-
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by a combination of hyperglycemia, reduced insulin sensitivity, and/or relative impairment of insulin secretion[1]. Renal damage, which is a major microvascular complication of DM and a progressive kidney disease, has been considered the most common cause of end-stage renal disease[2]. Renal fibrosis is a common outcome of progressive renal damage, which could be induced by renal inflammation due to sustained hyperglycemia stimulation that drives the kidney’s repair response to the damage process[3]. Hyperglycemia-activated inflammation plays a crucial role in the pathogenesis of renal damage.
Pterostilbene (PTE), a bioactive component of blueberries, has gained much attention recently due to its beneficial biological functions resembling to resveratrol on various chronic human diseases[4]. PTE has strong antioxidant, anti-inflammatory, and anti-DM activities[5]. A previous study on streptozotocin (STZ)-induced rat models of DM indicated that PTE treatment could reduce serum glucose and insulin levels. However, the potential molecular mechanism underlying the effects of PTE on renal damage remains unclear. Therefore, in this study, we examined the potential effects of PTE on renal damage in DM rats and its underlying mechanisms.
A total of 70 specific pathogen-free grade male Sprague–Dawley (SD) rats (about 4 weeks old; weight, 170–220 g) were purchased from the Henan Province Experimental Animal Center. All rats were housed under controlled temperature (22 ± 2 °C), in 50%–60% relative humidity, with a 12-h day/night cycle, and had free access to food and water.
All experimental protocols were approved by National Research Council Guide for the Care and Use of Laboratory Animals. After 1 week of adjustable feeding, rats were randomly divided into six weight-matched groups. Ten rats were randomly allocated to a normal control group that received normal feeding, while the remaining rats were allocated to the experimental group and fed a high-sugar and high-fat diet (basic food, 59.5%; sucrose, 20%; lard oil, 10%; yolk powder, 10%; and sodium cholate, 0.5%). Diabetes induction was performed using STZ (40 mg/kg body weight). In our study, 58 rats were successfully established as DM models and were then randomized into five groups (n = 10 per group): diabetic control group (DM group), PTE-treated DM groups (20, 40, or 80 mg/(kg∙day), PTE20, PTE40, and PTE80 groups), and RSG-treated DM group [5 mg/(kg∙day), RSG group]. The remaining DM rats and unsuccessful DM rats were sacrificed. PTE and RSG were dissolved in 0.5% sodium carboxymethyl cellulose (CMC-Na) and administered to rats by oral gavage, whereas the DM group received 0.5% CMC-Na aqueous solution. After 12 weeks, all rats were anesthetized and sacrificed. After the experiment, blood and rat kidneys were collected, and a part of each rat kidney was stained with hematoxylin and eosin (HE).
The levels of fasting blood glucose (FBG), fasting insulin (FINS), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were evaluated using the Excellence 360 Automatic Biochemical Analyzer (KHB Co., Shanghai, China). Blood urea nitrogen (BUN) and serum creatinine (SCR) levels were determined by assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), according to the manufacturer’s instructions.
Kidney tissues (100 mg) were homogenized in tissue protein extraction kits and quantified by bicinchoninic acid protein assay. Samples containing 30 μg of denatured protein were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. The membranes were blocked and incubated at 4 °C with respective antibodies against glyceraldehyde 3-phosphate dehydrogenase (1:5,000, Dingguo Changsheng Biotech, Beijing, China), peroxisome proliferator-activated receptor (PPAR-γ), NF-κBp65, transforming growth factor beta 1 (TGF-β1), and α-smooth muscle actin (α-SMA) (1:1,000, Proteintech, China). After incubation, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies and diluted (1:5,000, Dingguo Changsheng Biotech) for 2 h at room temperature. Protein bands were detected with an enhanced chemiluminescence reagent kit, and intensities were calculated using Quantity One software. The density of the immune-reactive bands was analyzed using Image J software.
All data are presented as mean ± standard deviation (SD). The significance of differences between groups was determined with a one-way analysis of variance and was followed by Bonferroni posthoc analysis using the GraphPad Prism 8.0 software (α = 0.05).
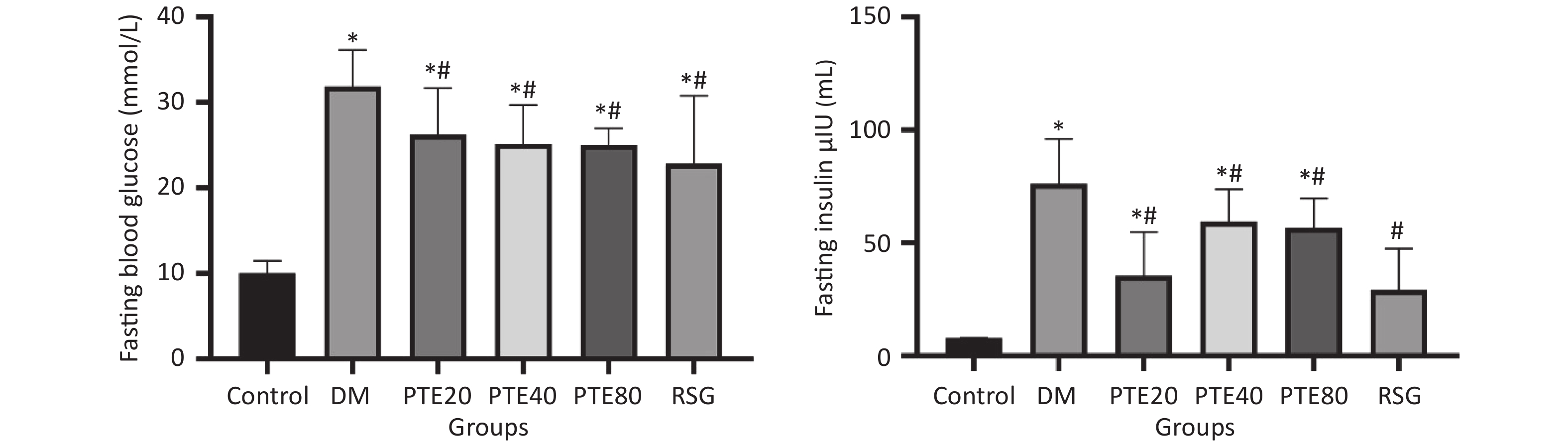
Hyperglycemia is one of the vital factors that are responsible for the structural alterations in the kidney and can induce renal damage directly or through hemodynamic modifications[6]. Thus, maintenance of glucose homeostasis is currently the mainstay of treatment for renal damage. Figure 1 shows the FBG and fasting serum insulin (FSI) levels in rats. The FBG and FSI levels of all treatment groups were markedly decreased when compared with those in the DM group after 12 weeks of treatment (P < 0.05). Moreover, PTE treatment was found to improve lipid metabolism disorders, such as reducing the levels of TC, TG, and LDL-C and increasing the level of HDL-C contents in DM rats (Table 1). These results indicated that PTE might be a high-efficient regulator of renal damage by regulating glucose and lipid metabolism. However, no dose–response relationship was found among these doses. Thus, it remains unknown whether the target sites of different concentrations of PTE are consistent, and with individual differences, the underlying mechanisms for this phenomenon need to be further explored.
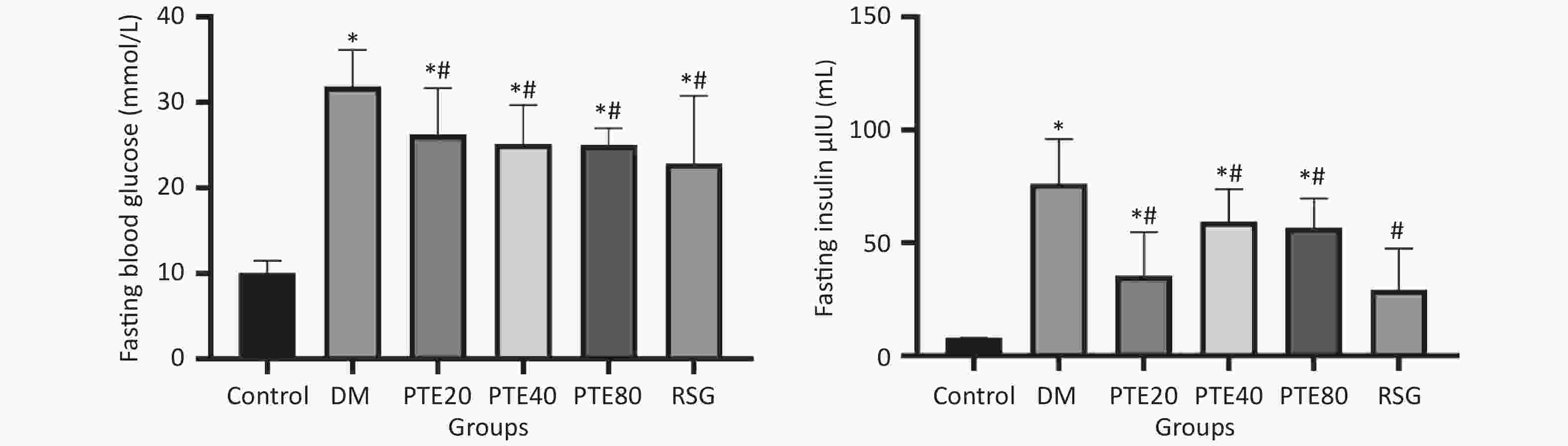
Figure 1. Effects of pterostilbene on fasting blood glucose and fasting insulin levels in diabetic rats. PTE intervention from week 0. All data are expressed as mean ± SD (n = 10 per group). *P < 0.05 compared with the normal control group. #P < 0.05 compared with the diabetic control group. Control group, normoglycemic control group; DM, diabetic control group; PTE, pterostilbene; RSG, rosiglitazone.
Groups TC (mmol/L) TG (mmol/L) LDL-C (mmol/L) HDL-C (mmol/L) SCR (mmol/L) BUN (mmol/L) Control 1.34 ± 0.23 0.65 ± 0.96 0.29 ± 0.19 1.12 ± 0.20 33.74 ± 8.68 6.17 ± 0.58 DM 3.24 ± 0.43* 4.60 ± 0.85* 1.97 ± 0.36* 0.33 ± 0.07* 163.41 ± 41.16* 26.45 ± 2.73* PTE20 2.21 ± 0.43*# 3.10 ± 0.24*# 0.71 ± 0.94*# 0.46 ± 0.58* 47.14 ± 21.61*# 20.28 ± 0.85*# PTE40 2.43 ± 0.68*# 2.20 ± 0.27*# 0.64 ± 0.33*# 0.73 ± 0.15*# 52.28 ± 15.50*# 17.15 ± 0.71*# PTE80 2.68 ± 0.14*# 1.58 ± 0.28# 0.57 ± 0.07*# 0.67 ± 0.30* 49.51 ± 13.42*# 12.52 ± 1.76*# RSG 2.63 ± 0.23*# 2.10 ± 1.41*# 0.67 ± 0.11*# 0.87 ± 0.31*# 56.57 ± 2.00*# 9.07 ± 0.35*# Note. Data are presented as mean ± SD (n = 10 per group); *P < 0.05 compared with the normal control group. #P < 0.05 compared with the diabetic control group. Control group stands, normoglycemic control group; DM, diabetic control group; PTE, pterostilbene; RSG, rosiglitazone. Table 1. Effects of PTE on serum lipid profile and kidney function
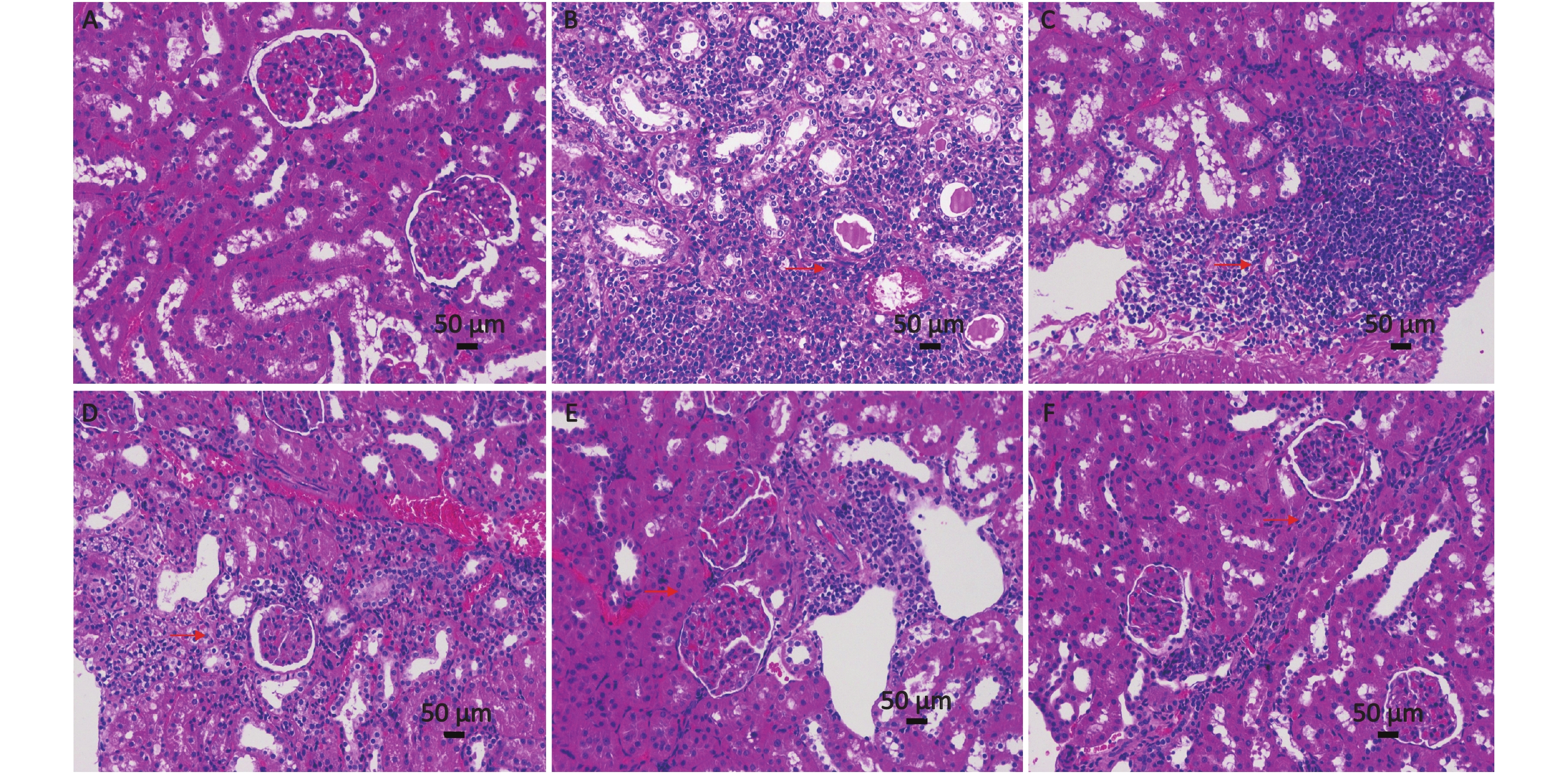
Increased levels of SCR and BUN in DM rats indicated decreased renal function. In the meantime, elevated SCR levels indicated the possibility of renal failure. SCR and BUN levels were measured to evaluate kidney function (Table 1), and HE staining of renal tissues was performed. The SCR and BUN levels in the model group were significantly higher than those in the control group, which indicates a renal function in model rats. The preservation of near-normal SCR and BUN levels after 12 weeks of PTE administration suggested that PTE was able to preserve near-normal kidney functions in DM. Moreover, the renal tissues of the control group were well organized, and no noticeable pathological change was observed (Supplementary Figure S1A available in www.besjournal.com). As for the DM group, a large number of inflammatory cells were seen in renal tissues, and the structure of the renal tubules was blurry or has disappeared (Supplementary Figure S1B). Moreover, after 12 weeks of PTE intervention, the structure of renal tissues was improved, with a small number of enlarged renal tubular epithelial cells, reduced inflammatory cell infiltration, and lessened pathological injury (Supplementary Figure S1C–E).
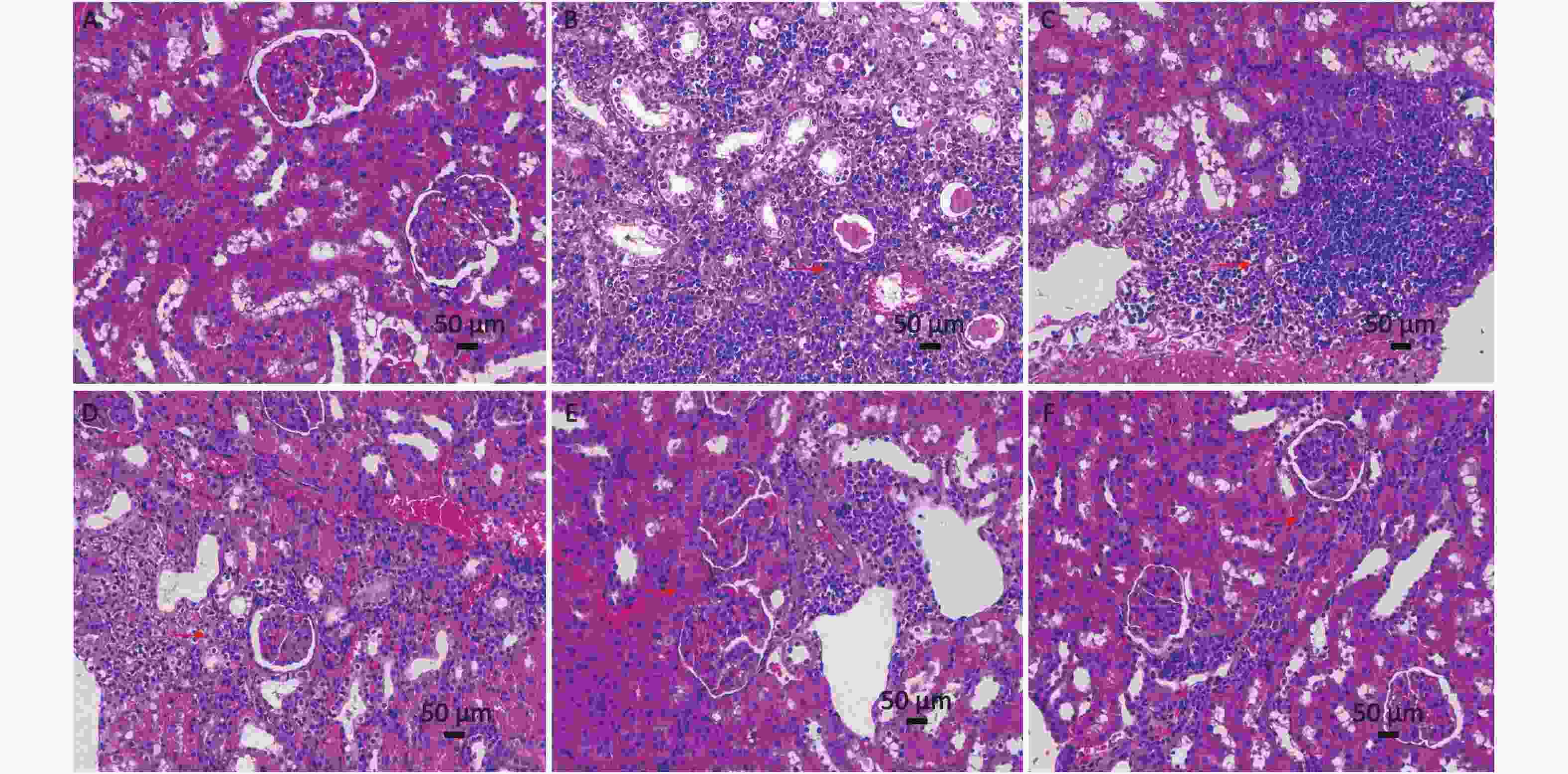
Figure S1. Effect of pterostilbene (PTE) on the histological morphology of kidney from diabetic rats. (A) Normal control rats, (B) diabetic control rats, (C) PTE 20 mg/kg treated rats, (D) PTE 40 mg/kg treated rats, (E) PTE 80 mg/kg treated rats, (F) rosiglitazone-treated rats (magnification, ×200).
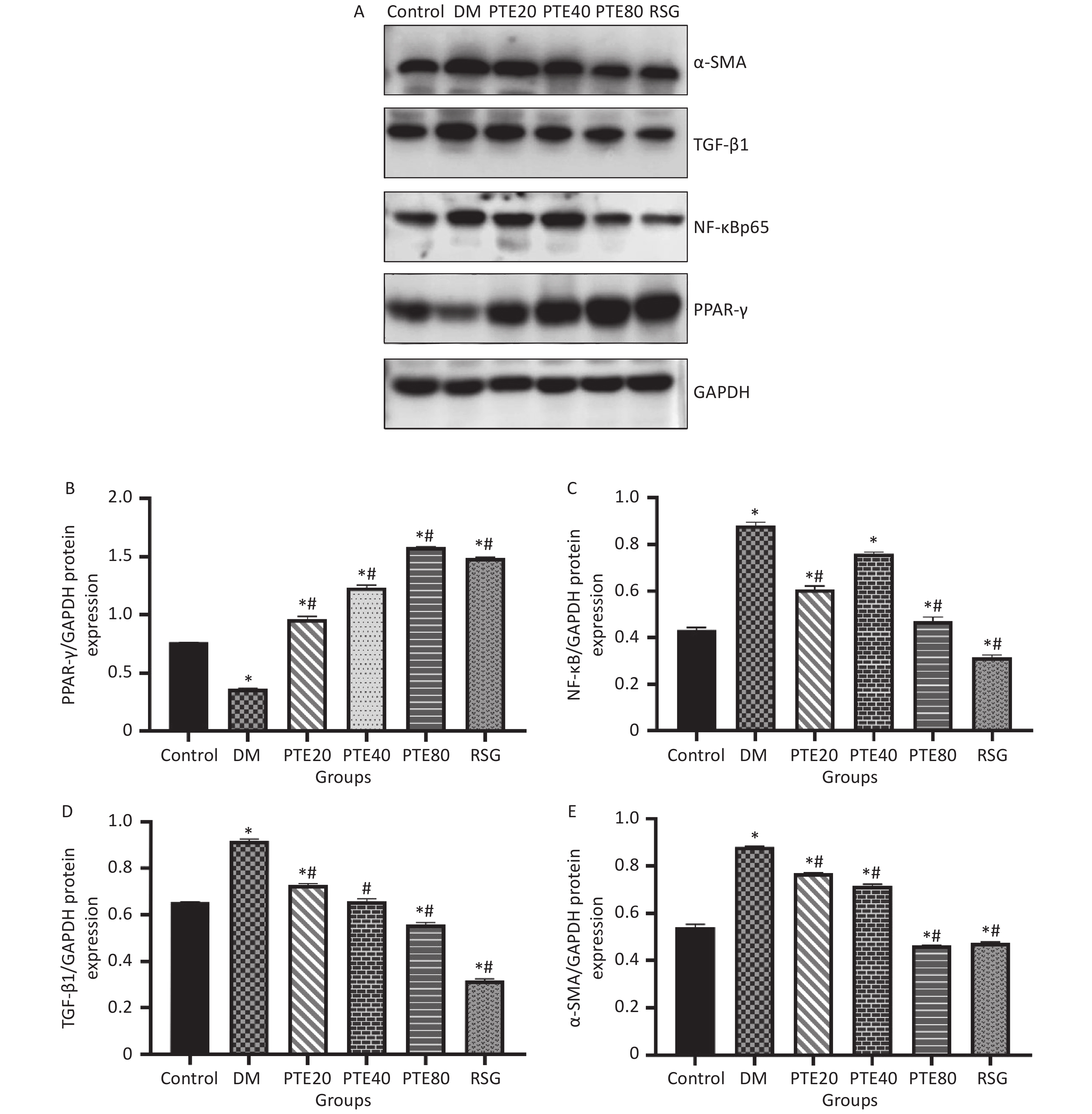
Hyperglycemia-induced inflammation and fibrosis play important roles in the pathogenesis of DN[7]. Chronic inflammation can lead to the synthesis of fibronectin cytokines and growth factors that accelerate the progression of glomerulosclerosis and tubulointerstitial fibrosis, which in turn aggravate renal damage and lead to DN. PPAR-γ is mainly expressed in the kidney and is believed to play a vital role in regulating insulin resistance, cell growth, and inflammation[8]. In recent years, cumulative evidence has demonstrated that PPAR-γ is an upstream regulator of NF-κB, and the activation of PPAR-γ can lead to the inhibition of NF-κB signaling[9]. Figure 2 shows that PTE administration to DM rats decreased the expressions of NF-κBp65 while upregulated the expressions of PPAR-γ (P < 0.05). These results were consistent with the previous finding of Qureshi, showing that PTE might be a potent proteasome inhibitor of IκBα (the inhibitory subunit of NF-κB), thus inhibiting the activation of several pro-inflammatory cytokine genes[10]. Considering the possible relationship between PPAR-γ and NF-κB, our results suggest that NF-κB may be regulated by PPAR-γ and exert subsequent biological functions. Under DM conditions, the activated NF-κB induces the expression of its target genes, including transforming growth factor-β1 (TGF-β1), resulting in extracellular matrix accumulation. In the case of DN, TGF-β1 overexpression may result in the transformation of fibroblasts into activated myofibroblasts, which is indicated by the excessive expression of α-SMA. In this study, PTE administration inhibited the expressions of TGF-β1 and α-SMA in renal tissues. Our present data suggest that PTE alleviates fibrotic response, and this effect might be caused by reductions in TGF-β1 and α-SMA expressions of DM rats.
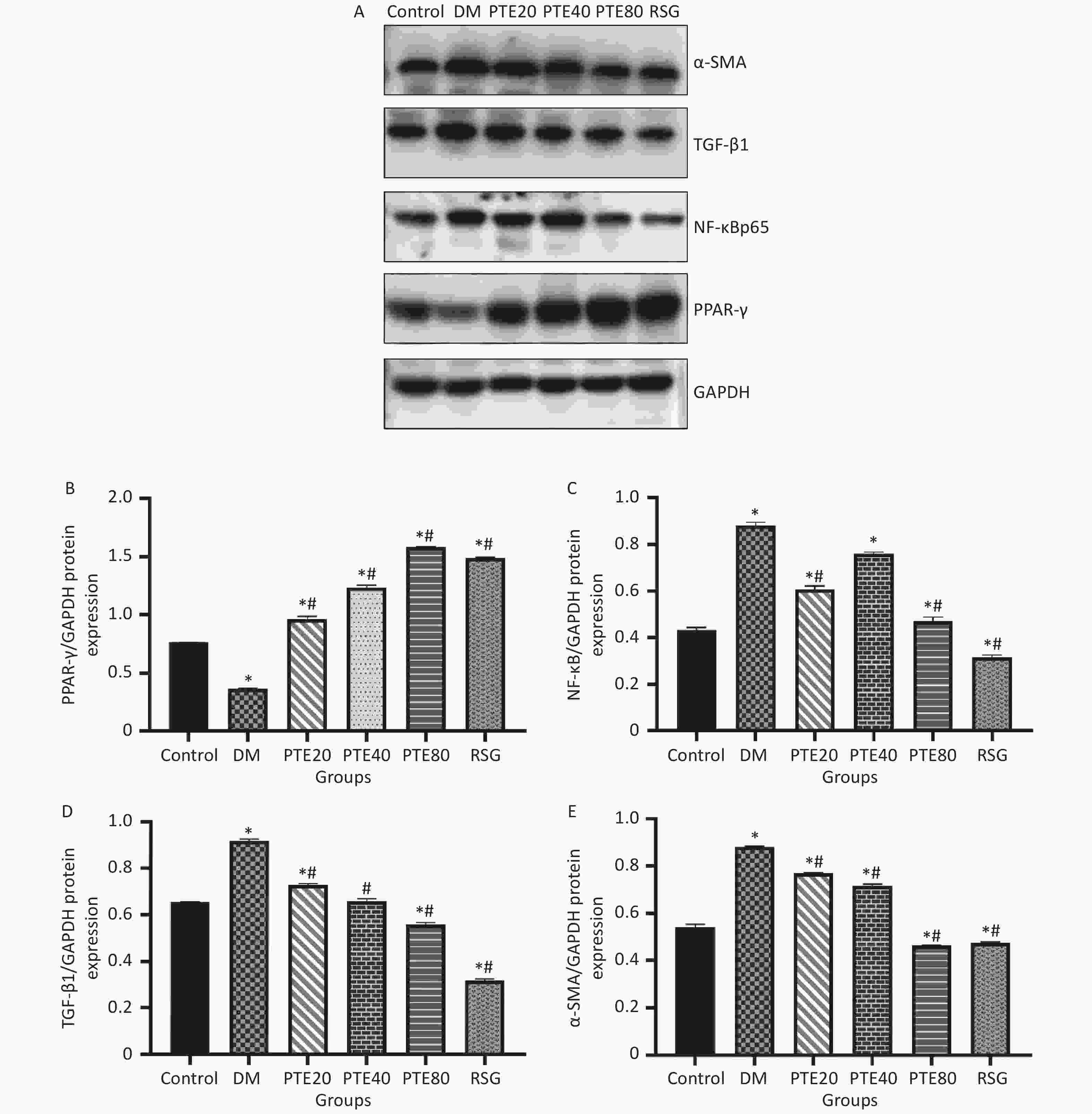
Figure 2. Effects of pterostilbene on the expression of PPAR-γ, NF-κBp65, TGF-β1, and α-SMA protein in kidney tissue of diabetic rats after 12 weeks of treatments. (A) Bands of western blot; Densitometry analysis of PPAR-γ (B), NF-κBp65 (C), TGF-β1 (D), and α-SMA (E). Values are represented as mean ± SD (n = 10). *P < 0.05 compared with the normal group. #P < 0.05 compared with the diabetic control group. Control group, normoglycemic control group; DM, diabetic control group; PTE, pterostilbene; RSG, rosiglitazone.
In summary, our study demonstrates that PTE owns ability to improve the FBG, metabolic parameters, and renal fibrosis of DM rats. PTE may activate the PPAR-γ pathway by promoting the expression of PPAR-γ and alleviating renal inflammation and fibrosis by inhibiting NF-κB, TGF-β1, and α-SMA signaling pathways and subsequently ameliorating the renal damage within DM rats. However, the specific process and mechanism need to be confirmed by further study.
Designing and drafting of the manuscript: LWJ, LX, and DRR. Acquisition of the data: HGY, ZYJ, LXX, LYM, and DRR. Statistical analysis: SHL, XZ, GJJ, and DRR. Revision of the manuscript: DRR. Study supervision: LX and LWJ. All authors read and approved the final manuscript.
HTML
Reference
 21091Supplementary Materials.pdf
21091Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: