-
Yersinia pestis, the causative agent of bubonic and pneumonic plague, is considered to be one of the most dangerous and deadly pathogenic bacteria in the world[1]. Y. pestis is classified as a category A pathogen by the United States Center for Disease Control and Prevention because of its ease of aerosol-to-human transmission, high lethality, and potential for mass casualties as a biological warfare agent[2]. Therefore, both prevention and treatment require the development of a rapid, sensitive, and specific method to detect Y. pestis.
Traditional methods for the detection of Y. pestis mainly include bacterial isolation and microscopic observation, enzyme-linked immunosorbent assay (ELISA) based on antibody-mediatedidentification of the F1 antigen, conventional polymerase chain reaction (PCR) detection, a phage lysis test, and optical fibers or an up-conversion fluorescence biosensor[3]. The fiber optic biosensor uses the purified antibody against antigen FI immobilized on polystyrene probes as the capture antibody and the monoclonal antibody-Cy5 conjugate as the detector[4]. The up-conversion fluorescence biosensor uses 400 nm up-converting phosphor particles as the reporter[5]. These methods play a significant role in the detection of Y. pestis. However, each technique has its own shortcomings, such as assay time, and the need for costly equipment and highly skilled personnel. Over the past few decades, several non-PCR isothermal amplification techniques have been developed, based on the molecular biology of DNA/RNA synthesis. These techniques do not require the use of thermal cyclers, making it easy to detect nucleic acids at a constant temperature. Two of the most successful methods areloop-mediated isothermal amplification (LAMP) technology and recombinase polymerase amplification (RPA). Feng et al.[6] took advantage of LAMP to detect Y. pestis, which would be an ideal assay if this technology were inexpensive and not time-consuming. Euler et al.[7] achieved good results using RPA to detect Y. pestis, but the plasmid-harbored pla gene is less labile. The target genes on chromosome might be more stable. In thisstudy, we constructed a real time recombinase-aided amplification (rt-RAA) method to detect Y. pestis based on the 3a sequence on chromosome, which might recognize a greater amount of samples. Consequently, a simple, rapid, and effective diagnostic method is still urgently needed for long-term surveillance of plague foci in poverty-stricken areas to predict future rodent-borne epidemics and human exposure risks, or for on-site investigation of suspected bioterrorism samples.
Recombinase-aided amplification (RAA) is a new isothermal amplification technology that is receiving much attention for its efficient amplification of DNA with high specificity and sensitivity under isothermal conditions of 39 °C–42 °C in less than 30 minutes[8]. rt-RAA uses the fluorescence signal collected during the amplification process to achieve real-time detection. This method only requires a simple water bath or similar stable heating method, making it suitable for field applications, especially in poor regions. In addition, many scholars have achieved satisfactory results using this technique to detect pathogens[8, 9].
In this study, we aimed to develop an rt-RAA assay for the detection of Y. pestis. We evaluated the analytical sensitivity and specificity of the rt-RAA assay, and tested whether it could be used to detect Y. pestis in simulated tissue samples.
The strains used for specificity test in this study were shown in Table 1. The 3a genetic sequence is commonly chosen as the target region for the detection of Y. pestis[10]. All of the 3a sequences available for Y. pestis were obtained from the National Center for Biotechnology Information (NCBI) database. The Oligo7 software was used to design the primers and probes (Table 2). Each rt-RAA reaction contained the following components in the reaction mixture: 1 μL DNA template, 25 μL rehydration buffer, 16.7 μL ddH2O, 2.1 μL primers (10 μmol/L), and 0.6 μL target-specific rt-RAA exo-probe (10 μmol/L). Finally, the 47.5 μL mixture/template solution was transferred to each lyophilized rt-RAA particle provided in the kit. In each reaction, 2.5 μL of 280 mmol/L magnesium acetate was dripped into the cap of the tube. The cap of the tube was carefully closed, the contents were mixed gently by swirling, followed by brief centrifugation. The magnesium acetate dripped into the reaction mixture during centrifugation, initiating the rt-RAA reaction. Then, rt-RAA fluorescence detection device rt-RAA-F1620 (Jiangsu Qitian Bio-Tech Co. Ltd., China) transferred the test tube to the test tube rack for 20 min of amplification. Y. pestis DNA and ddH2O were included as positive and negative controls, respectively. Conventional rt-PCR was performed according to the protocols of the Superreal premix Plus (SYBR green) FP205 (Tiangen Biochemical Technology Co. Ltd., China) in the rt-PCR fluorescence detection device CFX96 Real-Time system (Bio-Rad, USA).
Bacteria strain Description Y. pestis EV761 live attenuated vaccine strain Y. pestis 2012 biovar Microtus strain Y. enterocolitica ATCC96103 standard ATCC strain Y. enterocolitica 52204 Ya96 Y. enterocolitica 52207 Ya885 Y. pseudotuberculosis 3384 serotype O1a, isolated from bird, Denmark Y. pseudotuberculosis Pa36064 serotype O1b, isolated from human, Japan Y. pseudotuberculosis Kuratani serotype O1c, isolated from water, Japan Y. pseudotuberculosis NO.49 serotype O2a, isolated from human, Japan Y. pseudotuberculosis NO.1799 serotype O2b, isolated from human, From Dr. Knapp Y. pseudotuberculosis 274 serotype O2c, isolated from pig, Japan Y. pseudotuberculosis 83 serotype O3, isolated from human, France Y. pseudotuberculosis 51 serotype O4a, isolated from pig, Japan Y. pseudotuberculosis Pa3422 serotype O4b, isolated from human, Japan Y. pseudotuberculosis 204 serotype O5a, isolated from human, Japan Y. pseudotuberculosis 197 serotype O5b, isolated from pig, Japan Y. pseudotuberculosis DD110 serotype O6, isolated from dog, Japan Y. pseudotuberculosis 141 serotype O7, isolated from mouse, Japan Y. pseudotuberculosis 151 serotype O8, isolated from pig, Japan Y. pseudotuberculosis R708Lv serotype O9, isolated from wild rat, Japan Y. pseudotuberculosis 6088 serotype O10, isolated from raccoon dog, Japan Y. pseudotuberculosis R80 serotype O11, isolated from wild rat, Japan Y. pseudotuberculosis MW864-2 serotype O12, isolated from mountain water, Japan Y. pseudotuberculosis N916 serotype O13, isolated from house rat, China Y. pseudotuberculosis CN3 serotype O14, isolated from wild rat, China Y. pseudotuberculosis 93422 serotype O15, isolated from human, Korea E. coli DH5α engineerd E. coli strain Enterobacter aerogenes 3-SP5 clinical strain isolated from human, China Klebsiella pneumoniae W14 clinical strain isolated from human, China Serratia marcescens WK2050 clinical strain isolated from human, China Note. 1. Cui Y, Yang X, Xiao X, et al. Genetic variations of live attenuated plague vaccine strains (Yersinia pestis EV76 lineage) during laboratory passages in different countries. Infect Genet Evol, 2014; 26, 172–9.
2. Zhang Q, Wang Q, Tian G, et al. Yersinia pestis biovar Microtus strain 201, an avirulent strain to humans, provides protection against bubonic plague in rhesus macaques. Hum Vaccin Immunother, 2014; 10, 368–77.
3. Kot B, Blaszczyk M. The application of PCR fingerprinting to the differentiation of Yersinia enterocolitica strains isolated from humans and pigs. Acta Microbiol Pol, 2003; 52, 355–9. 4. Zhou Y, Zhou J, Ji Y, et al. Bioluminescent tracing of a Yersinia pestis pCD1(+)-mutant and Yersinia pseudotuberculosis in subcutaneously infected mice. Microbes Infect, 2018; 20, 166–75. 5. Chen Z, Li H, Feng J, et al. NDM-1 encoded by a pNDM-BJ01-like plasmid p3SP-NDM in clinical Enterobacter aerogenes. Front Microbiol, 2015; 6, 294.Table 1. Bacterial strains used in this study
Primer Type Sequence (5′–3′) 3a-F RT-RAA forward primer AAGATATGTGATGATTAAGTTCATGCTGCTTTA 3a-R RT-RAA reverse primer TACAACACGGATATGTACAACGACATTCTTA EV76-Ta RT-RAA probe ACAGACACTCAAGGCGCTTAACCGAGCCTT/i6FAMdT/C/THF/G/iBHQdT CCGGCAGCCGATGA (C3-spacer) 3aP-F RT-PCR forward primer ACTACCATCCCCTCAAGGTT 3aP-R RT-PCR reverse primer GAGGGCGTTTTGGTAGAGAA Note. aFor probe modifications i6FAMdT, 6-Carboxyfluorescein; THF, tetrahydrofuran; iBHQdT, black hole quencher; C3-spacer, 3′ phosphate blocker. Table 2. Primer and probe sequences used for RT-RAA and RT-PCR assays
To determine the sensitivity of the rt-RAA assay for the detection of Y. pestis EV76, we used sequential 10-fold dilutions of bacterial genomic DNA as a template for rt-RAA fluorescence detection. The results showed that all the dilutions of bacterial genomic DNA, from 1.74 × 105 to 1.74 × 101 copies per reaction, produced apositive result in the assays. Two of the nine replicates with 1.74 copies per reaction tested positive in the rt-RAA assay. Statistical analyses indicated that the detection limits of the rt-RAA assay were thus 17.4 copies per reaction for Y. pestis DNA (binomial distribution, P = 0.04 < 0.05). In addition, rt-RAA was able to detect a change in fluorescence signal at 2–4 min, with a final result available after 20 min.
To determine the specificity of rt-RAA detection for Y. pestis, we extracted 30 bacterial genomic DNAs extracted from pure culture and applied them as templates at 1 × 103 copies of DNA per reaction for rt-RAA and real-time quantitative PCR (rt-qPCR) fluorescence detection. Fluorescence signals were only detected in the reactions containing Y. pestis EV76 and Y. pestis 201 DNA, whereas no fluorescence signal was detected for any of the other bacterial DNA samples (Figure 1). Kappa test results (к = 1.0, P < 0.01) indicated that the rt-RAA and rt-PCR assays both demonstrate good consistency in the detection of Y. pestis.
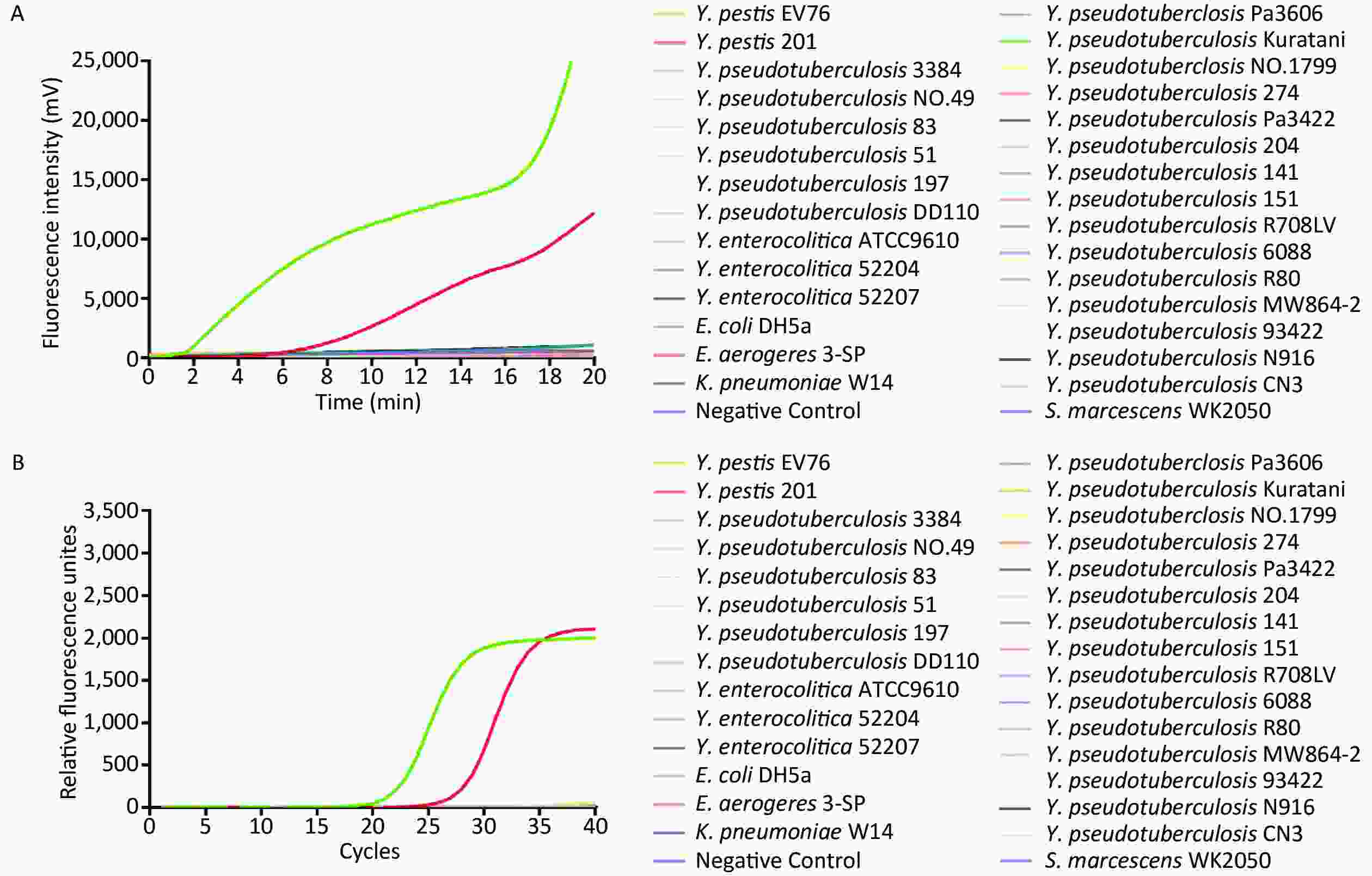
Figure 1. The specificity (A, B) of rt-RAA and rt-qPCR assays for the detection of Y. pestis using 30 bacterial genomic DNAs. (A) rt-RAA assay results. (B) rt-qPCR assay results.
To verify the feasibility of the rt-RAA assay for the detection of biological samples, we artificially contaminated mouse blood with different concentrations of bacteria and then extracted DNA from the mixed sample for analysis by rt-RAA. No fluorescent signal was detected in the negative control or PBS groups, but a fluorescent signal was detected in the samples contaminated with Y. pestis EV76. As the bacterial concentration increased in the biological samples, a concomitant increase in the fluorescence signal was detected, confirming that the rt-RAA assay can effectively detect Y. pestis in blood. We also used this method to test contaminated liver, lung, and spleen samples, and the results demonstrated that rt-RAA can be used to detect Y. pestis in a range of biological samples.
The 3a gene, located in a 41.7-kb Y. pestis-specific region, has been used as a target gene in previous studies for the detection of Y. pestis[10]. Amplification using a primer pair designed to the 3a gene sequence has been used to identify isolates of Y. pestis without producing false positives for closely related organisms, which is an important criterion for unambiguous bacterial identification[10]. In this study, we selected the 3a gene as a target sequence and established a new constant-temperature real-time fluorescence detection assay for the rapid detection of Y. pestis. This alternative chromosomal target (3a gene) is present at a lower copy number but has the advantage of being less labile, unlike the plasmid–harbored genechosen by Euler et al.[7]. Our assay had a detection limit of 17.4 gene copies per reaction. Yan et al.[11] and Chen et al.[8] employed RAA to detect coxsackievirus A6 and respiratory syncytial virus (RSV), respectively, and demonstrated that RAA could be used to quantitatively amplify the virus genome in real-time[9]. Our study drew similar conclusions, confirming that RAA could effectively and specifically detect Y. pestiswith the same specificity as rt-qPCR. In addition to the faster processing time, the requirement for a constant temperature of 39 °C is another advantage of rt-RAA over rt-PCR, which normally requires a range ofcycling temperatures performed under rigorous conditions. The rt-RAA detection system does not require a sophisticated laboratory setting or expensive equipment, and can be performed using a portable device. Furthermore, the results can be obtained by recording fluorescence without opening the sample tubes, which minimizes concern regarding contamination ofthe amplified products. Despite its high sensitivity and specificity, the expensive instrumentation and need for skilled operators restricts the wide clinical application of rt-qPCR, whereas RAA has the potential for rapid detection of Y. pestis in the field.
To verify that rt-RAA can be accurately and reliably applied forbiological sample detection, we tested the assay on mouse tissue samples. We selected the blood, liver, spleen, and lung tissues of mice for monitoring. It has been reported that the bacterial load of liver and lung can reach 1 × 104 and 1 × 106 CFU/g after 4 h of infection, and that of blood and spleen can reach 1 × 103 CFU/g after 36 h[12]. The rt-RAA assay was sensitive enough to detect the tissues which were blended with the 1 × 103 CFU bacterial suspension.These results showed that RAA technology can rapidly detect pathogens and the sensitivity of this technique shouldbe sufficient for clinical testing needs.
In conclusion, the rt-RAA assay described here is a sensitive and specific method for the rapid detection of Y. pestis. However, use of the RAA assay still has some limitations to overcome. For example, nucleic acid extraction must be performed before sample detection, and the premixing step is indispensable. Both of these processing steps can affect the results[11]. Optimizing the assay to overcome these shortcomings will be the focus of future studies.
The authors declare no competing financial interests.
WEI Xiao, LI Yan, and LU Xin performed the experiments and contributed equally to this study as joint first authors. ZHAO Rong Tao and YUAN Zheng Quan provided the bacterial strains. SHI Hua and ZHAO Xiang Na wrote the article.
Rapid Detection of Yersinia pestis by Real-time Recombinase-aided Amplification
doi: 10.3967/bes2021.040
- Received Date: 2020-06-11
- Accepted Date: 2020-08-31
| Citation: | WEI Xiao, LI Yan, LU Xin, ZHAO Rong Tao, YUAN Zheng Quan, SHI Hua, ZHAO Xiang Na. Rapid Detection of Yersinia pestis by Real-time Recombinase-aided Amplification[J]. Biomedical and Environmental Sciences, 2021, 34(4): 309-313. doi: 10.3967/bes2021.040 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: