-
Acute respiratory distress syndrome (ARDS) is a syndrome characterized by significant lung edema, impaired permeability of lung microvascular endothelial cells with the mal-expression of aquaporins (AQPs). In this study, a two-hit porcine model of ARDS induced by smoking and methicillin-resistant Staphylococcus aureus (MRSA) was developed, we investigate the AQPs expression and the influence of Ulinastatin (UTI) to them.
Forty-nine healthy male domestic pigs aged 8–10 weeks and weighing 30 ± 2 kg were used for the study. Anesthesia was maintained through continuous intravenous infusion of pentobarbital [8 mg/(kg∙h)]. A 5-Fr PiCCO catheter (Pulsiocath PV2015L20; Pulsion Medical Systems, Munich, Germany) and a central venous catheter was inserted for hemodynamic monitoring, including mean arterial pressure (MAP), cardiac index (CI), systemic vascular resistance index (SVRI) and extravascular lung water index (ELWI). Oxygen dynamics, including oxygen supply (DO2), oxygen consumption (VO2) and oxygen extraction rate (ERO2) were calculated by the data of blood gas analysis. Mechanical ventilation was provided by a cuffed 6.5-mm endotracheal tube to maintain an end tidal concentration of carbon dioxide (EtPCO2) of 35–40 mmHg and arterial oxygen saturation (SpO2) of more than 90%, respectively. The thoracopulmonary pressure-volume (P-V) curves were obtained by the constant low-flow method to calculate the thoracopulmonary compliance (Ctp)[1]. The percentage of dead space (Vd) was measured by the difference of PaCO2 and EtPCO2.
Animals were randomly assigned to one of five groups: a sham group (SH, n = 5); an ARDS group (RD, n = 11); an ARDS group treated with 25 mg/kg of vancomycin (500 mg, Eli Lilly, IN) (VM, n = 11); an ARDS group treated with 30,000 U/kg of UTI (50,000 U, Techpool, Guangdong, China) + 25 mg/kg of vancomycin (3U, n = 11) and an ARDS group treated with 50,000 U/kg of UTI + 25 mg/kg of vancomycin (5U, n = 11). No other procedures apart from catheter insertion and mechanical ventilation were performed in the SH group. Animals were exposed to smoke from 50 g of burning cotton using a bee smoker according to the previously described method except the sham group[2]. Following smoke-induced lung injury, 3 × 1011 colony forming units of live MRSA were instilled into the lungs of animals except sham group via a bronchoscope[2]. Afterwards, 30,000 U/kg and 50,000 U/kg of UTI in 100 mL of saline solution were infused into the central venous catheter within 30 min in the 3U and 5U group respectively. Twenty-five mg/kg of vancomycin in 100 mL saline was infused more than 60 min at the same time in the animals of VM, 3U and 5U group. All animals were continuously monitored for 24 h or until death. At the end of the protocol, blood culture was performed. AQPs expression were tested by Western blot and immunohistochemistry respectively.
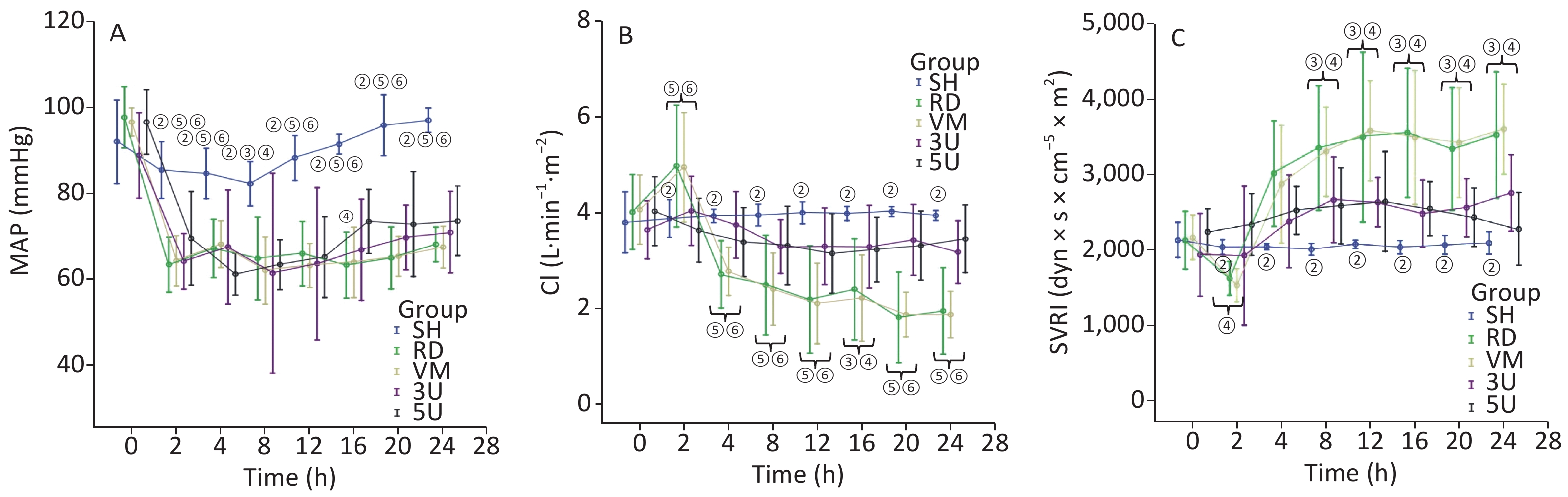
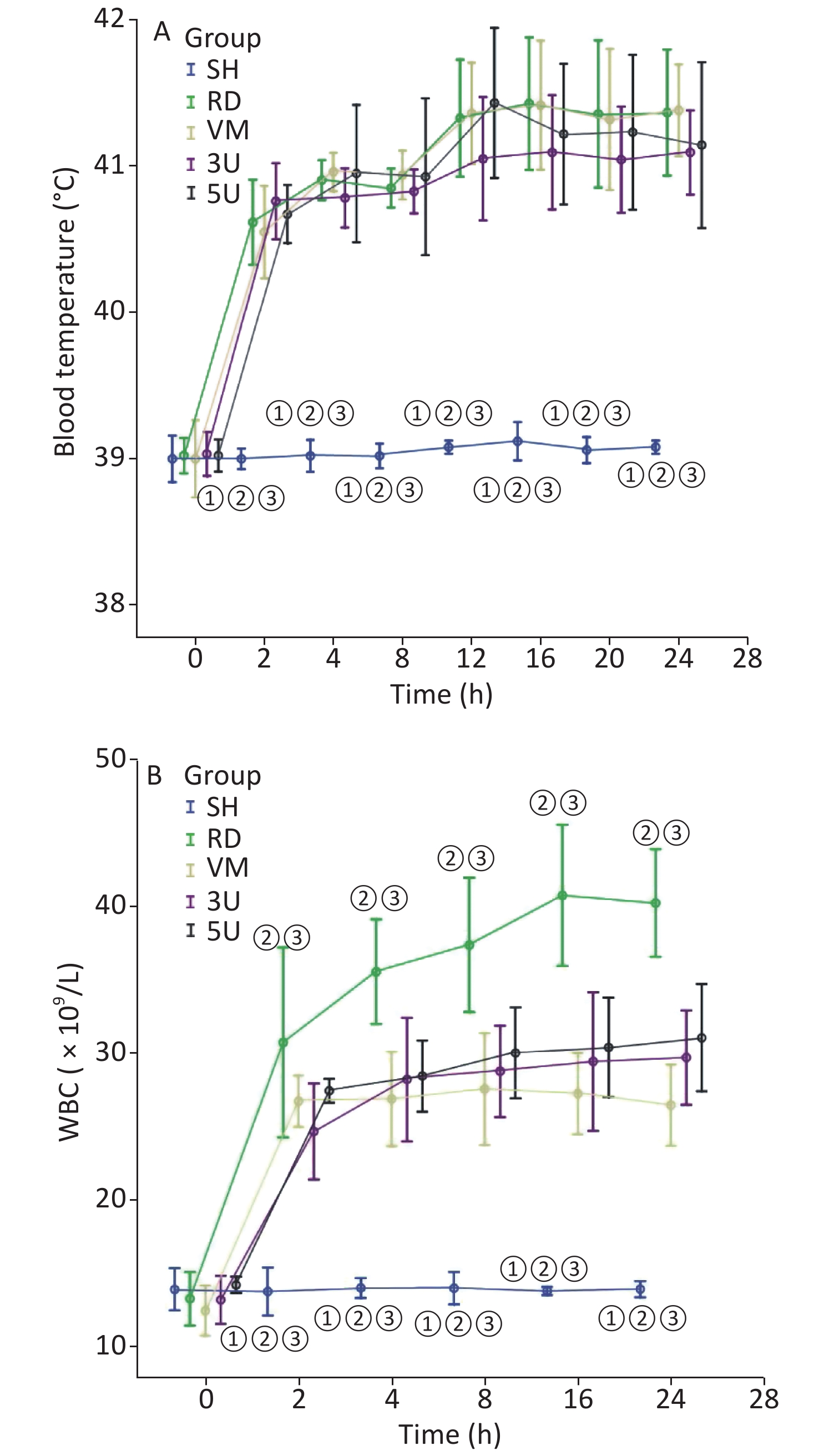
In this two-hit porcine model of ARDS induced by smoking and MRSA, three animals in the RD and VM groups died respectively during the protocol, whereas all animals in the other groups remained alive until the end of the protocol. Significant difference of mortality was found among the groups (X2 = 11.734, P = 0.019). The mortality was decreased by the treatment of UTI, but not vancomycin. MRSA was found in the blood of 9/11 (81.8%) animals in the RD group, whereas all other groups were negative for MRSA. The blood temperature increased rapidly to more than 40 °C 2 h after lung injury in all groups except the SH group (Supplementary Figure S1A, available in www.besjournal.com). We use MRSA to make the model, because it is a usual Gram positive bacteria that has the worst prognosis in septic shock. In the RD group, white blood cell (WBC) count gradually increased, and a significant difference was found between each drug treated group and the RD group at each time point 2 h after lung injury (all P < 0.001; Supplementary Figure S1B). The hemodynamics changes characterized by the low MAP and SVRI with high CI at 2 h after lung injury and the high SVRI with low CI two hours after that, which were the typical changes of septic shock in clinic. Changes in all of the above parameters were attenuated in the two UTI treatment groups, but not in the RD and VM group. Significant differences to the RD or VM group 2 h in CI and 8 h in SVRI after lung injury (P < 0.05 or 0.01, Figure 1) were found. From the current experimental results, it can be deduced that UTI ameliorates the fluctuation of hemodynamic forces.
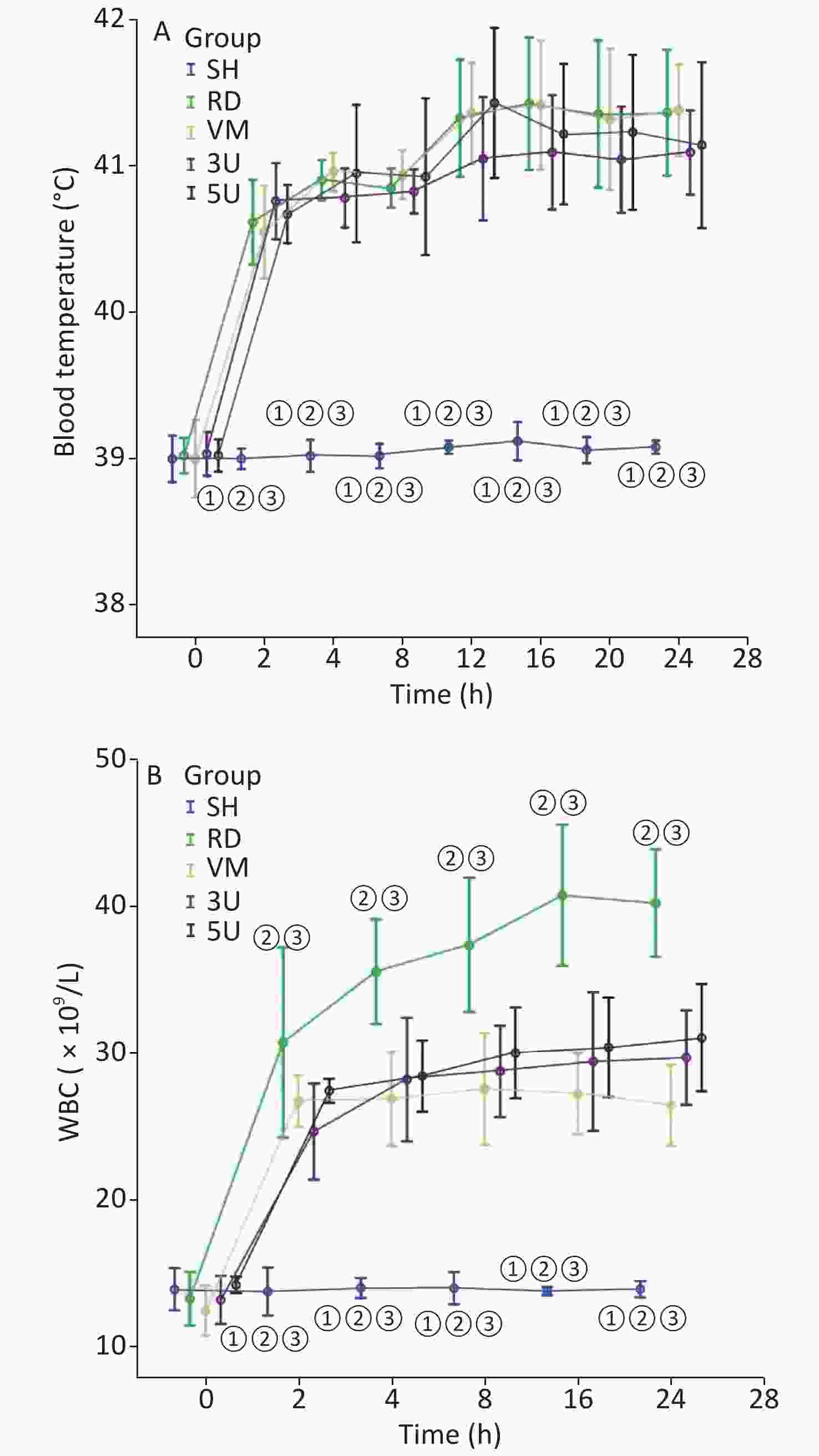
Figure S1. Changes in blood temperature and WBC. (A) There was a rapid increase in blood temperature in the RD group to more than 40 °C 2 h after lung injury. It stayed within normal limits in the SH group. (B) The WBC count gradually increased in the RD groups, and a significant difference was found between each drugs treatment group and the RD group after 2 h (all P < 0.001). WBC, white blood cell; ARDS, acute respiratory distress syndrome; UTI, ulinastatin; SH, sham group; RD, ARDS group; VM, vancomycin treatment group; 3U, 30,000 U/kg UTI treatment group; 5U, 50,000 U/kg UTI treatment group. ① P < 0.01 vs. the RD group; ② P < 0.01 vs. the VM group; ③ P < 0.01 vs. the 3U & 5U group.
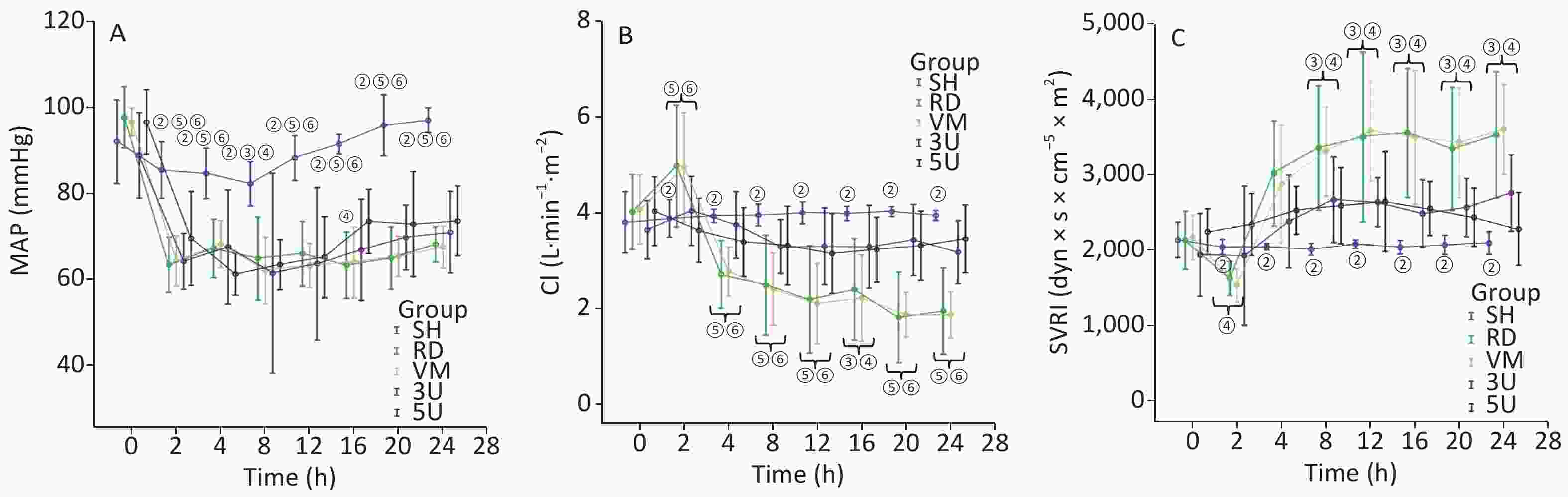
Figure 1. Hemodynamic changes. All parameters in the SH group stayed within normal limits. In other groups, MAP decreased significantly exactly 2 h after lung injury (A); the changes in CI (B) and SVRI (C) were opposite: the CI increased 2 h after lung injury and then gradually decreased, whereas the SVRI decreased 2 h after lung injury and then gradually increased. All changes in the two UTI treatment groups were attenuated, and significant differences were found between each of them and the RD or VM group 2 h (CI) and 8 h (SVRI) after lung injury (P < 0.05 or 0.01, Figure 2). CI, cardiac index; MAP, mean arterial pressure; SVRI, systemic vascular resistance index; ARDS, acute respiratory distress syndrome; UTI, ulinastatin; SH, sham group; RD, ARDS group; VM, vancomycin treatment group; 3U, 30,000 U/kg UTI treatment group; 5U, 50,000 U/kg UTI treatment group. ① P < 0.05 vs. the RD & VM group; ② P < 0.01 vs. the RD & VM group; ③ P < 0.05 vs. the 3U group; ④ P < 0.05 vs. the 5U group; ⑤ P < 0.01 vs. the 3U group; ⑥ P < 0.01 vs. the 5U group.
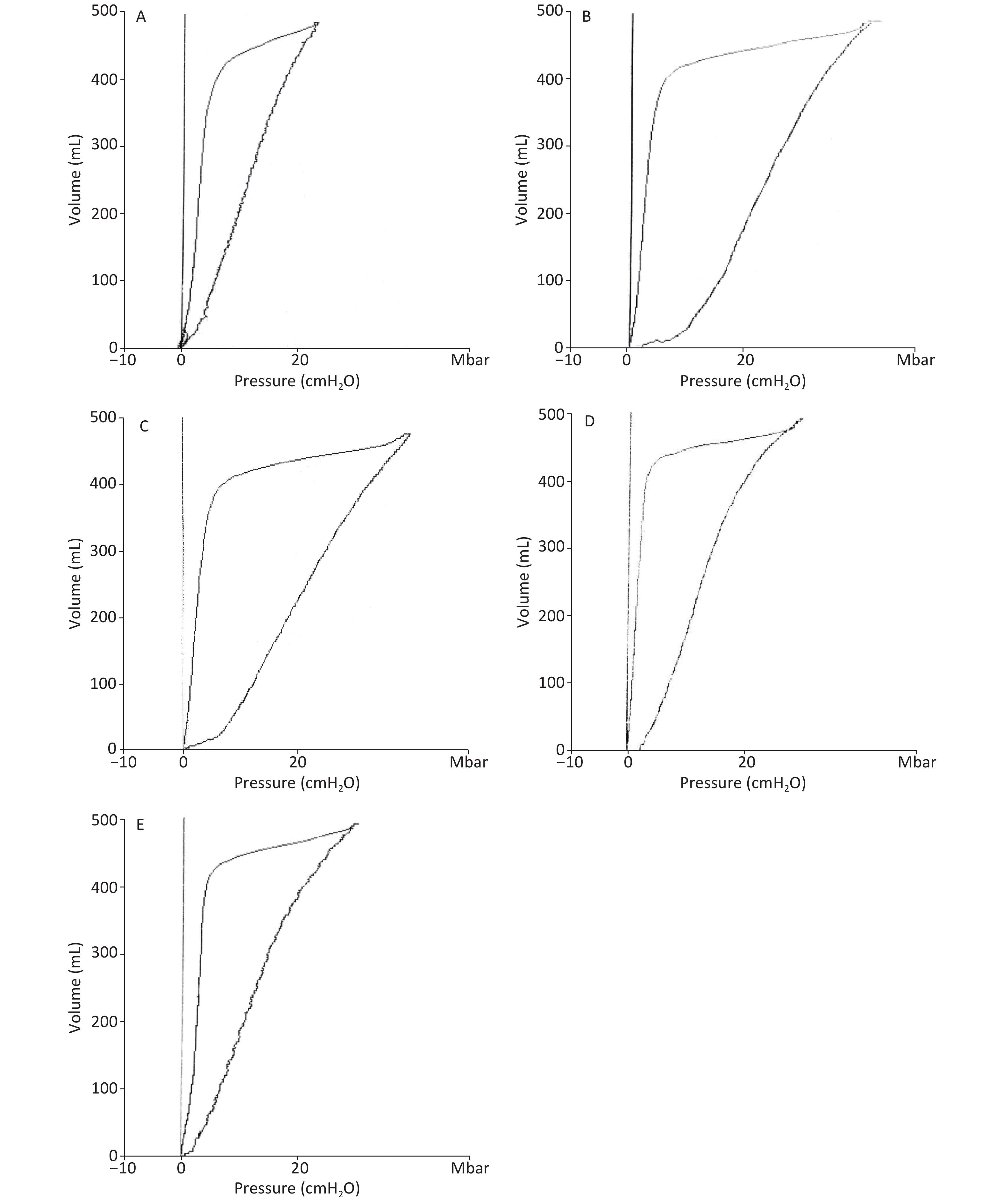
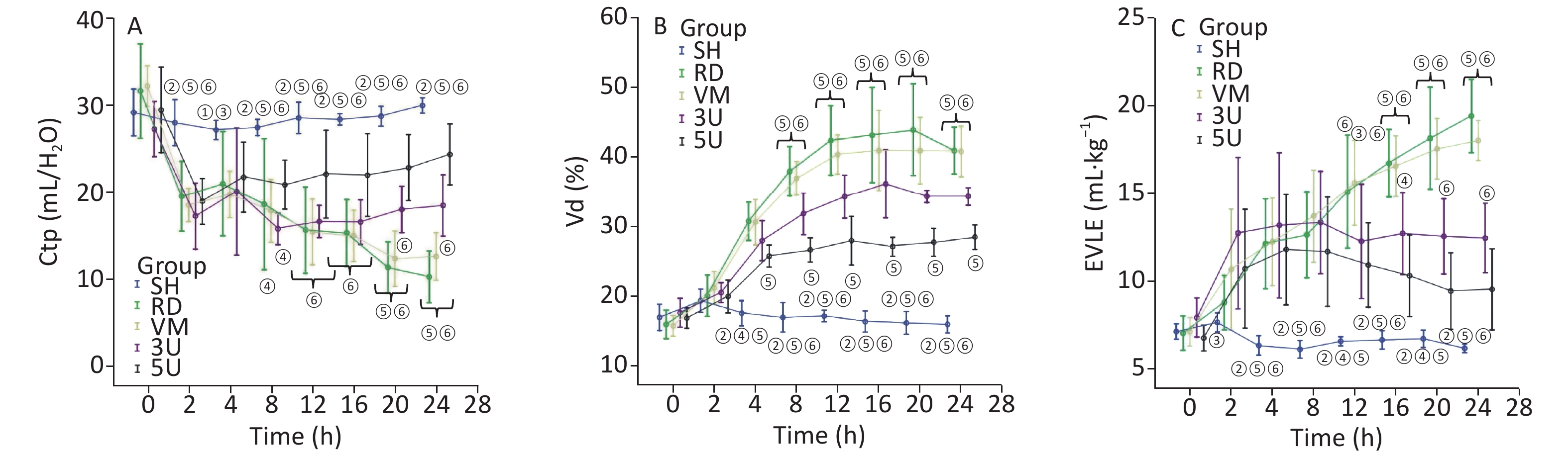
For the lung function, Vd and ELWI increased, whereas Ctp decreased after injury in the RD and VM groups. The slope of the P-V curves revealed that Ctp in the UTI treatment groups was improved (Supplementary Figure S2, available in www.besjournal.com), and significant differences were found between each UTI treatment group and the RD or VM group 20 h and 8 h after lung injury respectively (P < 0.05). Vd and ELWI were also decreased in the UTI treatment groups 12 h after lung injury compared with the RD or VM group (P < 0.05). All of the above parameters were significantly improved in the 5U group compared with those in the 3U group (Figure 2). Improvements in Ctp after lung injury might be due to the decrease in ELWI related to UTI dosing, since there was a more obvious reduction in ELWI in the 5U group. In the VM group, no significant changes of hemodynamics, oxygen dynamics and respiratory parameters were found compared with the RD group, because vancomycin can kill MRSA and treat pneumonia, but cannot improve the lung function.
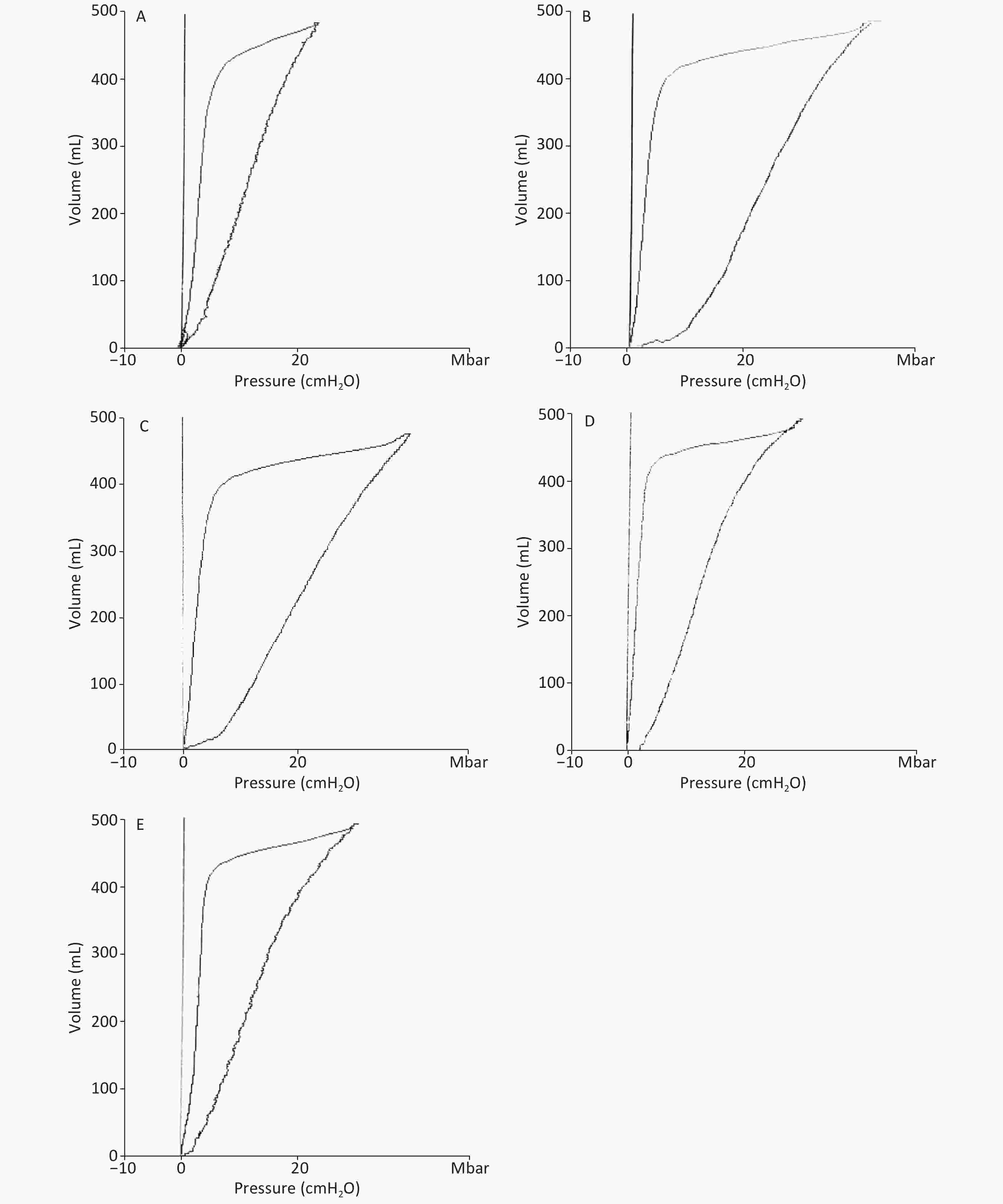
Figure S2. Ctp was calculated as the slope rate of the linear portion of the P-V curve in (A) the SH group, (B) the RD group, (C) the VM group, (D) the 3U group and (E) the 5U group. At 24 h, the outline of P-V curves in the RD and VM group showed an obvious shift to the right. Ctp: thoracopulmonary compliance; P-V: pressure–volume; ARDS, acute respiratory distress syndrome; UTI, ulinastatin; SH, sham group; RD, ARDS group; VM, vancomycin treatment group; 3U, 30,000 U/kg UTI treatment group; 5U, 50,000 U/kg UTI treatment group.
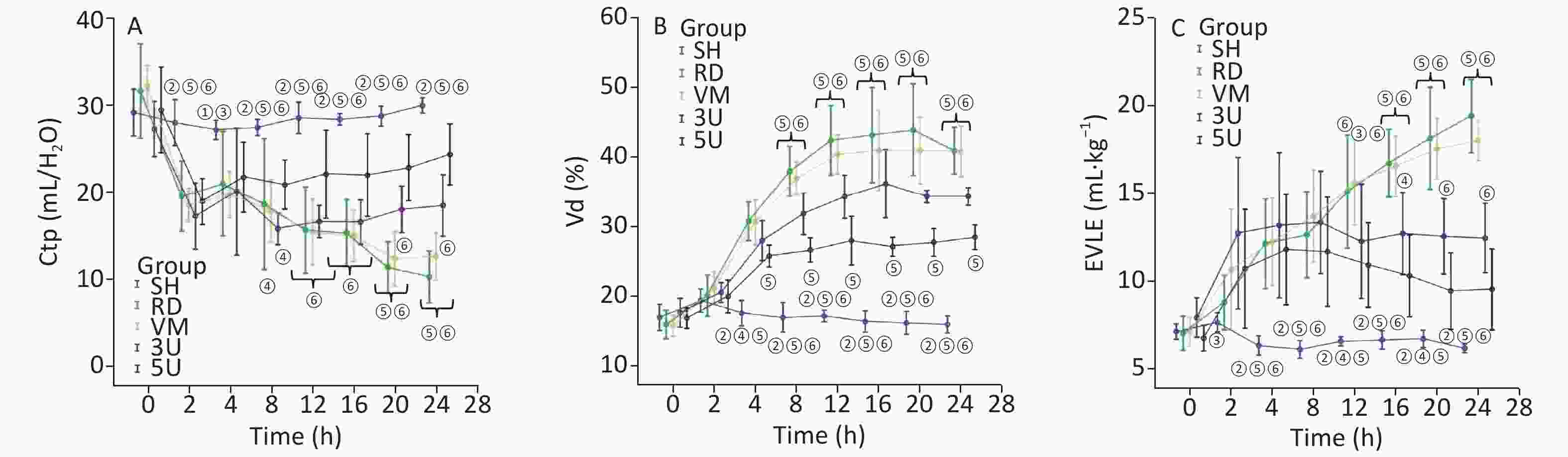
Figure 2. Changes in respiratory parameters. Ctp (A) decreased, Vd (B), and ELWI (C) increased after lung injury in the RD and VM groups. Ctp improved in the UTI treatment groups, and significant differences were found between the 3U group, 5U group and the RD or VM group 20 h and 8 h after lung injury (P < 0.05). ELWI also improved in the UTI treatment groups 12 h after lung injury vs. the RD and VM groups (P < 0.05). Greater improvement in airway resistance was found in the 5U than in the 3U group, and significant differences were found between the 3U and 5U groups 8 h after lung injury.
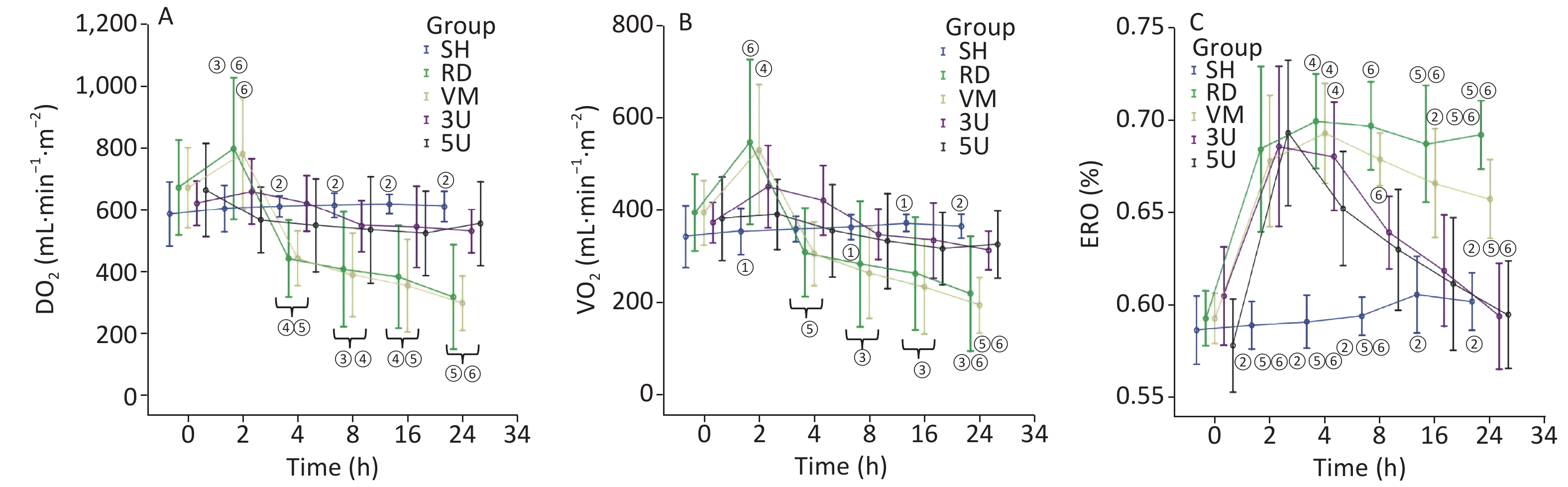
In the RD and VM group, there were the sharp increases in DO2 and VO2, peaking 2 h after lung injury and then decreasing gradually, but ERO2 stayed at a high level constantly until the end of the protocol. In the two UTI treatment groups, fluctuations in DO2 and VO2 disappeared. The elevated ERO2 decreased to normal at the end of the protocol. Significant differences in ERO2 were found between the RD or VM group and the two UTI treatment groups after 8 h after lung injury (P < 0.01; Supplementary Figure S3, available in www.besjournal.com). The imbalance of oxygen metabolism, as demonstrated by a mismatch between DO2 and VO2, i.e. the DO2 cannot maintain a high ERO2, was improved by UTI.
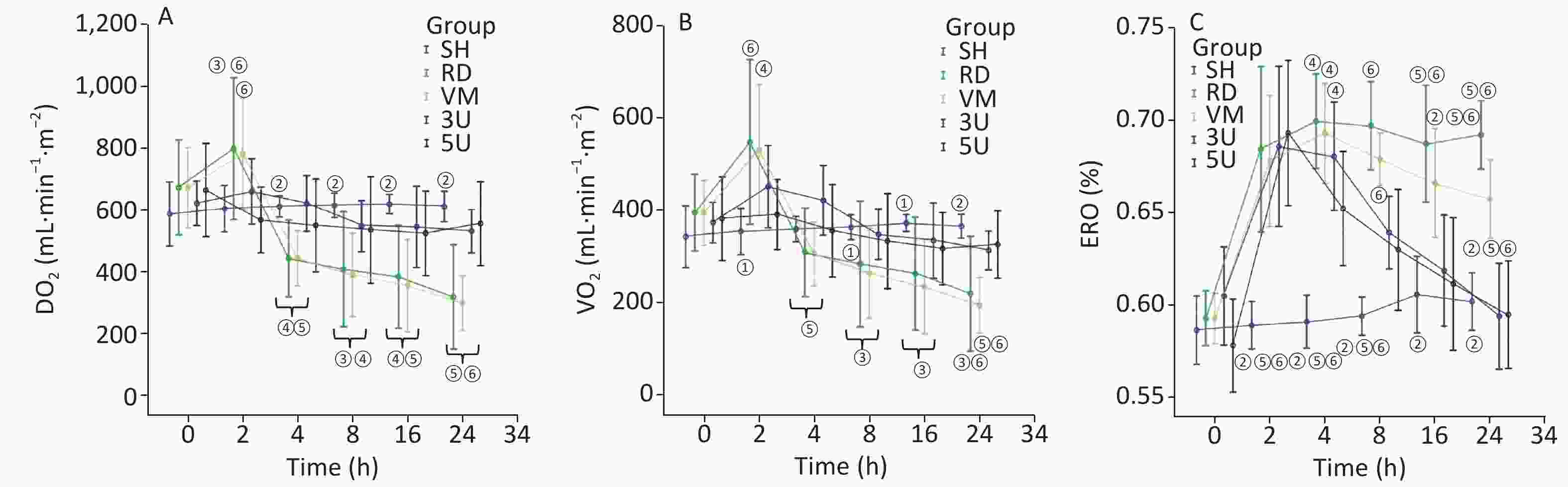
Figure S3. Changes in oxygen dynamics. In the RD group, there were sharp increases peaking in DO2 (A) and VO2 (B) 2 h after lung injury and then decreasing, but ERO2 (C) stayed at a high level constantly until the end of the protocol. The changes of parameters in the VM group were similar with the RD group. In the two UTI treatment groups, fluctuations in DO2 and VO2 were absent. The elevated ERO2 fell back to normal at the end of the protocol. Significant differences in ERO2 were found between the RD or VM group and the two UTI treatment groups 8 h after lung injury (P < 0.01). DO2, oxygen supply; VO2, oxygen consumption; ERO2, oxygen extraction rate; ARDS, acute respiratory distress syndrome; UTI, ulinastatin; SH, sham group; RD, ARDS group; VM, vancomycin treatment group; 3U, 30,000 U/kg UTI treatment group; 5U, 50,000 U/kg UTI treatment group. ① P < 0.05 vs. the RD & VM group; ② P < 0.01 vs. the RD & VM group; ③ P < 0.05 vs. the 3U group; ④ P < 0.05 vs. the 5U group; ⑤ P < 0.01 vs. the 3U group; ⑥ P < 0.01 vs. the 5U group.
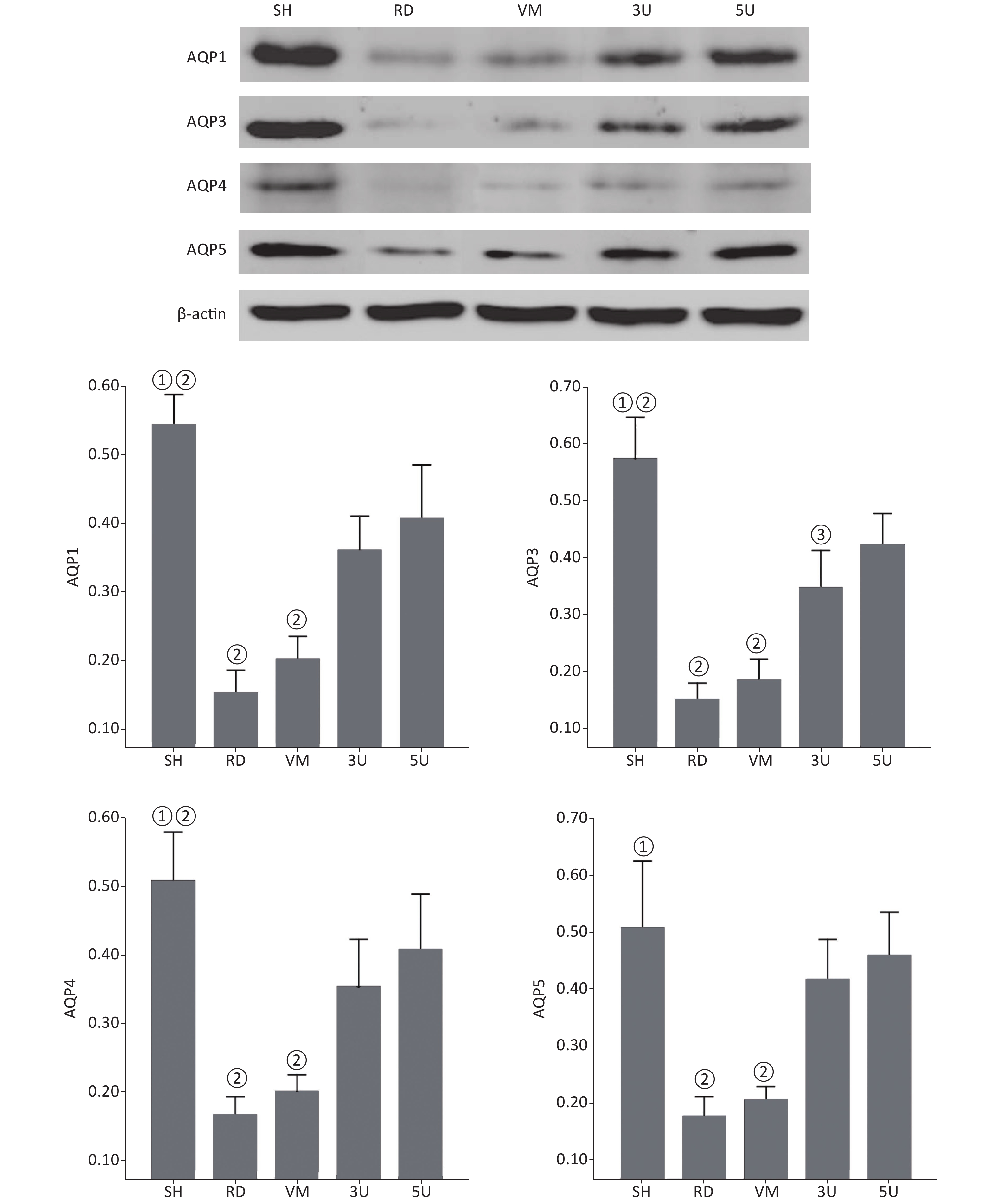
Western blot (Supplementary Figure S4, available in www.besjournal.com) and Immunohistochemistrical analysis (Supplementary Table S1, Supplementary Figure S5, available in www.besjournal.com) indicated that there was a significant decline of AQPs expression in the RD and VM group, whereas they were up-regulated by the UTI treatment in the 3U and 5U groups. All of these increased following treatment of UTI but not vancomycin. These offered an explanation for the decrease in ELWI and improvement in lung function in UTI-treated animals.
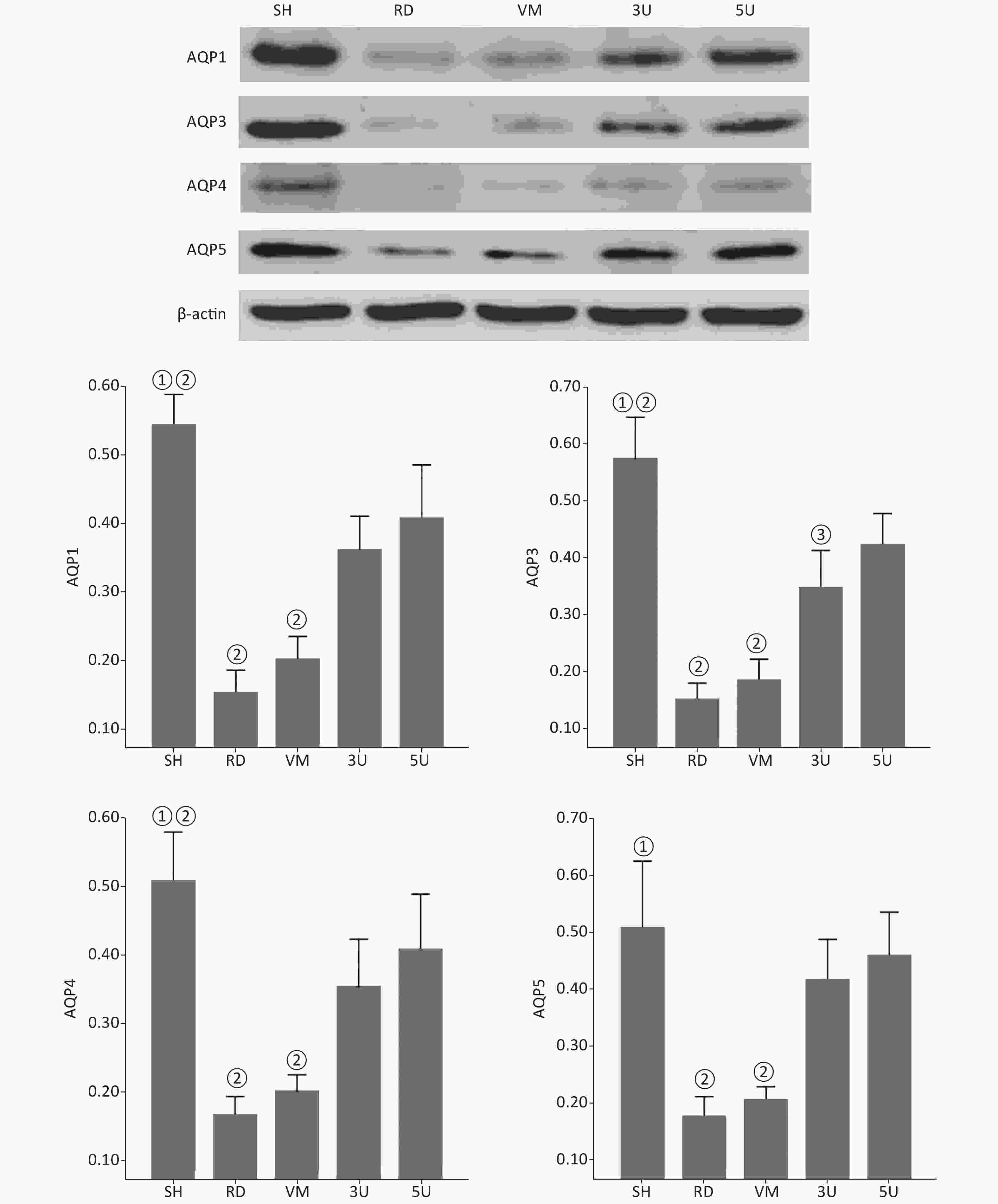
Figure S4. Western blot analysis of AQPs. There was a significant decline in the expression of AQPs in the lungs of the RD and VM groups, whereas there was increased expression following UTI treatment in the 3U and 5U groups. A significant difference was found between the RD or VM group and the UTI treatment groups (P < 0.01). AQPs, aquaporins; ARDS, acute respiratory distress syndrome; UTI, ulinastatin; SH, sham group; RD, ARDS group; VM, vancomycin treatment group; 3U, 30,000 U/kg UTI treatment group; 5U, 50,000 U/kg UTI treatment group. ① P < 0.01 vs. the RD & VM group; ② P < 0.01 vs. the 3U & 5U; ③ P < 0.01 vs. the 5U group.
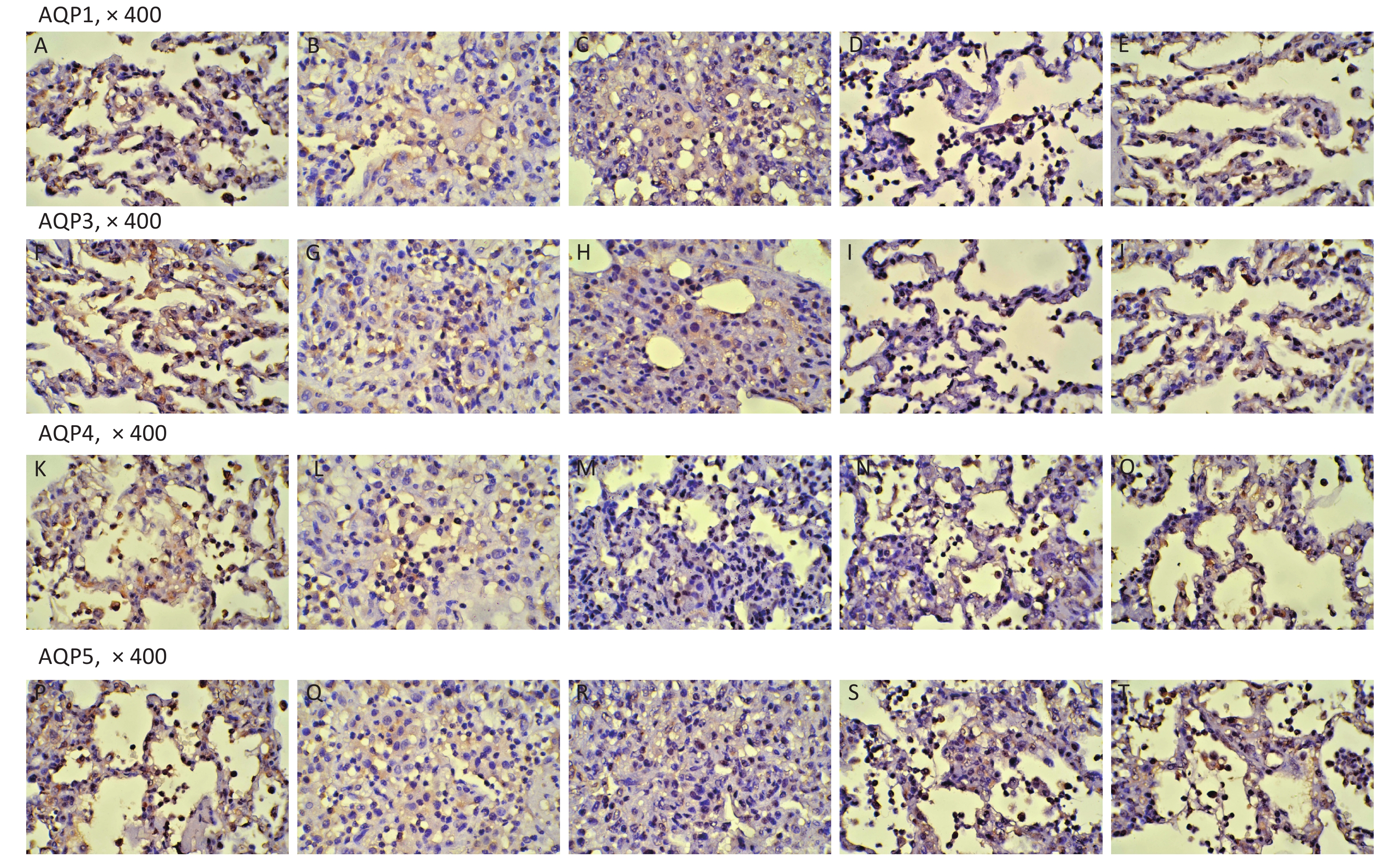
Variables SH (n = 5) RD (n = 11) VM (n = 11) 3U (n = 11) 5U (n = 11) F P AQP1 0.57 ± 0.12ab 0.39 ± 0.05cd 0.39 ± 0.04cd 0.51 ± 0.10 0.53 ± 0.11 8.605 < 0.001 AQP3 0.64 ± 0.03abc 0.35 ± 0.03cd 0.36 ± 0.03cd 0.51 ± 0.11 0.53 ± 0.14 15.346 < 0.001 AQP4 0.62 ± 0.03abc 0.30 ± 0.03cd 0.31 ± 0.03cd 0.47 ± 0.13 0.54 ± 0.12 21.448 < 0.001 AQP5 0.63 ± 0.01abc 0.31 ± 0.04cd 0.32 ± 0.03cd 0.49 ± 0.13 0.55 ± 0.12 22.378 < 0.001 Note. AQP, aquaporin; ARDS, acute respiratory distress syndrome; UTI, ulinastatin; SH, sham group; RD, ARDS group; VM, vancomycin treatment group; 3U, 30,000 U/kg UTI treatment group; 5U, 50,000 U/kg UTI treatment group. aP < 0.05 vs. the RD group; bP < 0.05 vs. the VM group; cP < 0.05 vs. the 3U group; dP < 0.05 vs. the 5U group. Table S1. Immunohistochemistry (AQP positive area percentage)
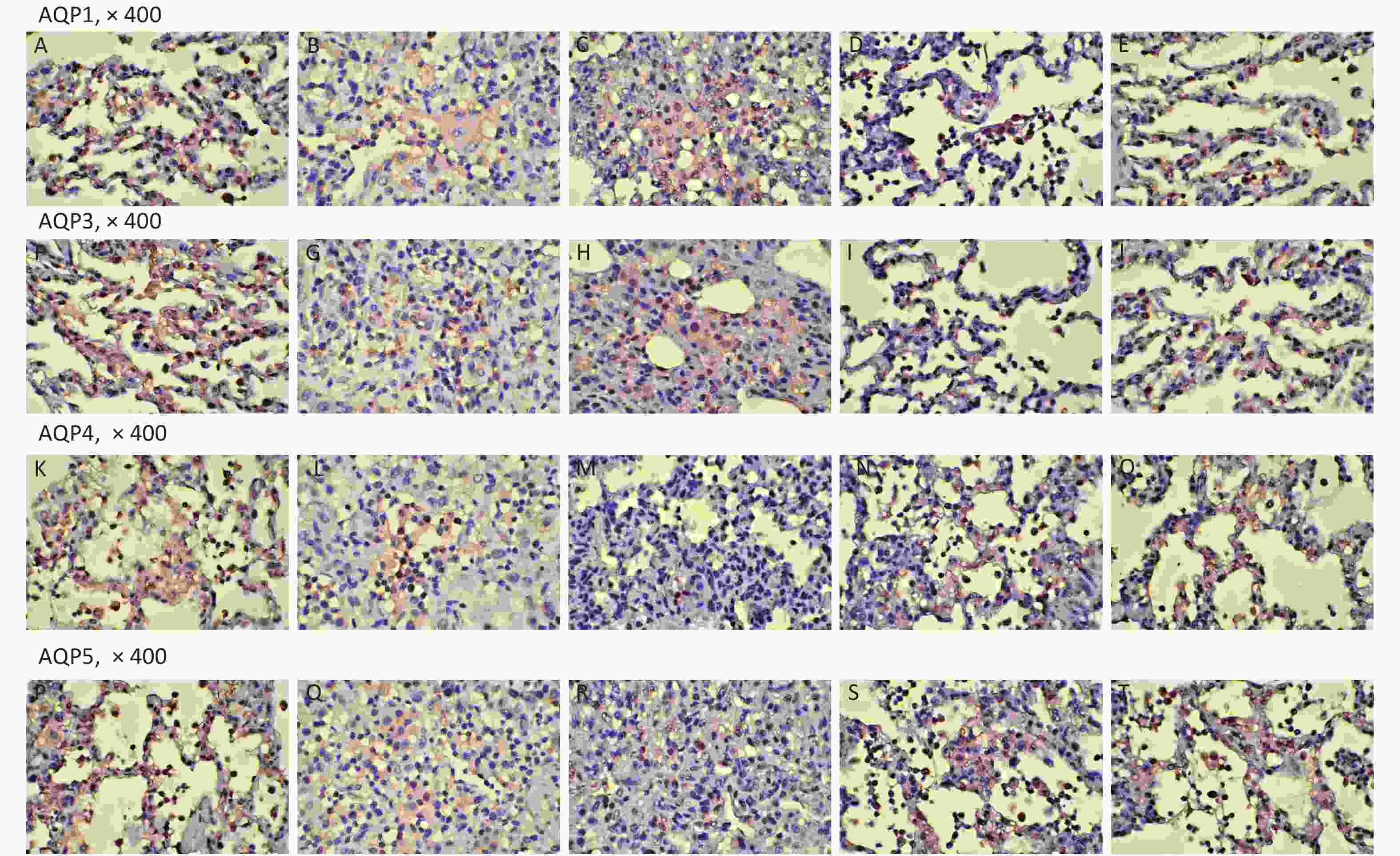
Figure S5. Immunohistochemistry. AQP1: (A) the SH group; (B) the RD group; (C) the VM group; (D) the 3U group; (E) the 5U group. AQP3: (F) the SH group; (G) the RD group; (H) the VM group; (I) the 3U group; (J) the 5U group. AQP4: (K) the SH group; (L) the RD group; (M) the VM group; (N) the 3U group; (O) the 5U group. AQP5: (P) the SH group; (Q) the RD group; (R) the VM group; (S) the 3U group; (T) the 5U group. AQPs, aquaporins; ARDS, acute respiratory distress syndrome; UTI, ulinastatin. SH, sham group; RD, ARDS group; VM, vancomycin treatment group; 3U, 30,000 U/kg UTI treatment group; 5U, 50,000 U/kg UTI treatment group.
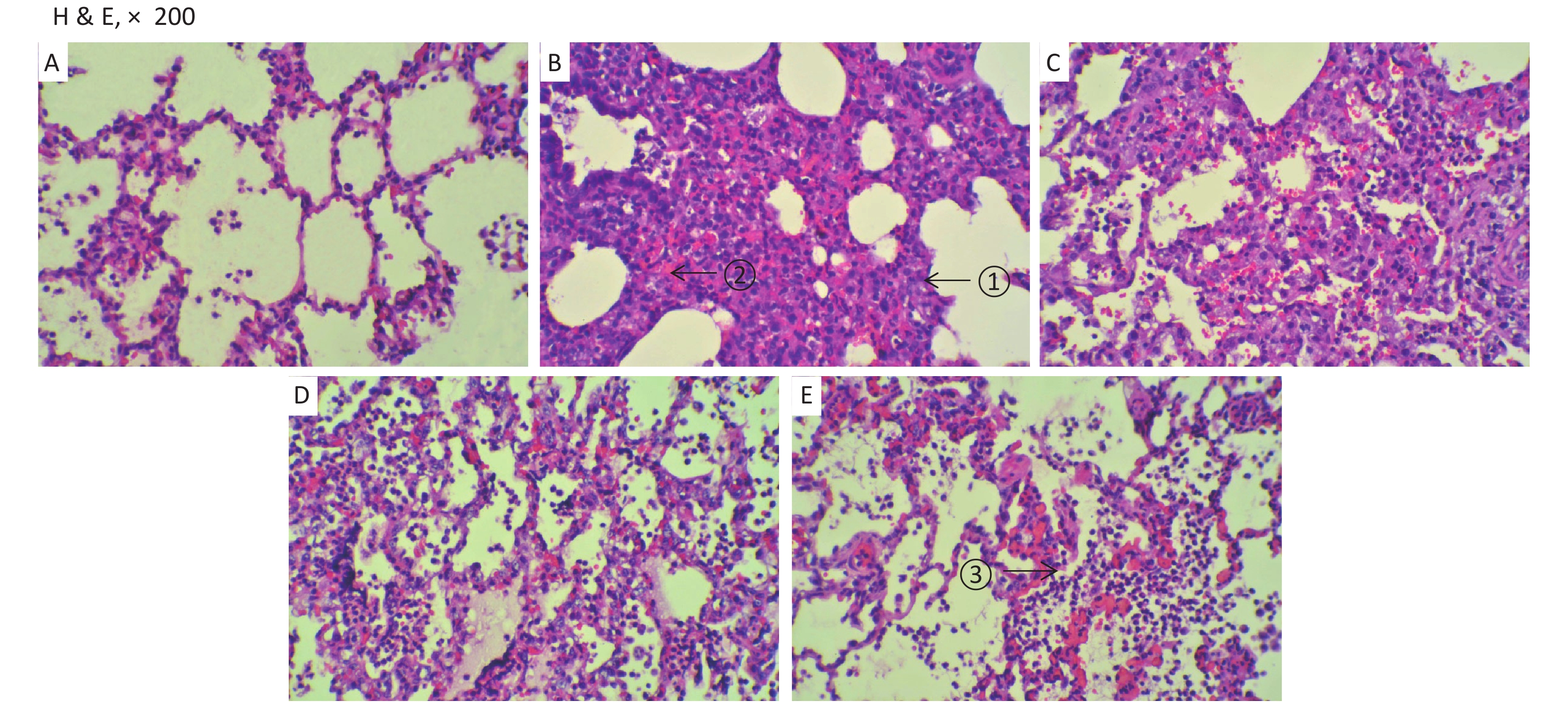
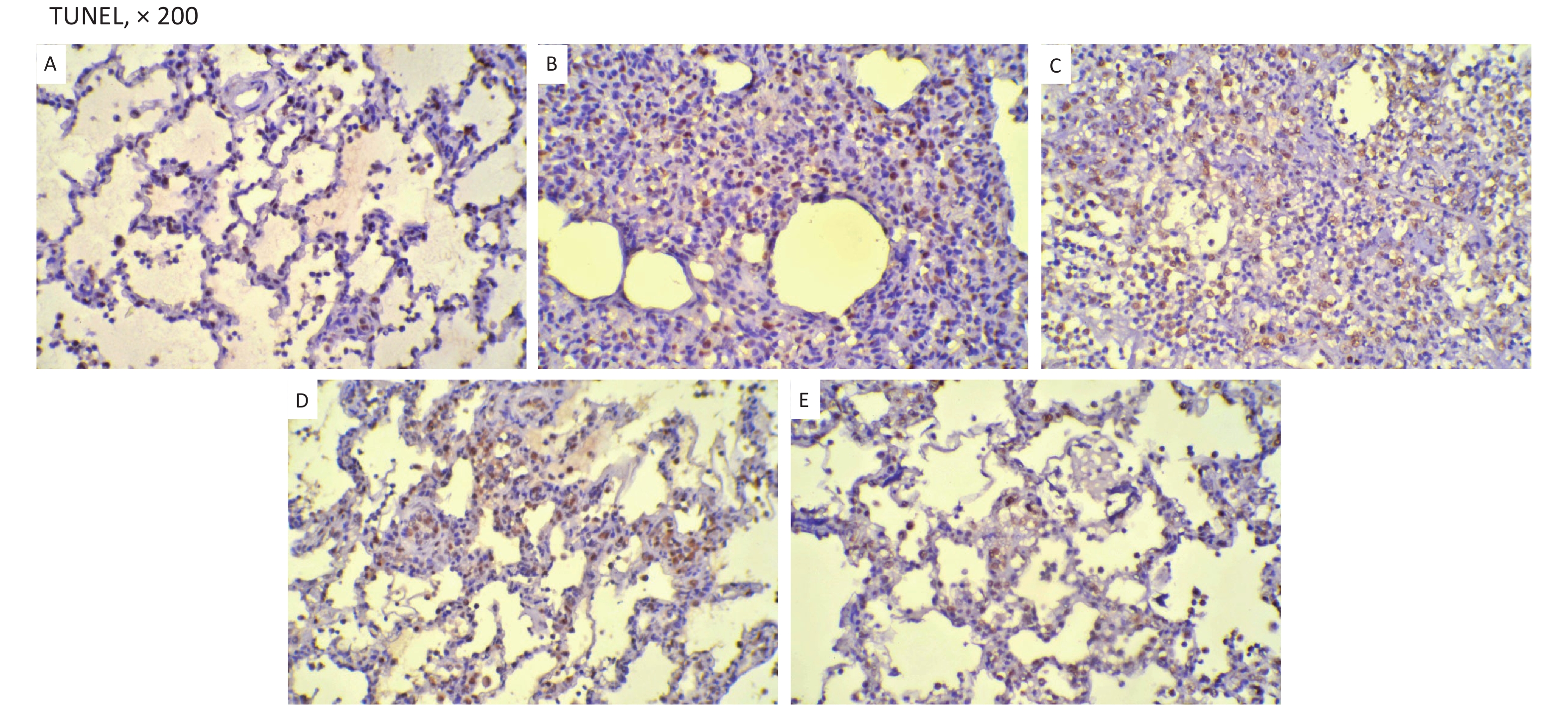
The changes of histopathology confirmed that an ARDS model was successfully developed. Significant histopathologic lung injuries were found in the RD group, including infiltration of neutrophils, lymphocytes and macrophages in alveoli and thickening of the interalveolar septum by infiltration of inflammatory cells (Supplementary Figure S6, available in www.besjournal.com). There was not significant improvement of lung injuries in the VM group when compared with the RD group. Significant differences in lung injury scores were found between the RD or VM and the UTI treatment groups (P < 0.01, Table 1). In the treatment groups (RD, VM, 3U and 5U), the numbers of apoptosis cells were greater than those in the SH group, but decreased in the two UTI treatment groups, with significant differences being found between the RD or VM and the two UTI treatment groups (P < 0.01, Table 1, Supplementary Figure S7, available in www.besjournal.com). The improvement of Ctp, which might lead to the improvements in oxygenation and DO2, and eventually to decreased anaerobic glycolysis. On the other hand, both the improved oxygenation and lung edema decreased the apoptosis of epithelium and then mitigated the lung injury, which was confirmed by the results of TUNEL and lung injury score
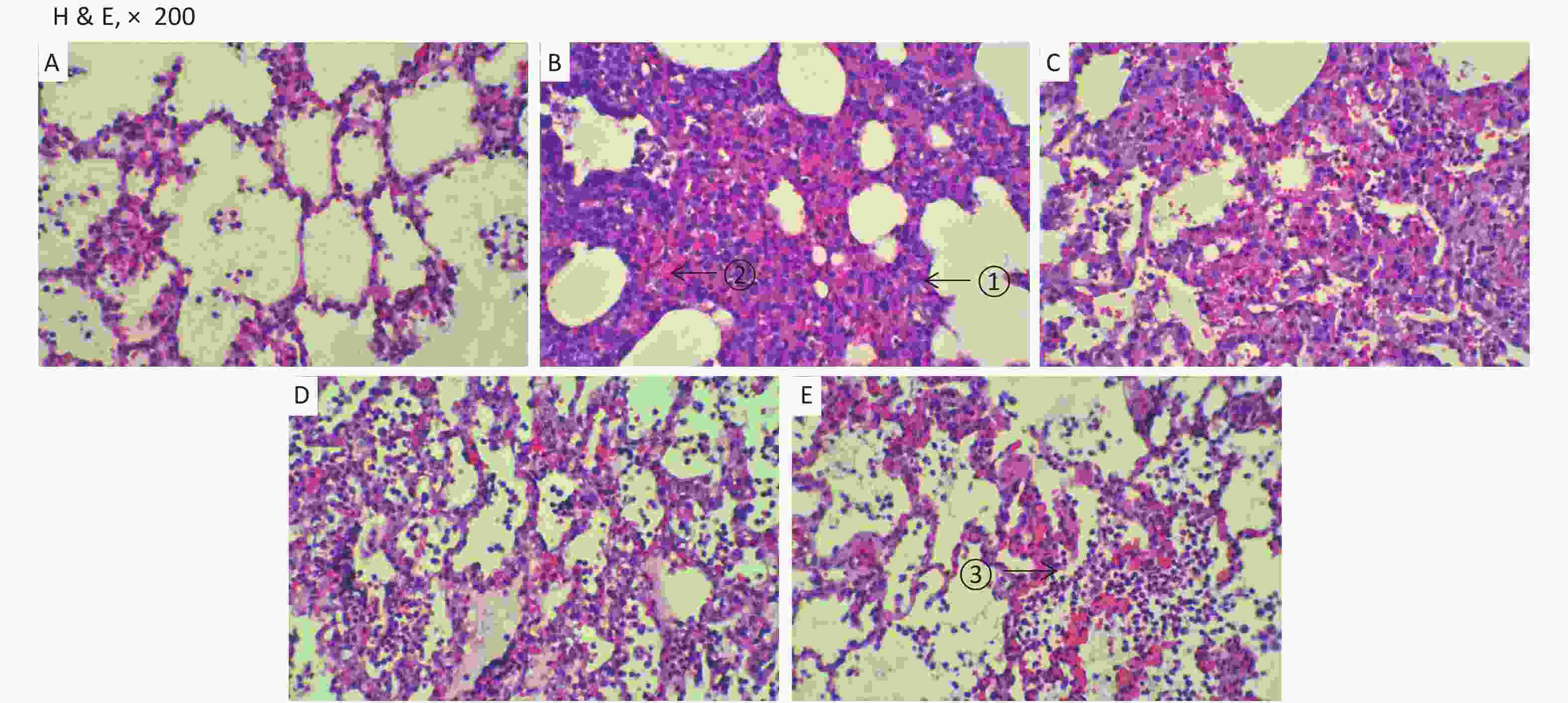
Figure S6. Histopathology. Very significant histopathologic lung injuries were found in the RD and VM groups under light microscopy: ① thickening of the interalveolar septum by infiltration of inflammatory cells; ② microthrombus formatted in the capillaries; ③ infiltration of neutrophils, lymphocytes and macrophages in alveoli. (A) the SH group; (B) the RD group; (C) the VM group; (D) the 3U group and (E) the 5U group. H & E, hematoxylin and eosin staining; ARDS, acute respiratory distress syndrome; UTI, ulinastatin. SH, sham group; RD, ARDS group; VM, vancomycin treatment group; 3U, 30,000 U/kg UTI treatment group; 5U, 50,000 U/kg UTI treatment group.
Variables SH (n = 5) RD (n = 11) VM (n = 11) 3U (n = 11) 5U (n = 11) F P Lung injury scores 0.40 ± 0.55abcd 3.55 ± 0.52cd 3.64 ± 0.67cd 2.82 ± 0.75 3.00 ± 0.77 22.870 < 0.001 TUNEL (%) 1.50 ± 0.16abcd 31.64 ± 1.43cd 30.36 ± 2.87cd 15.91 ± 2.12d 12.00 ± 2.05 304.638 < 0.001 Note. TUNEL, deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling; ARDS, acute respiratory distress syndrome; UTI, ulinastatin; SH, sham group; RD, ARDS group; VM, vancomycin treatment group; 3U, 30,000 U/kg UTI treatment group; 5U, 50,000 U/kg UTI treatment group. aP < 0.05 vs. the RD group; bP < 0.05 vs. the VM group; cP < 0.05 vs. the 3U group; dP < 0.05 vs. the 5U group. Table 1. Histopathology outcomes
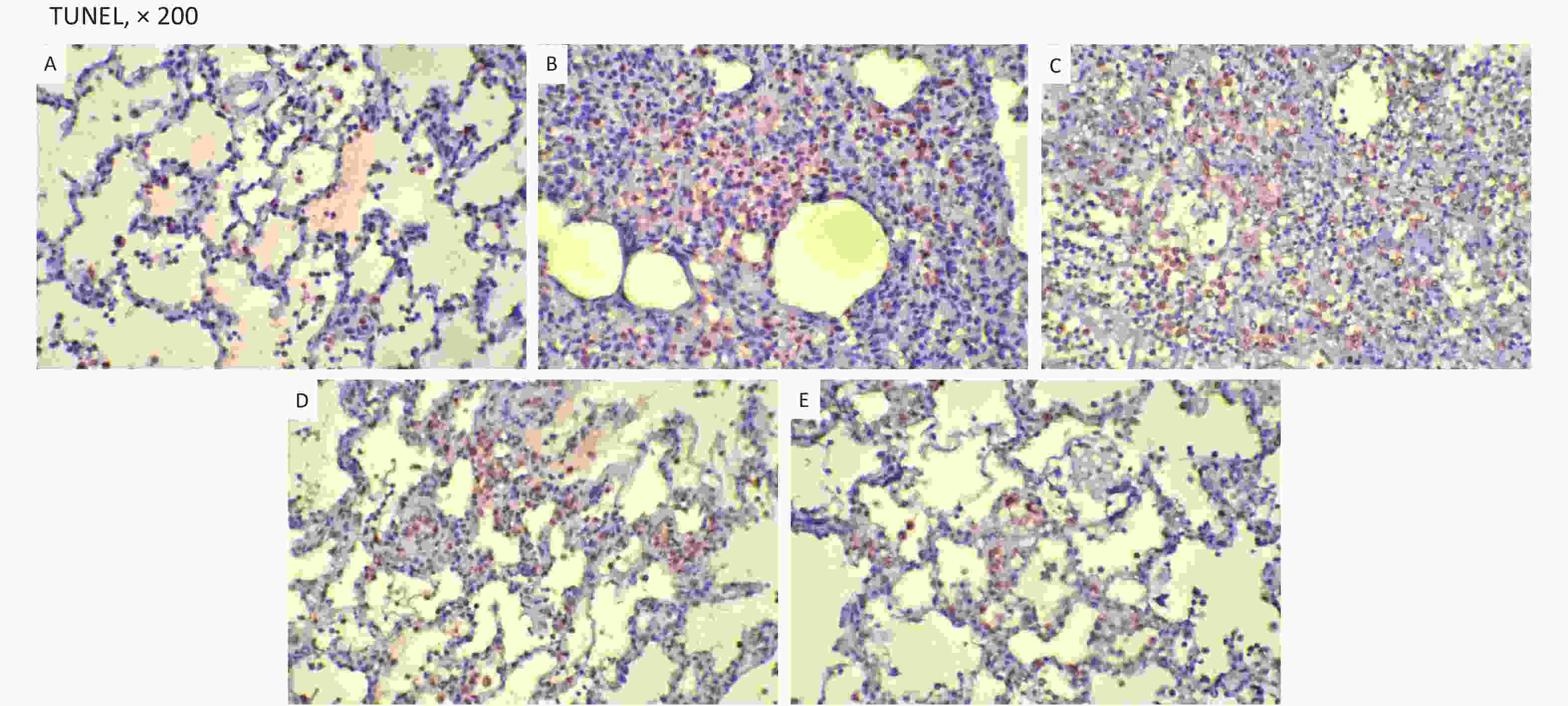
Figure S7. TUNEL. TUNEL-positive cells were stained brown. They were decreased in the two UTI treatment groups. (A) the SH group; (B) the RD group; (C) the VM group; (D) the 3U group and (E) the 5U group
ARDS is characterized by progressive dyspnea and refractory hypoxemia caused by a variety of events occurring within and outside the lungs, mainly as the result of an excessive inflammatory response. Damage and dysfunction of the pulmonary vascular endothelium, especially around alveoli, as well as increased pulmonary vascular permeability, are the important pathological features[3, 4]. Water transport between the air space and the alveolar capillaries plays an important physiological role in the regulation of airway hydration, reabsorption of alveolar fluid, and the resolution of pulmonary edema[5, 6]. Fluid movement malfunction across epithelial and endothelial barriers occurs in the presence of interstitial and alveolar pulmonary edema, such as in cases of congestive heart failure, ARDS, pneumonia, and acute lung injury due to physical and chemical factors.
From the current experimental results, it can be deduced that UTI ameliorates the fluctuation of hemodynamic forces, effectively blocks the cascade of systemic inflammation, inhibits pulmonary edema, improves alveolar capillary permeability, transmembrane fluid transport and pulmonary gas exchange by up-regulating the expression of AQPs. These improvements were dose dependent.
Whereas, ARDS is a complicated syndrome, and there may be other mechanisms that influence its pathophysiology. From experimental results[7], UTI can mitigate vascular endothelial injury at an ultra-early lung injury stage by reducing serum vascular endothelial growth factor (VEGF) levels and increasing pulmonary vascular permeability, thereby playing an important role in lung protection. It was also shown that UTI can directly inhibit the release of neutrophils and other inflammatory mediators, thus reducing damage to endothelial cells and reducing capillary permeability[8, 9]. Further studies should be done, especially the cytokines changing in bronchoalveolar lavage fluid (BALF) and the pathway of the regulation in special protein.
In conclusion, UTI can dose dependent improve lung injury in a two-hit porcine model of ARDS by up-regulating the expression of AQPs. Hemodynamics and oxygen dynamics were also enhanced by resultant improvements in oxygenation.
Competing Interests The authors declare that they have no competing interests.
Ethics Approval The use of all animals that received treatment was in compliance with the National Research Council’s 1996 Guide for the Care and Use of Laboratory Animals.
Author Contributors HCC: operating and manuscript writing. GYH: operating, data acquisition and statistical analysis. LCS: final approval of the manuscript. WS: conception, design and final approval of the manuscript. All authors read and approved the final manuscript.
HTML
 21071Supplementary Materials.pdf
21071Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: