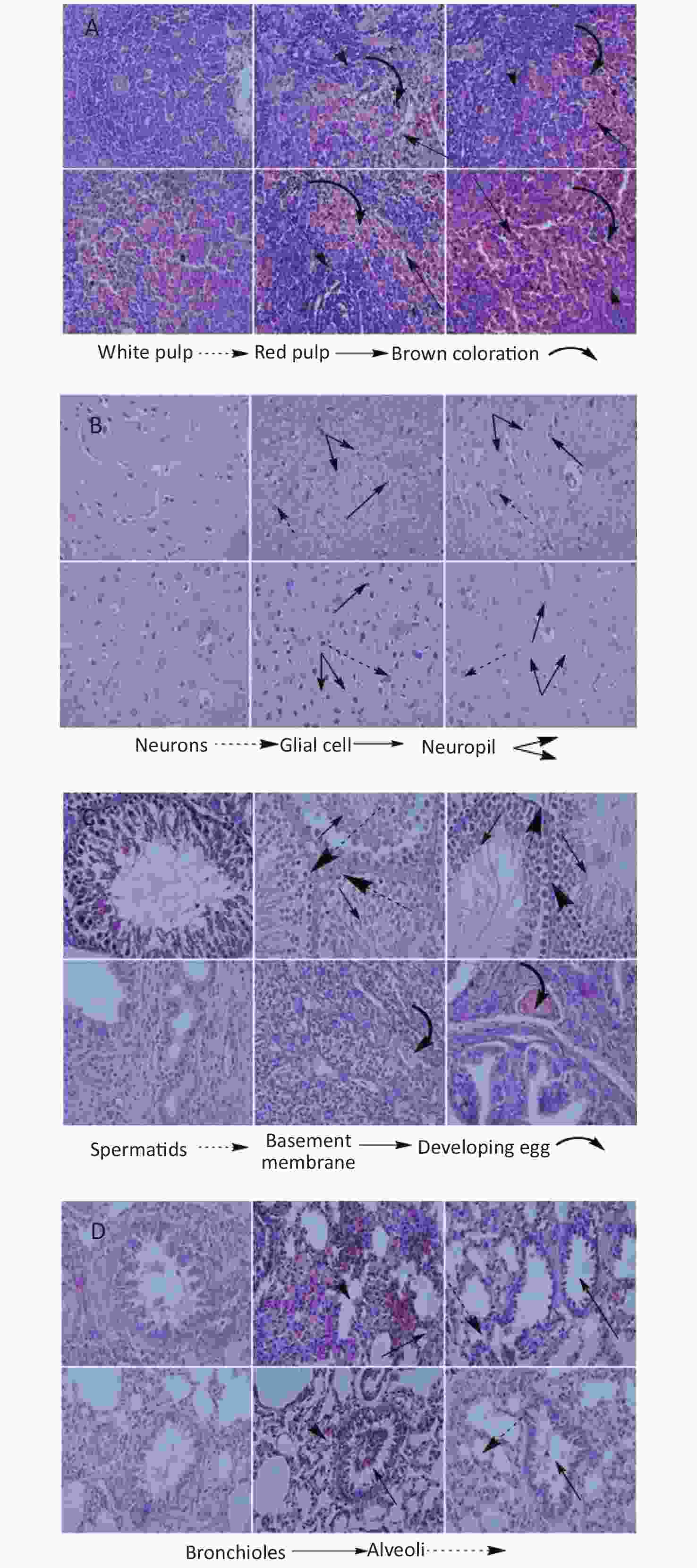
Figure 2. Histology of organs of rats treated with high (200 mg/kg) and low (100 mg/kg) dose of Fe2O3 biosynthesized NPs. (A) Histology of Pancreas, (B) Brain, (C) Gonads, (D) Lungs. From left to right control, high and low dose male in the upper row and control, high and low dose female in lower row. Slides were examined under Nikon Microscope Eclipse 80i and photographed were captured at 40X.
Plant based synthesis is ecofriendly and therefore reduces the associated toxic effects linked with conventional synthetic approaches. Male rats were more affected as compared to female at low dose administration. It can also be concluded that plant based synthesis of NPs overcome toxic effects associated with chemically synthesized NPs, and this was probably due to the capping of NPs with plant based materials. However, toxicity and biocompatibility of NPs is concentration and gender dependent. Moreover, these outcomes explains the significance of green chemistry, and supports their use in future biomedical research.
Toxicity Evaluation of Green Synthesized Iron Oxide Nanoparticles in Sprague-Dawley Rats: Biochemical and Histological Assessment
doi: 10.3967/bes2020.121
- Received Date: 2020-04-21
- Accepted Date: 2020-05-15
| Citation: | Sania Naz, Muhammad Majid, Muhammad Zia. Toxicity Evaluation of Green Synthesized Iron Oxide Nanoparticles in Sprague-Dawley Rats: Biochemical and Histological Assessment[J]. Biomedical and Environmental Sciences, 2020, 33(11): 882-886. doi: 10.3967/bes2020.121 |







 Quick Links
Quick Links
 DownLoad:
DownLoad:
