-
Infectious diseases would always threaten human health, especially emerging infectious diseases (EIDs), such as the coronavirus disease-2019 (COVID-19). In 2019, COVID-19 spread rapidly worldwide. On January 30, 2020, World Health Organization (WHO) declared its outbreak a public health emergency of international concern. By the end of June 2022, the number of confirmed cases had reached over 500 million, including more than 6 million deaths[1]. EIDs pose a significant burden on global economies and public health due to their rapid transmission, high toxicity, high fatality, and low inhibition rate [2]. Obstruction of the transmission route by interventional means is critical for preventing and restricting infectious diseases. Disinfection is one of the main interventional means to obstruct transmission, and it plays a key role in epidemic control.
The science of disinfection is an application-oriented discipline. Disinfection refers to a physical or chemical process that eliminates pathogens, except for bacterial spores, on inanimate objects and surfaces. It is a method of killing pathogenic microorganisms on objects or in the environment, to make the surroundings harmless[3]. Disinfection is widely used in the control of infectious diseases and healthcare-associated infections, food sanitation, environmental sanitation, and drinking water sanitation etc[4].
Disinfection has always been a powerful tool for humans to fight against pathogenic microorganisms. Upon the outbreak of severe acute respiratory syndromes, Middle East respiratory syndrome, and influenza in birds, people have increased their awareness of disinfection and the demand for disinfectants. With the spread of COVID-19 around the world, WHO has suggested washing hands regularly with soap and water, or cleaning hands with an alcohol-based hand rub[5]. Disinfectants have been gradually popularized in families, and the market size has increased rapidly. At present, the disinfectants are widely used, and their novel forms are constantly updated and iterated.
According to the “Report on Market Prospects of China’s Disinfectant Industry in 2020” released by the China Business Industry Research Institute, the output value of China’s disinfectant industry was RMB 10.34 billion (USD 1.54 billion) in 2019, which further increased significantly in 2020 due to COVID-19, reaching RMB 11.8 billion (USD 1.76 billion). With the widespread use of disinfectants, various forms of novel disinfectants are emerging, such as liquid, spray, gel, and paper towels. At the same time, the problem of disinfectant resistance is increasingly emerging. In this context, there is an increasing demand for pathogenic microorganism standard strains to evaluate the disinfection efficacy of disinfectants.
Similar to antibiotic resistance, the widespread use of disinfectants would also led disinfectant resistance[6]. Therefore, it is essential to evaluate the efficacy of disinfectants. The pathogenic microorganism standard strains are used as the indicators for efficacy evaluation of the disinfectants.
Standard strains have quintessential characteristics of the bacteria or virus species. In addition, their genetic characteristics should be identified, guaranteed, and traceable, for they can represent the species and subspecies. Such strains are reviewed and identified by national collection institutions and recorded with national collection numbers[7-8]. Standard strains provide a reference for quality control in daily work and scientific research. Standard strains in the International Organization for Standardization (ISO) and other standards are used for positive and negative controls, standard verification, medium quality control, and production process quality control, etc.
The present study focused on exploring the approach of screening pathogenic microorganism standard strains, which are used for evaluating the disinfection efficacy. This technical process of screening standard strains is expected to provide a reference for studies on standard strains, and also provide an approach for using the resources from collection institutions to improve their quality. At the same time, such a process also lays a foundation for meeting the application requirements of standard strains in various fields.
The disinfection efficacy was evaluated using various pathogenic microorganism standard strains as indicators. Standard strains used in the disinfection test mainly included bacterial propagules, mycobacteria and spores, such as Staphylococcus aureus ATCC 6538, Salmonella ATCC 10708, Mycobacterium bovis (BCG), and Bacillus subtilis ATCC 19659. Most standard strains were collected from the American Type Culture Collection (ATCC).
The science of disinfection in China has accumulated much experience in the prevention and control of the epidemic of several infectious diseases. It has developed rapidly and steadily with the demand in the fields of medical treatment, agriculture, food, and animal husbandry[4]. Currently, the standard strains frequently used in the disinfection practice in China include E. coli 8099, S. aureus ATCC 6538, M. chelonae ATCC 93326, and B. subtilis ATCC 9372, some of which are identical to those in the United States and the European Union.
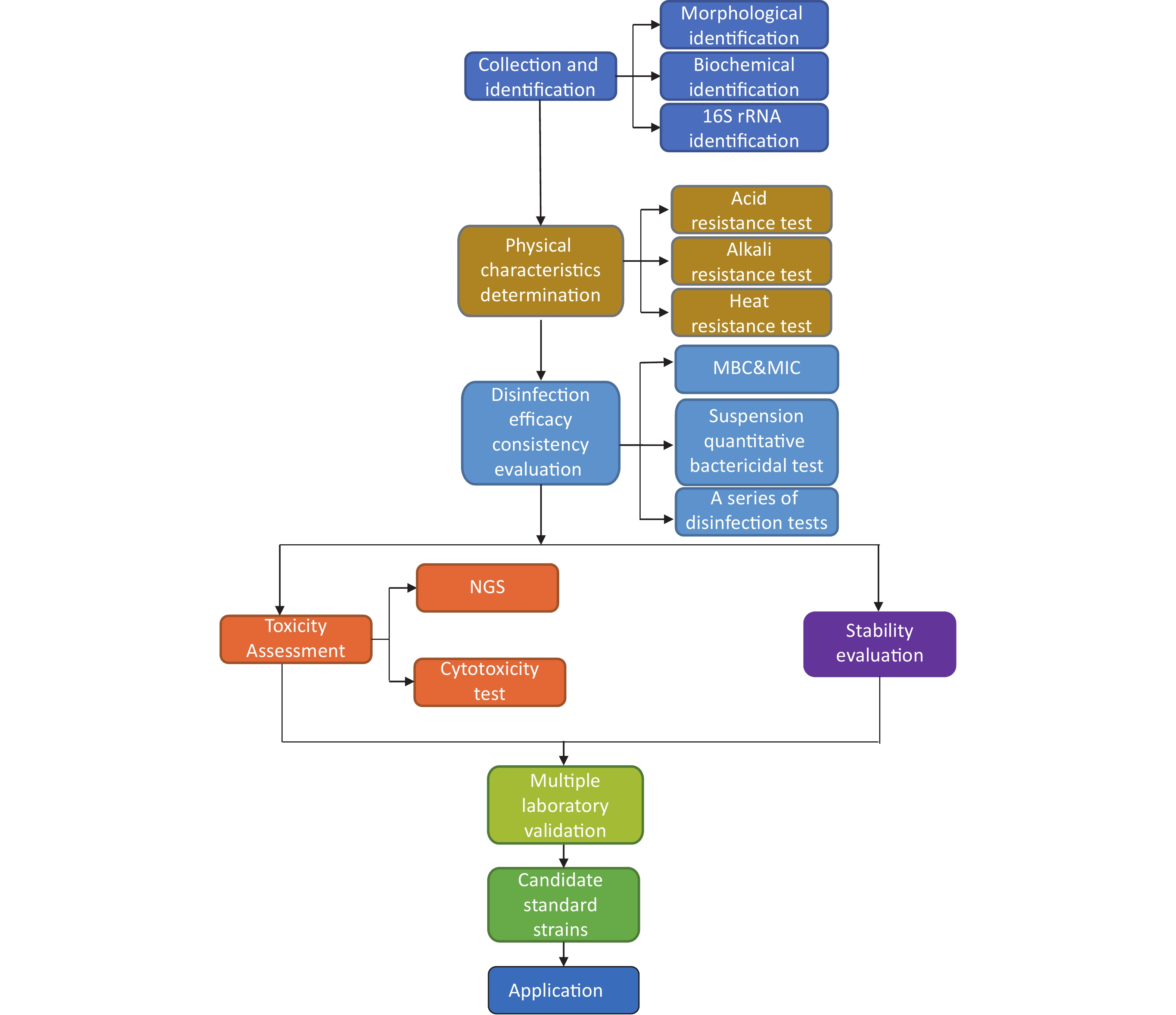
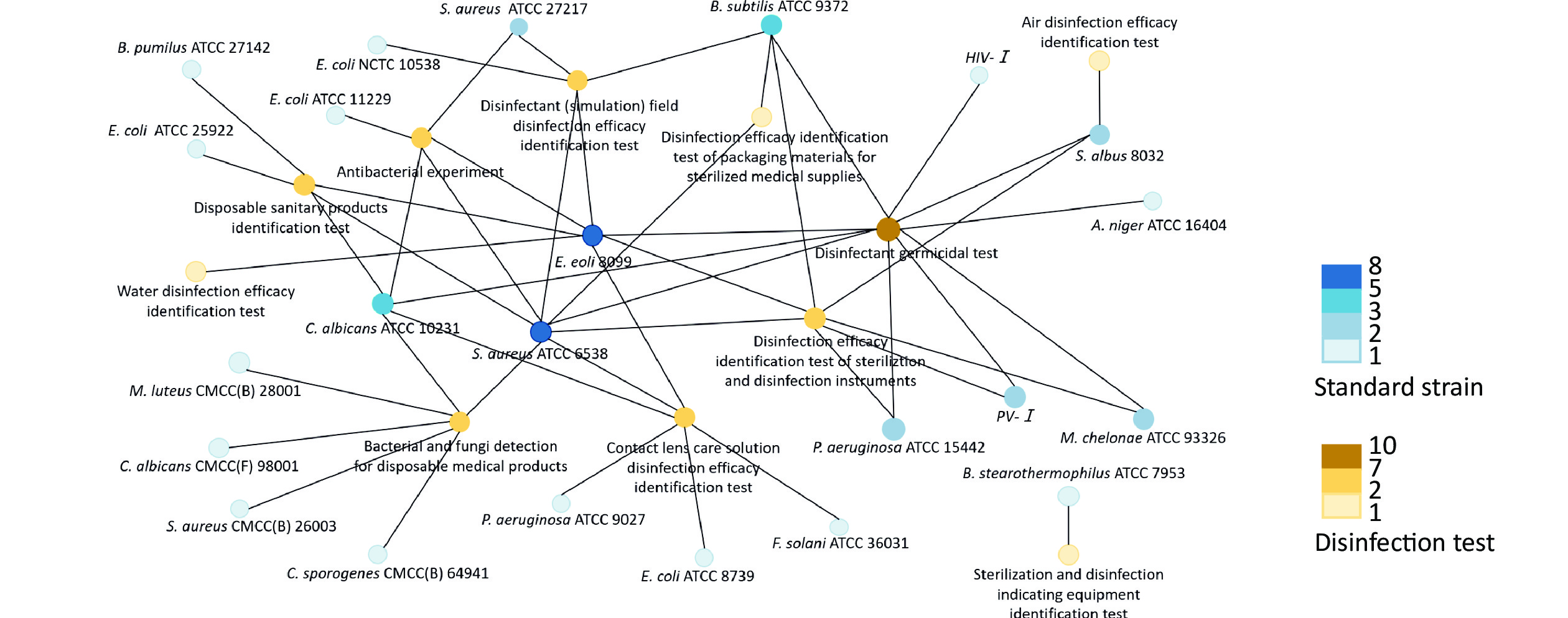
According to the “Technical Specifications for Disinfection” in China, the corresponding pathogenic microorganism standard strains used in the specific disinfection tests are depicted in Figure 1.
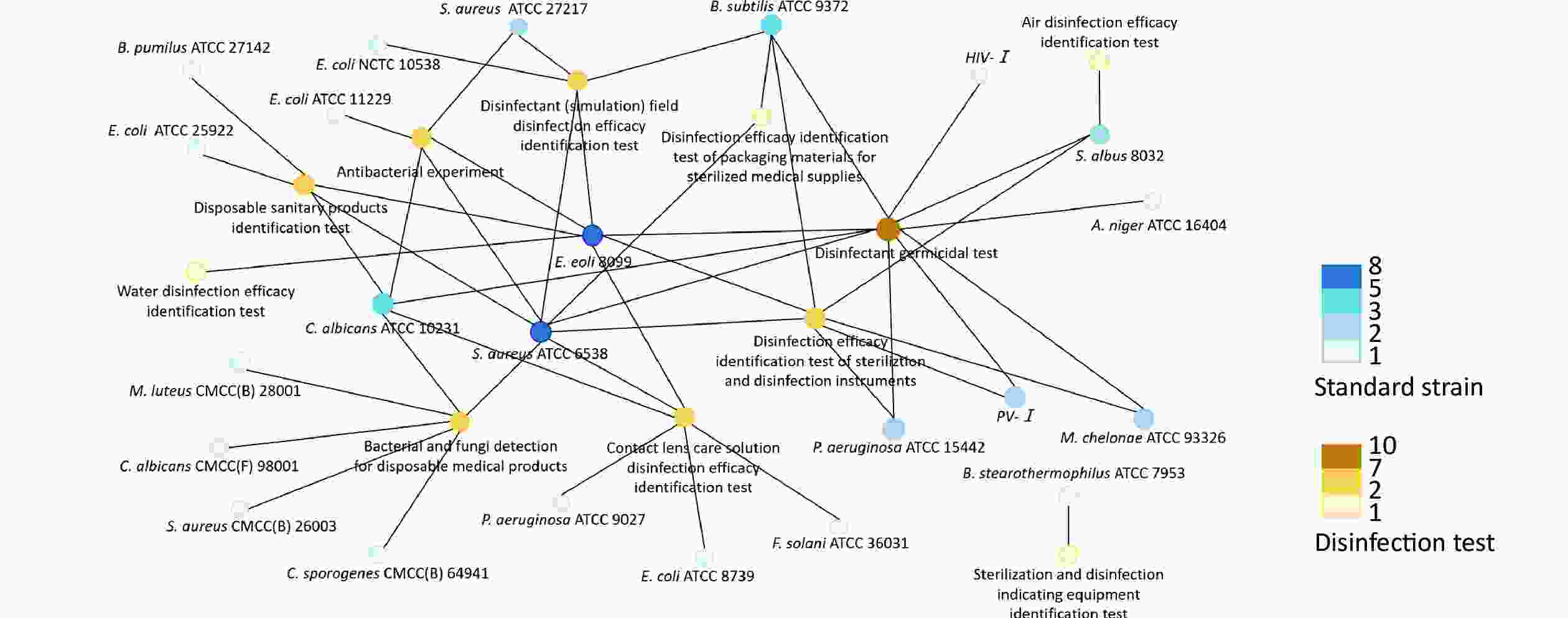
Figure 1. Correlation network for the use of pathogenic microorganism standard strains as described in the “Technical Specifications for Disinfection”
In the process of evaluating the disinfection efficacy, which following the principles of efficacy evaluation, and the demands of scientific research and teaching, it is essential to discuss the method of screening pathogenic microorganism standard strains based on the existing resources of collection institutions. At present, the international documents, such as Standard Operating Procedure, do not have the term on the manner of formulating standard strains. Hence, the following six-step technical process for formulating standard strains has been proposed (Figure 2).
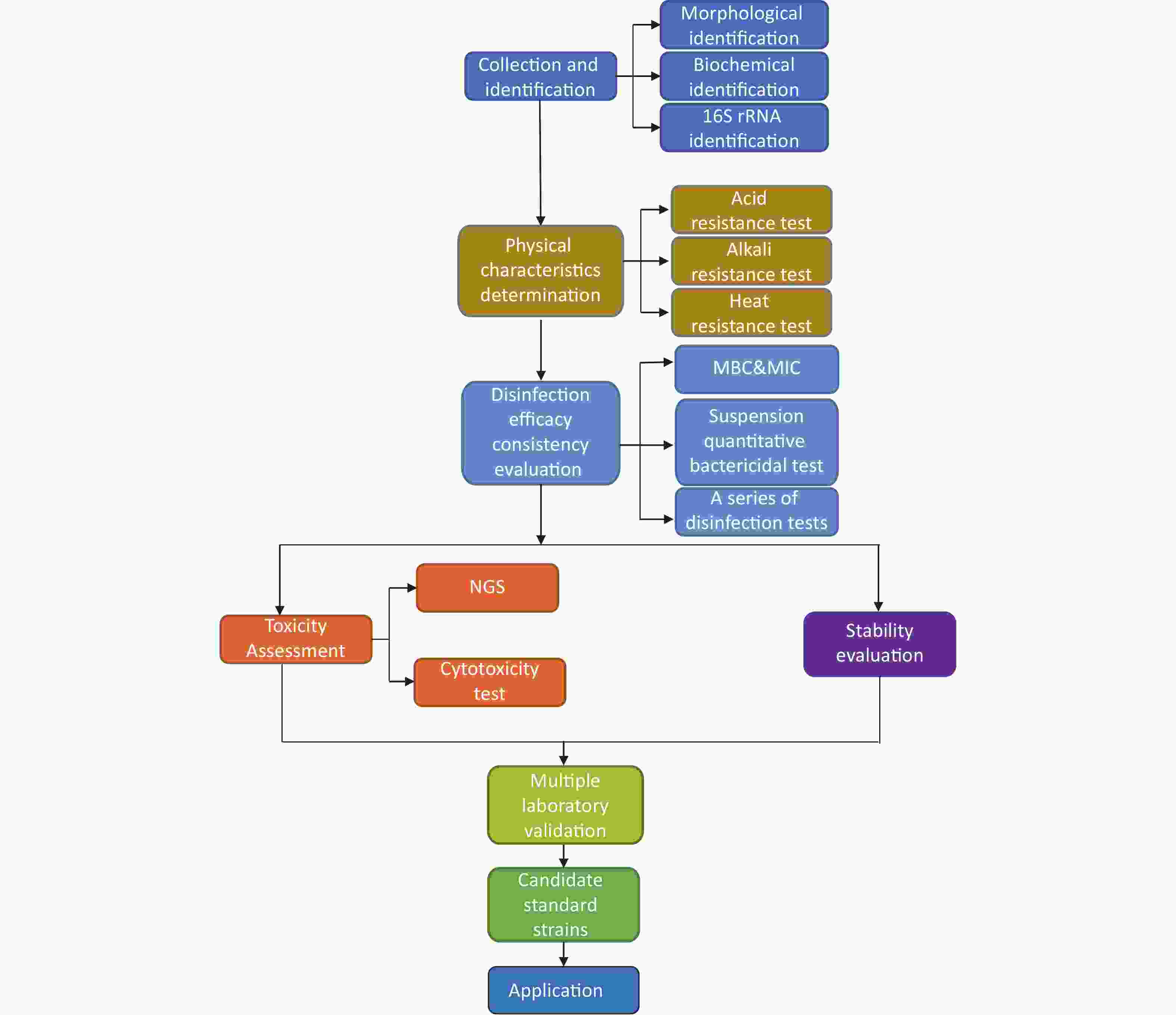
Figure 2. Technical schematic diagram for screening pathogenic microorganism standard strains for the evaluation of disinfection efficacy
The candidate standard strains need to be collected from different substrates and geographical regions based on the resources of national collection institutions, so as to ensure their representativeness. In the case of S. aureus, candidate strains are required to encompass clinical, food, environmental, and other sources from various geographical regions.
Subsequently, species should be identified, and the identification tests should include morphological, biochemical, and 16S rRNA analysis, to ensure that the phenotype and genotype of candidate standard strains and existing standard strains belong to the same species.
As tested, the physical characteristics of strains would affect disinfection efficacy. Therefore, they must be similar to those of the existing standard strains. In the screening process, it is required to determine whether the physical characteristics (such as pH and temperature) of candidate standard strains are consistent with those of the existing standard strains.
For ensuring the consistency of disinfection efficacy between the candidate and existing standard strains, a series of disinfection tests (Figure 1), including minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and suspension quantitative bactericidal, are required to be conducted during screening, to determine the survival conditions of the candidate standard strains in different scenarios and distinct chemical disinfection factors compared to the existing standard strains. Subsequently, the candidate standard strains with the same disinfection effect as the existing standard strains are retained.
Standard strains in disinfection tests are mostly pathogenic microorganisms, such as S. aureus, Pseudomonas aeruginosa, and Candida albicans. Since improper operation during the experiment may infect the operators, candidate standard strains should be safe with low toxicity.
In the screening process, the distribution of virulence and drug resistance genes of candidate standard strains should be detected by Next Generation Sequencing (NGS), and the in vitro toxicity to the cells should be detected by cytotoxicity assay. Finally, the strains containing resistant genes should be excluded, and those with fewer pathogenic genes, less toxicity, and less pathogenicity should be retained.
To assess the stability, the candidate standard strains should be preserved by glycerol lyophilization at –80 ℃ (–112℉) and then picked and activated at 1 d, 10 d, 30 d, 60 d, and 180 d to observe the morphology, thus ensuring that the strains meet the stability requirements.
The disinfection efficacy of candidate strains is not accidental. In order to eliminate the contingency of experimental instruments, operators, time and region, the selected candidate strains should be verified by multiple laboratories, preferably from different regions and institutions, so as to clarify the disinfection efficacy of candidate strains.
Pathogenic microorganism standard strains are crucial biological resources. In this study, a method for screening pathogenic microorganism standard strains was proposed, which can be used for the evaluation of disinfection efficacy. The selected standard strains are used for evaluating disinfection efficacy in China, as designed based on the “Technical Specifications for Disinfection”. This specification is consistent with EN 14885, as revised according to a series of standards issued by the Association of Official Analytical Chemists (AOAC) [9].
This method is expected to play a role in screening standard strains. In this way, the screened standard strains gradually connect with the existing disinfection standards and meet the requirements of standard strains. In addition, the screening process and technical route have the potential to provide strong technical support for screening pathogenic microorganism standard strains with robust safety and high stability.
On the other hand, disinfection with a certain concentration of disinfectants, and that with many disinfectants work differently. Screening pathogenic microorganism standard strains for the evaluation of disinfection efficacy is performed based on the current standard strains. In this process, the strains with stronger resistance may be found. It can provide an idea for standard revision and provide strains for subsequent studies on resistance genes of disinfectants.
Most pathogenic microorganism standard strains for evaluation of disinfection efficacy are obtained from ATCC in many countries, such as China. The World Data Center for Microbiology (WDCM) cooperated with ISO in the field of microbial resources and some strains were upgraded for ISO detections. The screening of standard strains is performed based on a wide range of resources. Presently, China is making efforts to promote the construction of the National Pathogen Resource Center to ensure the availability of rich candidate strains. Now, the relevant collection centers have been put into operation, forming a network of national collection institutions[10]. This progress lays a foundation for screening national pathogenic microorganism standard strains. The collection institutions would prioritize quality and standards; therefore, they should continue to strengthen the development of a national system of strand strains and promote their application, including the evaluation of disinfection efficacy. Gradually, a complete and systematic standard system will be formed to adapt to the developmental needs of biotechnology.
No potential conflicts of interest were disclosed.
The authors would like to thank Dr. YU Li (Beijing Center for Disease Prevention and Control, China), Dr. SHEN Jin (National Institute of Environmental Health of China CDC), and Dr. SUI Zhi Wei (National Institute of Metrology, China) for their help and support.
Screening Pathogenic Microorganism Standard Strains for Disinfection Efficacy Evaluation
doi: 10.3967/bes2022.135
- Received Date: 2022-07-05
- Accepted Date: 2022-09-20
| Citation: | LI Yi Xiao, SONG Yang, MEI Li, JIANG Meng Nan, WANG Duo Chun, WEI Qiang. Screening Pathogenic Microorganism Standard Strains for Disinfection Efficacy Evaluation[J]. Biomedical and Environmental Sciences, 2022, 35(11): 1070-1073. doi: 10.3967/bes2022.135 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: