-
Ulcerative colitis (UC) is a chronic inflammatory disease affecting the colon, with an increasing incidence worldwide. Disease pathogenesis is multifactorial and involves genetic predisposition, epithelial barrier defects, dysregulated immune responses, and environmental factors [1,2]. Long-standing UC leads to several complications such as megacolon, inflammation of the eye and joints, and/or colon cancer [3]. Eaden et al. revealed that 25-year chronic UC increases colorectal cancer risk by as much as 34% [4]. Their study further indicated that UC etiology is related to the environment, gene variation, intestinal microflora, and altered immune response, but precise mechanisms and pathogenesis remain unclear. Several drugs and surgical approaches are used to treat UC, but may present drug resistance issues or severe side effects [5]. Therefore, novel UC treatments are demanded.
Based on Strachan’s hygiene hypothesis, the possible protective effects of parasites in autoimmune disease have been explored. It is generally accepted that early exposure to helminths promote Th2-type immune responses, which may prevent excessive Th1-type inflammatory reactions which cause autoimmune disease in genetically predisposed individuals [6]. Recent research has indicated that balancing Treg/Th17 status is a key factor in UC development [7].
Schistosoma japonicum, one of the five major schistosoma species, infects humans. The adult worm lives in the host’s liver-portal system and mesenteric vein. Studies have reported that S. japonicum elicits pathological damage in UC [1,8]. In this study, we compared the prognosis and differential mechanisms between pre-existing S. japonicum eggs (pre-infection) and intervention in the acute phase of dextran sulfate sodium (DSS)-induced colitis (post-infection).
Experimental animal procedures were approved by the local ethics committee at Central South University (Changsha, China). Female C57BL/6 mice aged 7 weeks were provided by the Center of Experimental Animal of Xiangya School of Medicine of Central South University (Hunan, China); animals were free from specific pathogens, housed in ventilated cages, and fed a normal chow diet. DSS (36,000–50,000 Da, MP Biomedicals, BP 50067 IIIkirch, France) was dissolved in doubly distilled water (DDW) at 2.5% and presented as drinking water to mice for 7 days to induce colitis. Fresh DSS solutions were prepared daily. Then, mice were given DDW for the next 21 days. After this, animals were randomly divided into three groups of five mice each. Group I: DSS control; Group II: pre-infection + DSS; Group III: post-infection + DSS. S. japonicum eggs were prepared from the liver homogenate of a S. japonicum intraperitoneally infected rabbit. In the pre-treatment group, mice were treated once with S. japonicum eggs (2,000 eggs/mouse) 21 days before DSS treatment. In the post-infection group, mice were treated on day 7 after DSS treatment. An equal volume of saline was intraperitoneally injected into DSS control mice.
During the 7-day UC modeling course, changes in body weight and fecal occult blood levels were recorded, and experimental mice were observed daily. Body weight changes were normalized to the initial values recorded on day one. Fecal occult blood was identified using a Hemoccult assay kit (Zhuhai Beisuo Biotechnology Co. Ltd, Zhuhai, China). These observations were changed to once a week when the UC model was established. Percentage weight loss, occult blood test results, and fecal traits were used to indicate disease activity. The disease activity index (DAI) was based on accumulated scoring data (Supplementary Table S1, available in www.besjournal.com).
Score Weight loss (%) Character of Stoll FOBTa 0 0 Normal Negative 1 1–5 Looseb OB 2 5–10 Looseb OB 3 10–15 Wateryc Gross blood 4 > 15 Wateryc Gross blood Note. aFOBT, fecal occult blood test; OB indicates occult blood. bLoose mush that is not adherent to the anus of mice. cStools are liquid and adherent to the anus of mice. Table S1. Disease Activity Index (DAI) scoring
Mice were euthanized with 10% chloral hydrate via intraperitoneal injection (0.06 mL/10 g body weight), and 6–8 min after unconsciousness, serum and colon tissue samples were collected. Blood was placed at 37 °C for 1 h followed by centrifugation at 1,200 rpm for 15 min. Serum was then collected and stored at –20 °C. Next, 1 cm colon tissue (2.5–3.5 cm distal to the end of the appendix) was removed and fixed in 4% formalin, embedded in paraffin, and 5 μm sections generated for hematoxylin & eosin staining. Colon tissue homogenization times were limited to 5 min in a glass homogenizer. Stool was evaluated using stool characteristics and fecal occult blood test results (Supplementary Table S1); partial scores were added to provide final scores. After mice were sacrificed, macroscopic scores were based on colon length, hyperemia, wall-thickening, ulceration, inflammation extension, and damage. Sections from mice were analyzed by a pathologist blinded to groupings and treatments. Histopathological scores were calculated as the percent involvement score multiply the sum of the inflammation score, the extent score and the crypt damage score, which ranged from 0–40 according to Supplementary Table S2 (available in www.besjournal.com).
Feature graded Grade Description Inflammation 0 None 1 Slight 2 Moderate 3 Severe Extent 0 None 1 Mucosa 2 Mucosa and submucosa 3 Transmural Crypt damage 0 None 1 Basal 1/3 damaged 2 Basal 2/3 damaged 3 Only surface epithelium intact 4 Entire crypt and epithelium lost Percent involvement (%) 1 1–25 2 26–50 3 51–75 4 76–100 Table S2. The quantitative histological grading of colitis
Enzyme-linked immunosorbent assays for interleukin 1β (IL-1β) (Cat#: E-EL-M0037c), IL-4 (Cat#: E-EL-M0043c), IL-17 (Cat#: E-EL-M0047c), and transforming growth factor-β1 (TGF-β1) (Cat#: E-EL-M0051c) were supplied by Elabscience® (Houston, TX, USA), and used to quantify cytokine levels. Assays were performed following manufacturer’s protocols. Remaining colon tissue was frozen in liquid nitrogen and stored at –80 °C to prepare colonic tissue homogenates.
SPSS 21.0 (Chicago, IL, USA) and GraphPad Prism 7.04 were used for data analysis and presentation. One-way analysis of variance was used for the two-way factorial design, and Student’s t-tests were used for unpaired comparisons after Levene’s tests. P values for each comparison were annotated. Three cohorts were analyzed. Data are shown as the mean ± standard deviation and P < 0.05 was considered significant.
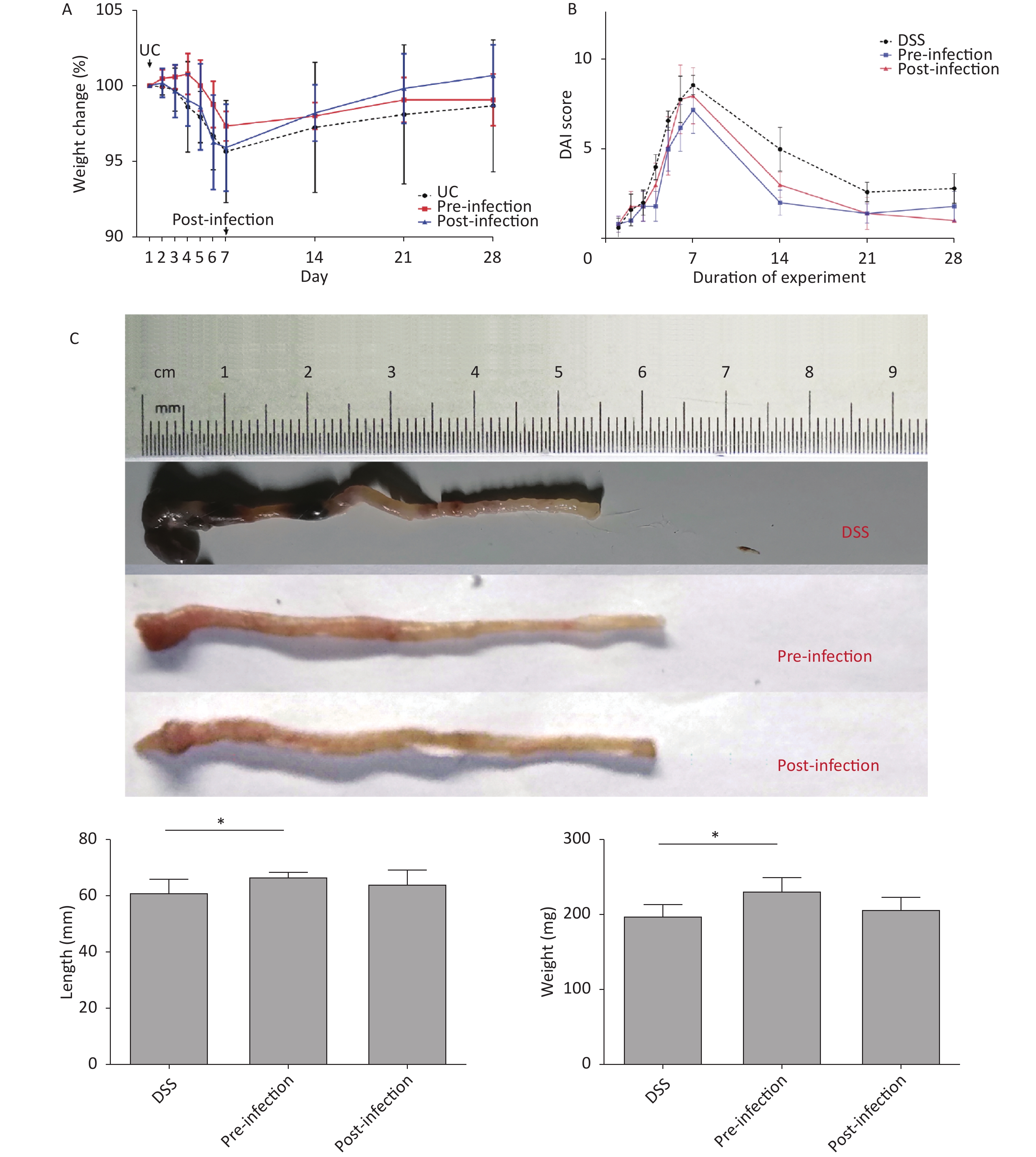
Preliminary studies indicated that male mice were more sensitive to DSS, had more severe symptoms, and had higher mortality rates when compared with females. Therefore, female mice were chosen for the current study. Mouse body weight in DSS and post-treatment groups decreased significantly from days 2 to 7. However, body weight in the pre-treatment group did not decrease until day 5. Mouse weight gradually increased after DSS withdrawal. Interestingly, mice in the post-treatment group exhibited a more significant increase in body weight at this stage (Figure 1A). UC mice presented general UC manifestations in accordance with observations in the literature. DAI scores in S. japonicum egg pre-treatment animals increased more slowly than in the control group. Mice in the post-treatment group had profound lower DAI scores (Figure 1B).
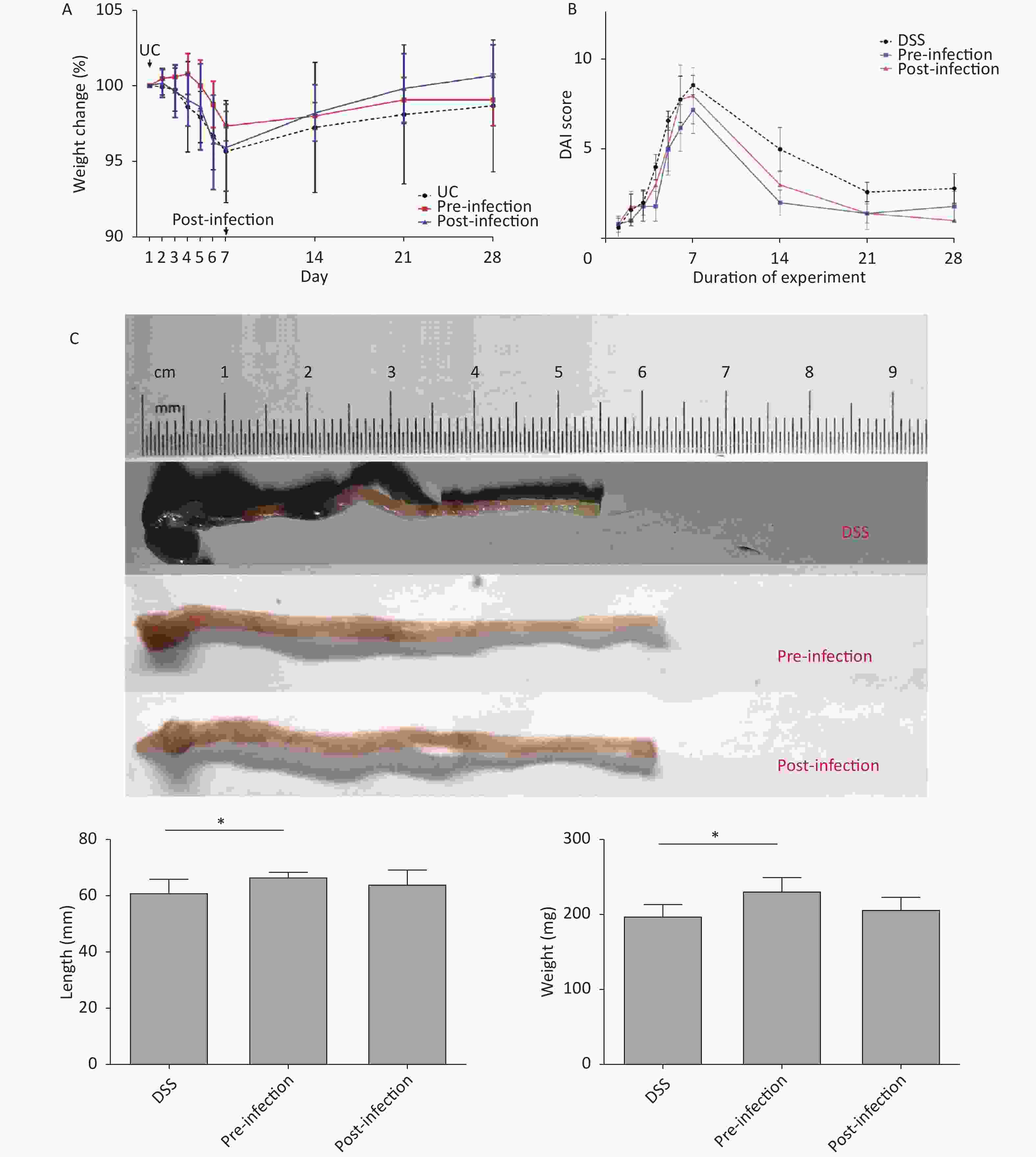
Figure 1. Body weight, DAI change and colon length comparison. (A) Weight loss observed in the last 3 days in UC induction time, groups without pre-infection of S. japonicum decreased more steeply than groups with S. japonicum. The post-infection group got a more significant increase in body weight in the last 21 days. (B) Increase of disease activity index observed in all groups, DAI score of group with pre-treatment of S. japonicum increase more slowly. DAI score of mice with post-treatment tended to alleviate more rapidly than other groups. (C) Shortened and fragile colons with thickened edematous intestinal walls were observed in UC-control mice. Pre-exposure appeared to abrogate colon shortening and edema and restore colon weight. UC, ulcerative colitis. DAI, disease activity index. DSS, disease activity index. *P < 0.05
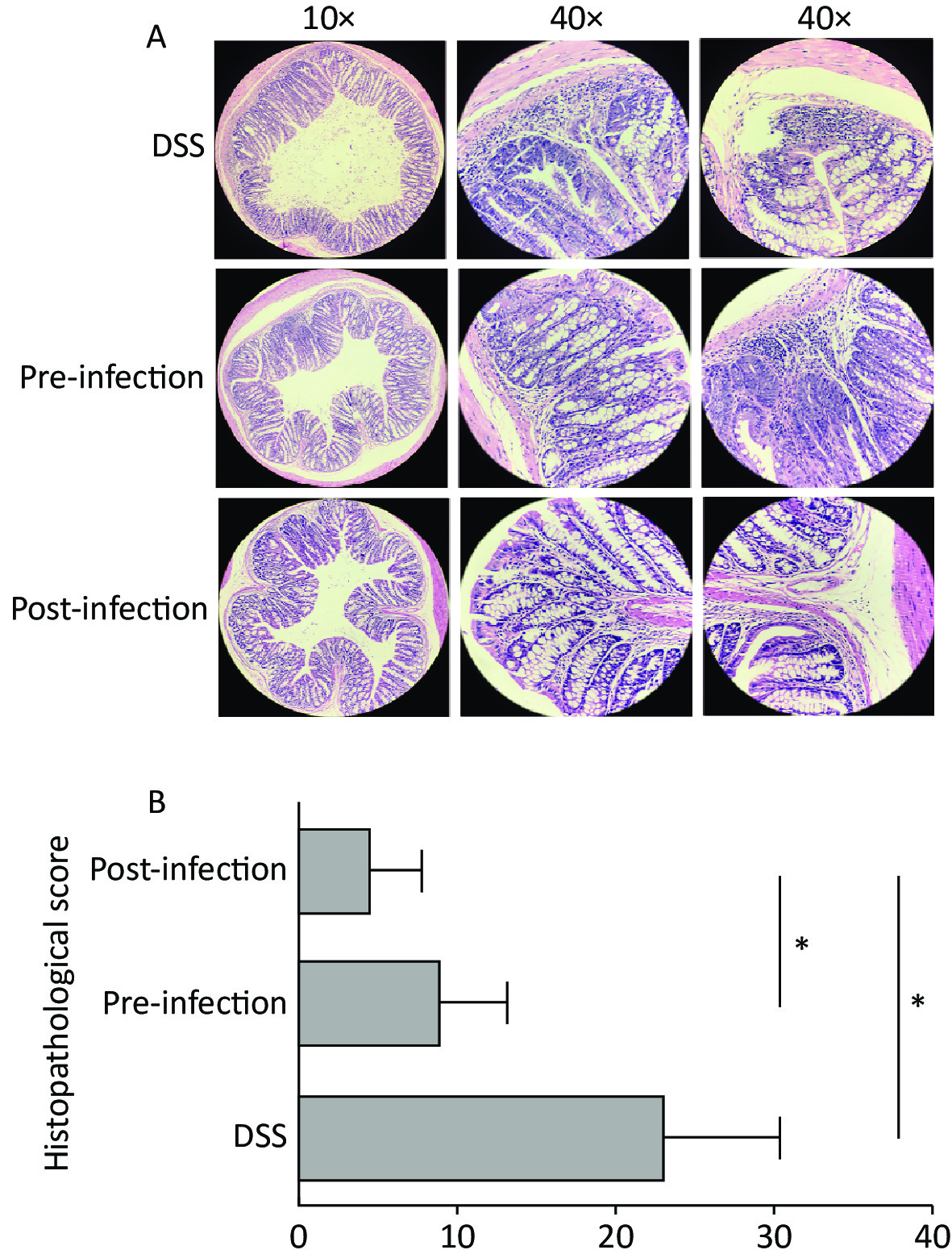
Colon weight and length usually decrease in colitis. In our study, colons in pre- and post-treatment groups had increased lengths and weights when compared with DSS animals. Although not statistically significant, post-treatment with S. japonicum eggs had a trend of alleviation in colon shortening and weight loss from DSS-induced UC (Figure 1C). At the micro level, colon tissue in pre- and post-treatment groups had significantly lower microscopic scores when compared with DSS animals, suggesting treatment with S. japonicum eggs protected colon tissue from DSS-induced damage (P < 0.05). Post-treatment animals had lower scores than pre-treatment animals (Figure 2).
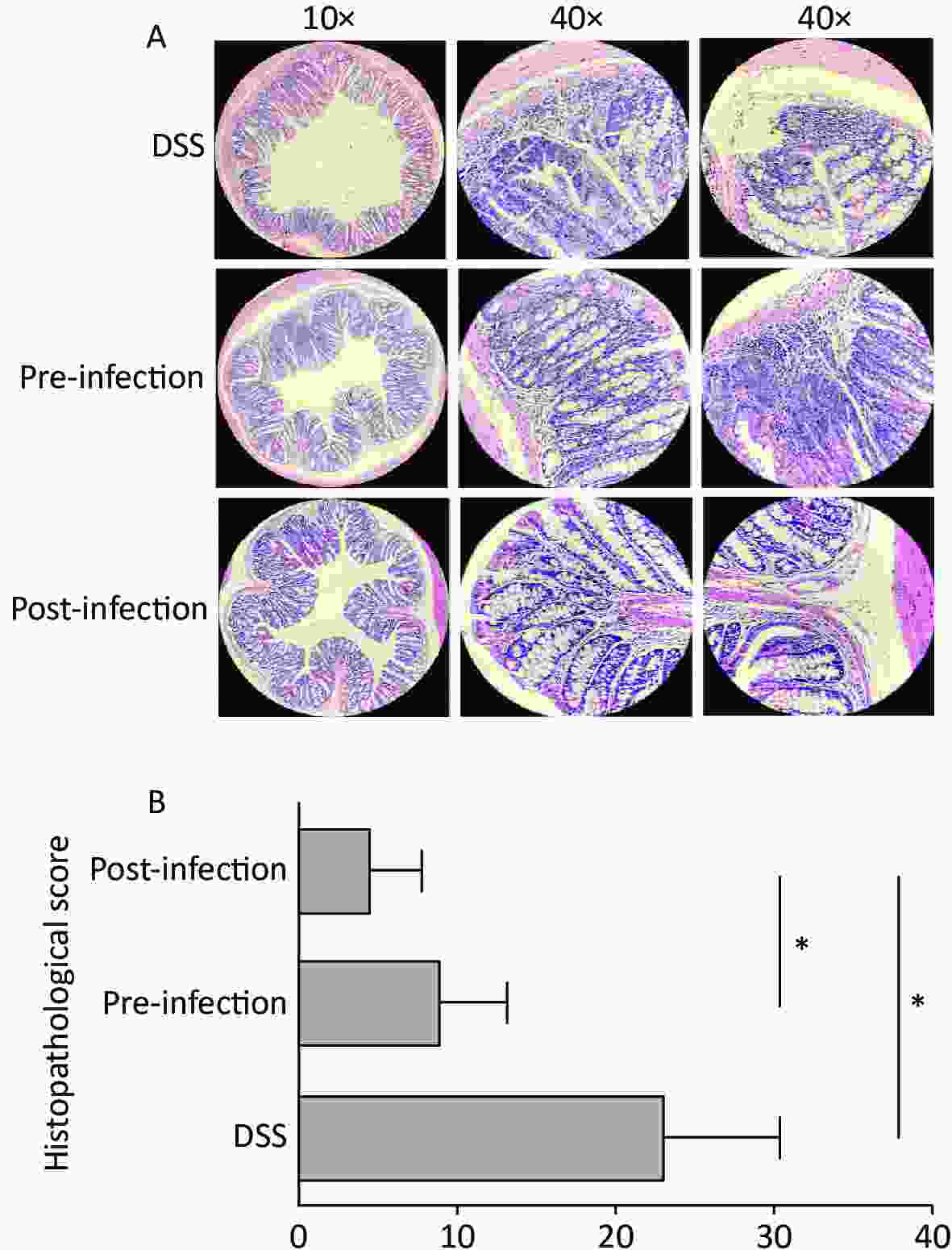
Figure 2. Histological grading of colitis comparison. (A) Cross-section of colon shown in ×10 magnification, representative damage of crypts or epithelium shown in ×40 magnification. Abnormal crypt structure and disrupted mucous membrane observed in UC-only mice. More lesions in the mucous membrane, along with cellular infiltration and extensive edema was observed in the pre-exposed group, while re-epithelialization and regeneration of crypt structure was more apparent in post-infected group. (B) The quantitative histological grading of colitis. Exposure to S. japonicum significantly reduced the score, while timing of intervention had a minor effect. Data is represented as mean ± SD. Significant differences are indicated by asterisks, P < 0.05 was annotated in the figure as * (n = 5 in each group). UC, ulcerative colitis. DSS, disease activity index.
Our previous results show that Trichinella improved colitis, and that post-treatment had better effects than a pre-treatment intervention [9]. In the current study, pre- and post-treatments improved UC pathology. Interestingly, post-treatment effects were better than pre-treatment effects. Thus, S. japonicum egg antigens had both preventative and therapeutic effects on UC, and the therapeutic effect seems to be more distinguishable. Further studies are required to determine how long these protective effects persist.
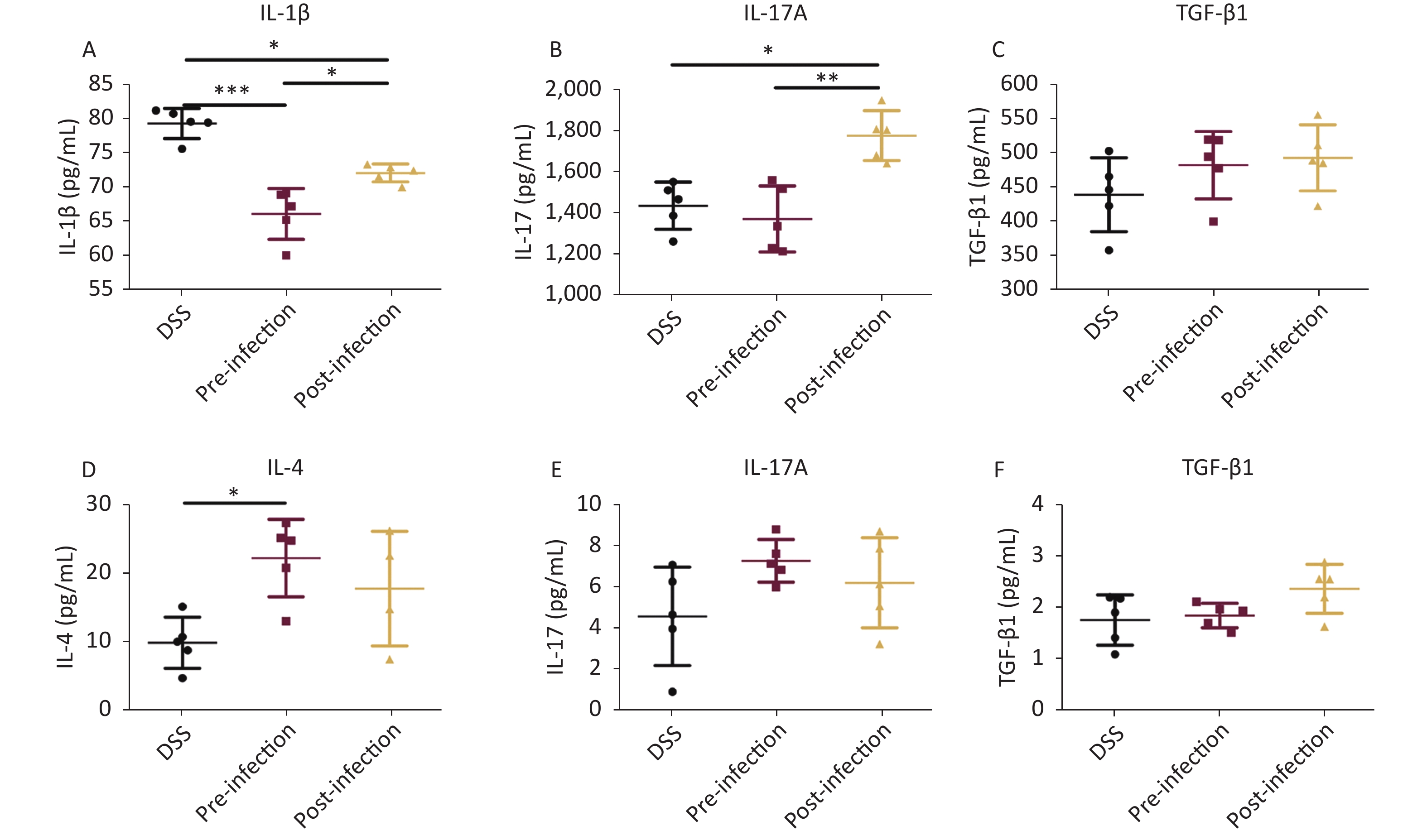
Cytokine analyses showed significant decreases in the Th1 pro-inflammatory cytokine IL-1β, especially in pre-treatment animals. Similarly, TGF-β1 serum levels in post-treatment mice were also increased when compared with DSS animals. Interestingly, proinflammatory IL-17A cytokine levels were decreased in pre-treatment animals, but increased in post-treatment mice when compared with DSS animals (Figure 3). Levels of the representative Th2 cytokine IL-4 were much higher in pre-treatment mice when compared with DSS mice. In colon tissue, IL-17A was significantly upregulated in pre- and post-treatment groups, but no significant differences were observed among all groups. Similarly, TGF-β1 levels in post-treatment colons were also higher when compared with DSS animals. Other cytokines were not significantly changed (Figure 3). These data suggested that the immune balance between Th2 and Th1 cytokines was important for UC prevention and treatment, and appeared to be the main mechanism in the prevention group, while TGF-β, playing a regulatory role, may have had a more obvious effect in treatment processes.
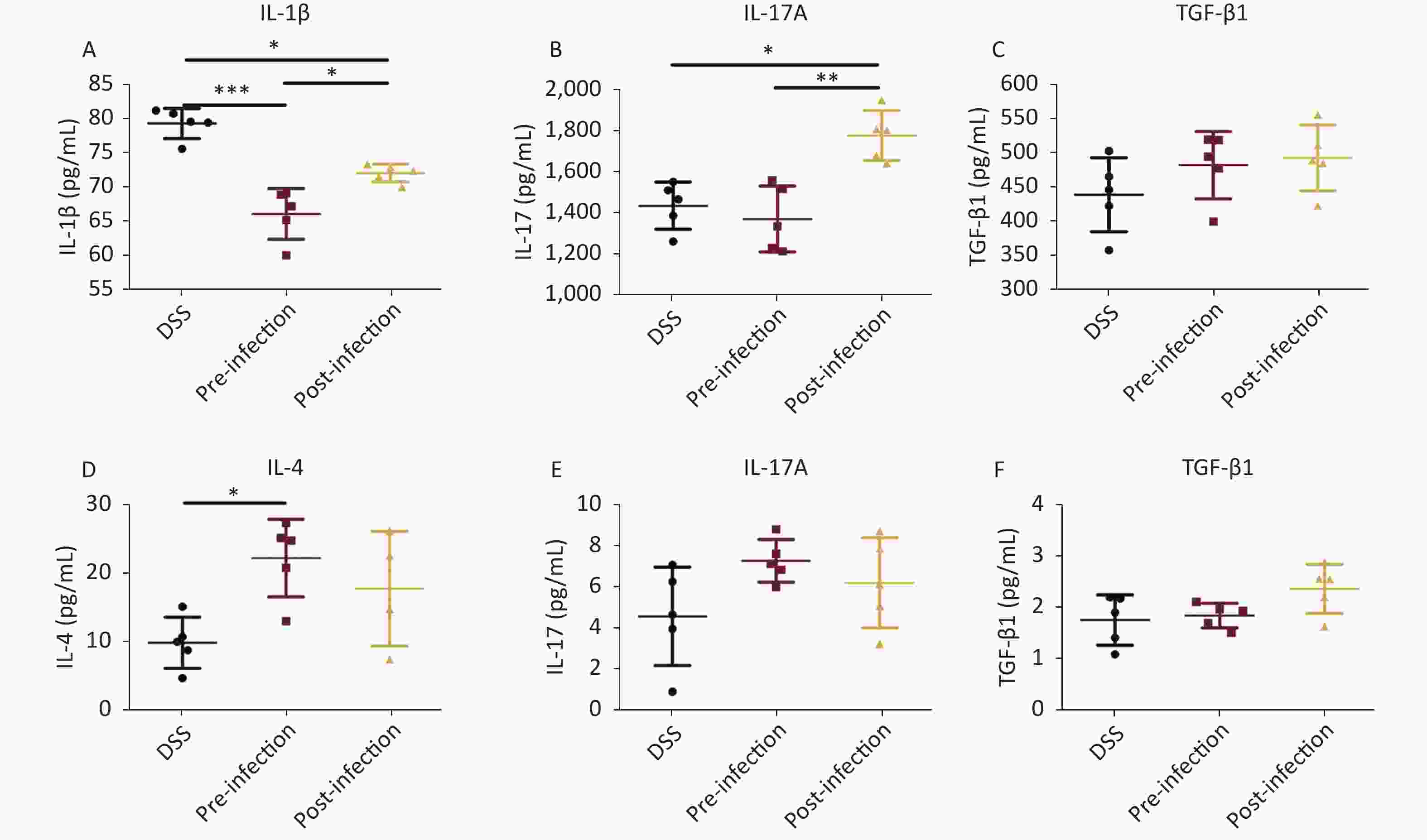
Figure 3. Cytokine detection. Serum levels of IL-1β/IL-17/TGF-β1 in serum (A–C), levels of IL-4/IL-17/TGF-β1 (D–F) in colon tissue homogenate were quantified using an ELISA assay. Data is represented as mean ± SD. Significant differences are indicated by asterisks, P < 0.05, P < 0.01, P < 0.001 were respectively annotated in the figure as *, **, *** (n = 5 in each group).
In recent years, several studies investigated other schistosomiasis molecules for UC; S. japonicum soluble egg antigen (SEA) activated Signal transducer and activator of transcription 6 (STAT6) and phosphatidylinositide 3-kinases (PI3K) signaling pathways to induce macrophage polarization toward M2 stages, which exerted anti-inflammatory functions by secreting IL-10. S. japonicum SEA also down-regulated tumor necrosis factor and inducible nitric oxide synthase gene expression in inflammatory cells [10]. We observed increased levels of IL-17A in colon tissues after intervention with S. japonicum eggs, suggesting a beneficial role of this cytokine in commensal tissue homeostasis, in agreement with an early study (PMID: 18411338). Understandably, due to human function complexity, animal models cannot be used to fully recapitulate the human condition; however, animal models provide deeper insights into human disease. In future studies, we will collect human clinical samples to characterize UC. In conclusion, we identified a role for S. japonicum eggs in treating UC, and provided an experimental basis for further in-depth research in the future. Such studies will include identifying how long the protective effects of S. japonicum eggs last, suitable doses, treatment durations, and single mono-therapeutic component screening.
Acknowledgments
We thank Dr. Modeste Judes and Dr. Shuaiqin Huang for language modification in this manuscript. Conflicts of Interest
All authors declare no conflict of interest in this work. Ethics Statement
Experimental animals were used according to and with the approval of the local ethics committee for the use of animals at the Central South University (Changsha, China). The microorganisms included in this study were handled according to the General Biosafety Standard for Microbiological and Biomedical Laboratories of the People’s Republic of China, with protocol number WS233-2002.
HTML
 22193Supplementary Materials.pdf
22193Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: