-
With the rapid progress of urbanization, atmospheric fine particulate matter with an aerodynamic diameter of less than 2.5 μm (PM2.5) has attracted increasing concern because of its hazardous effects as an air pollutant on human health[1]. Epidemiological studies have shown that PM2.5 has adverse effects on the respiratory, cardiovascular, nervous, and immune systems. The known toxic effects of PM2.5 on cells mainly involve cell viability, oxidative damage, inflammatory responses, and genotoxicity. Some reports have demonstrated that cells exposed to PM2.5 produced excessive ROS, which is considered the key mediator of PM2.5 toxicity[2-4]. PM2.5 has been documented to decrease the activity of the intracellular antioxidant enzymes catalase (CAT), superoxide dismutase, and glutathione peroxidase and increase the content of malondialdehyde (MDA).
Some reports suggested that PM2.5 induced systemic inflammation and oxidative stress in a rat model and significantly increased the gene expression of inflammatory factors in mouse macrophages. Although numerous studies on the mechanism of PM2.5-induced cell damage or apoptosis exist[5], only a few addressed the mitigation or alleviation of PM2.5 damage to cells by using ω-3 polyunsaturated fatty acids (PUFAs), and none discussed the possible protective function of ω-6 PUFA γ-linolenic acid (GLA) against the cellular damages caused by PM2.5.
GLA is abundant in some higher plants (e.g., the seeds of borage and evening primrose plants) and blue-green algae (e.g., Spirulina spp. and Nostoc commune). Several studies have demonstrated that ω-3 or ω-6 PUFAs can reduce oxidative stress and inflammatory responses by inhibiting inflammatory cytokine signaling pathways[6] and confirmed the therapeutic effects of ω-3 or ω-6 PUFAs on arthritis, asthma, and other related diseases[7]. Spirulina is a single-cell organism that is a source of protein and is rich in essential nutrients, vitamins, and GLA. We previously demonstrated that in mice, the combination of fish oil (FO) and Spirulina ethanol extract (SE) rich in GLA resulted in the inhibition of airway inflammation [8]. In this study, we investigated the protective effects of SE combined with FO on oxidative stress and inflammatory reactions caused by PM2.5 exposure. We utilized Spirulina SE as the source of the ω-6 PUFA GLA and FO as the source of the ω-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) to study the alleviation of PM2.5 toxicity to the human lung cancer alveolar epithelial cell line A549.
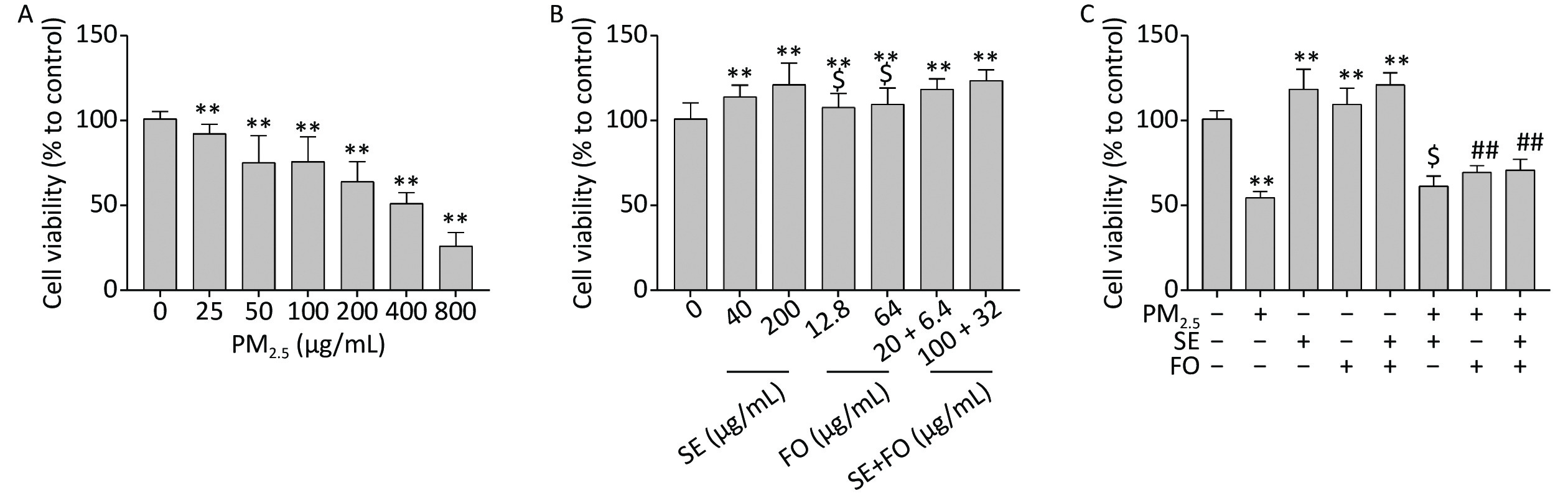
We first tested the effects of PM2.5 on the viability of A549 cells by exposing the cells to different concentrations of PM2.5 for 24 h. Cell viability decreased as the PM2.5 concentration was increased from 25 μg/mL to 800 μg/mL. Approximately 50% of the cells survived after 24 h exposure to 400 μg/mL PM2.5 (Figure 1A). The viability of A549 cells treated with SE, FO, or SE+FO for 24 h slightly increased compared with that of the cells treated with the control (Figure 1B). On the basis of these results, we selected the PM2.5 concentration of 400 μg/mL and high concentrations of SE (200 μg/mL), FO (64 μg/mL), and SE+FO (100 + 32 μg/mL) for subsequent studies (the fatty acid profiles of SE, FO, and SE+FO are described in Supplementary Table S1, available in www.besjournal.com).
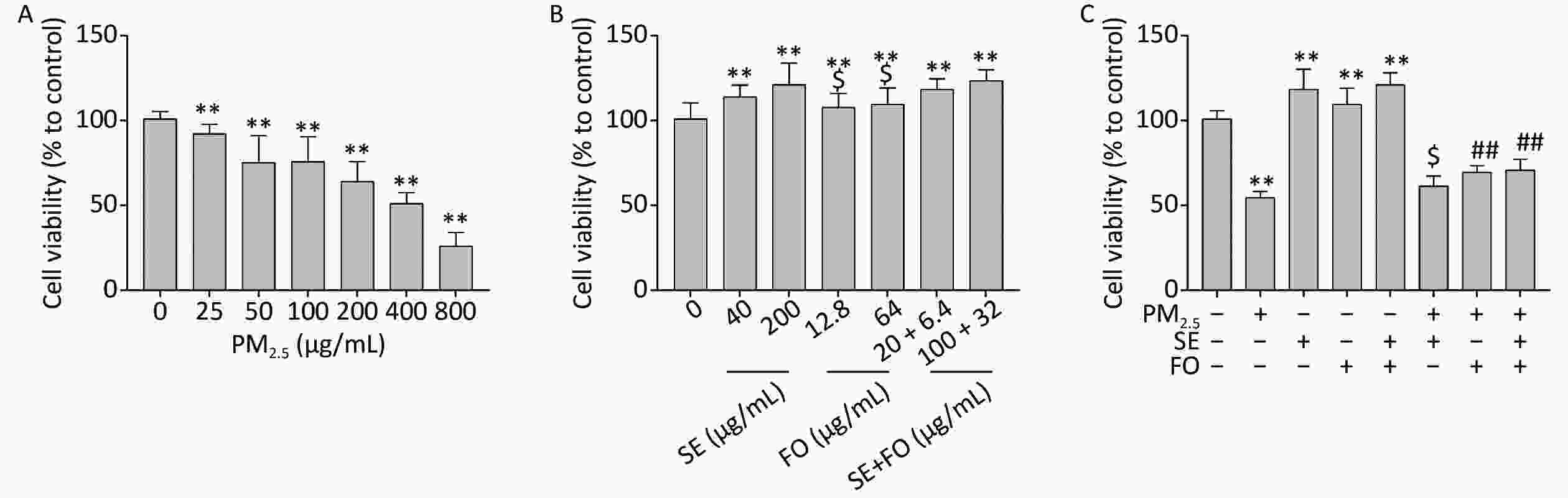
Figure 1. Effects of PM2.5 (A), SE/FO (B), and PM2.5 combined with SE/FO (C) on cell viability. A549 cells were treated with different concentrations of PM2.5 and SE/FO for 24 h, and their survival rate was determined by MTT assay. Means ± SD, n = 5. **P < 0.01 vs. normal control; ##P < 0.01 vs. PM2.5 control; $P < 0.05 vs. SE+FO treatment.
The viabilities of A549 cells pretreated with SE, FO, or SE+FO for 24 h before PM2.5 exposure were higher than those of the cells treated with PM2.5 alone (Figure 1C). This result demonstrated that SE, FO, or SE+FO can alleviate the damages caused by PM2.5 to A549 cells. In addition, pretreatment with FO or SE+FO resulted in higher cell viability than pretreatment with SE alone. This finding suggested that FO might have better protective effects against PM2.5 damage than SE.
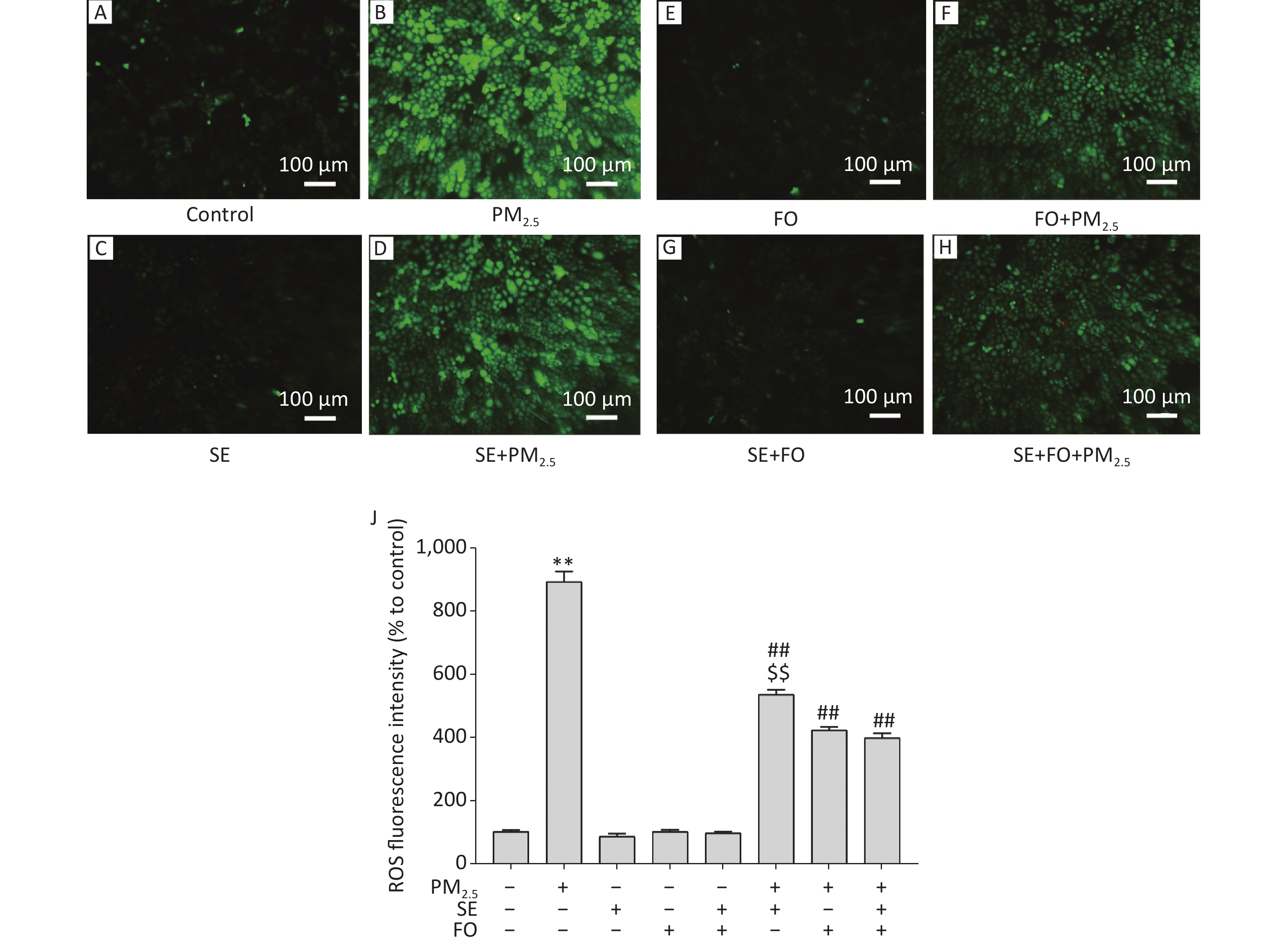
A549 cells were exposed to PM2.5 after pretreatment with SE, FO, or SE+FO for 24 h. Then, their intracellular ROS levels were detected by using the fluorescent probe H2DCFDA to monitor the changes in their intracellular ROS contents. Fluorescence microscopy observation revealed that intracellular ROS content, measured in the form of fluorescence intensity, in the cells exposed to PM2.5 for 24 h significantly increased relative to that in the control cells (Figure 2A, B, & J). However, treatment with SE, FO, or SE+FO did not induce any ROS production (Figure 2C, E , G, & J).
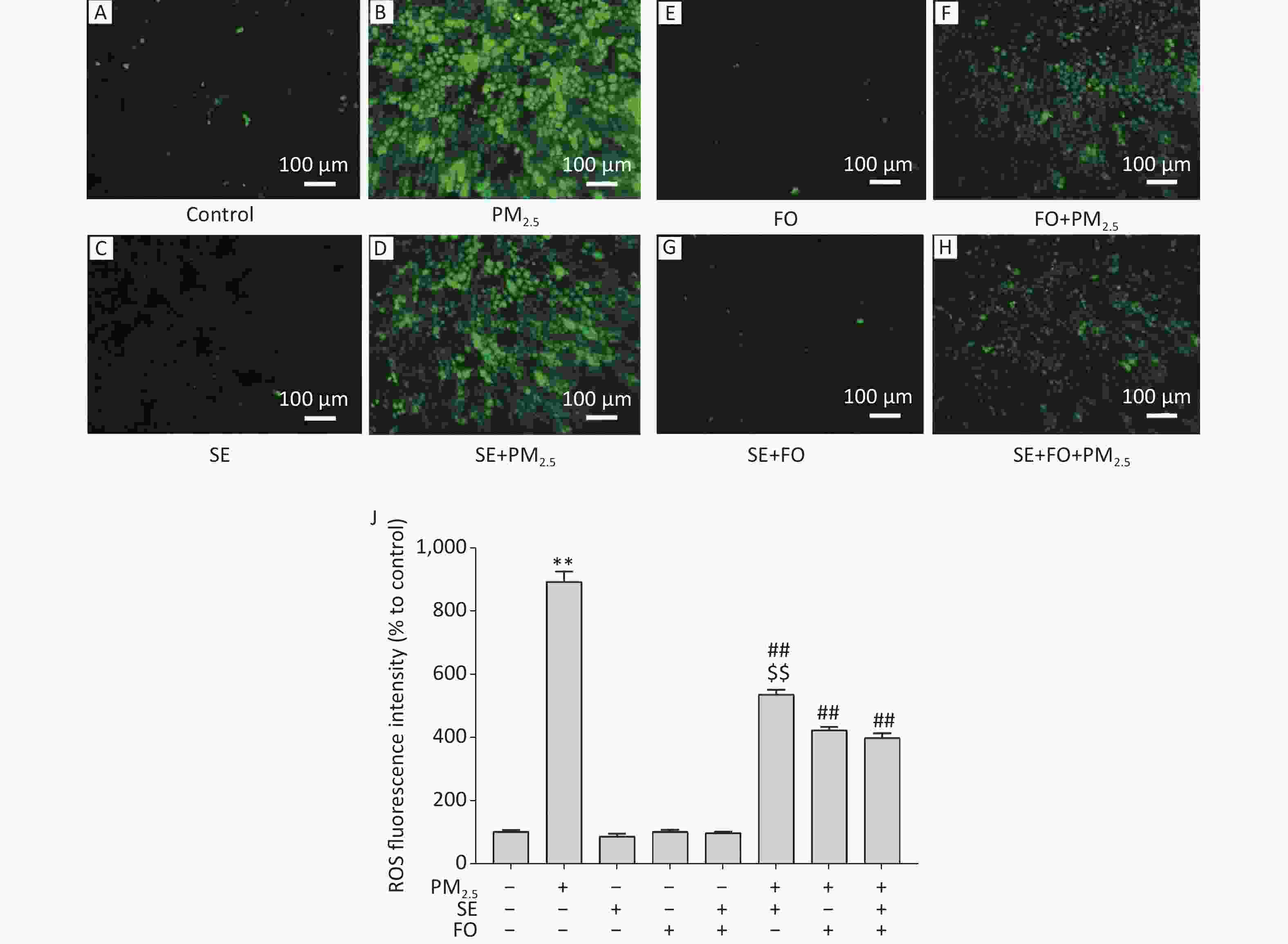
Figure 2. ROS production induced by exposure to PM2.5 and/or SE, FO, and SE+FO. Representative images (A–H) and the quantitative results (J) of ROS generation in A549 cells detected with H2DCFDA fluorescein. The green fluorescence intensity of A549 cells was analyzed by using Image software. Means ± S.D., n = 3. **P < 0.01 vs. normal control; ##P < 0.01 vs. PM2.5 control. $$P < 0.01 vs. SE+FO treatment.
For the detection of the effects of SE and FO on ROS production induced by PM2.5 exposure, A549 cells were pretreated with SE, FO, or SE+FO for 24 h before PM2.5 exposure. ROS fluorescence intensity in the cells pretreated with SE was significantly reduced compared with that in the cells without pretreatment (Figure 2B, D, & J). FO or SE+FO pretreatment caused greater reductions in ROS fluorescence intensity than SE pretreatment (Figure 2D, F, H, & J). These results demonstrated that in A549 cells, SE and FO can reduce the ROS production induced by PM2.5 exposure and that FO showed better inhibitory effects than SE on ROS production.
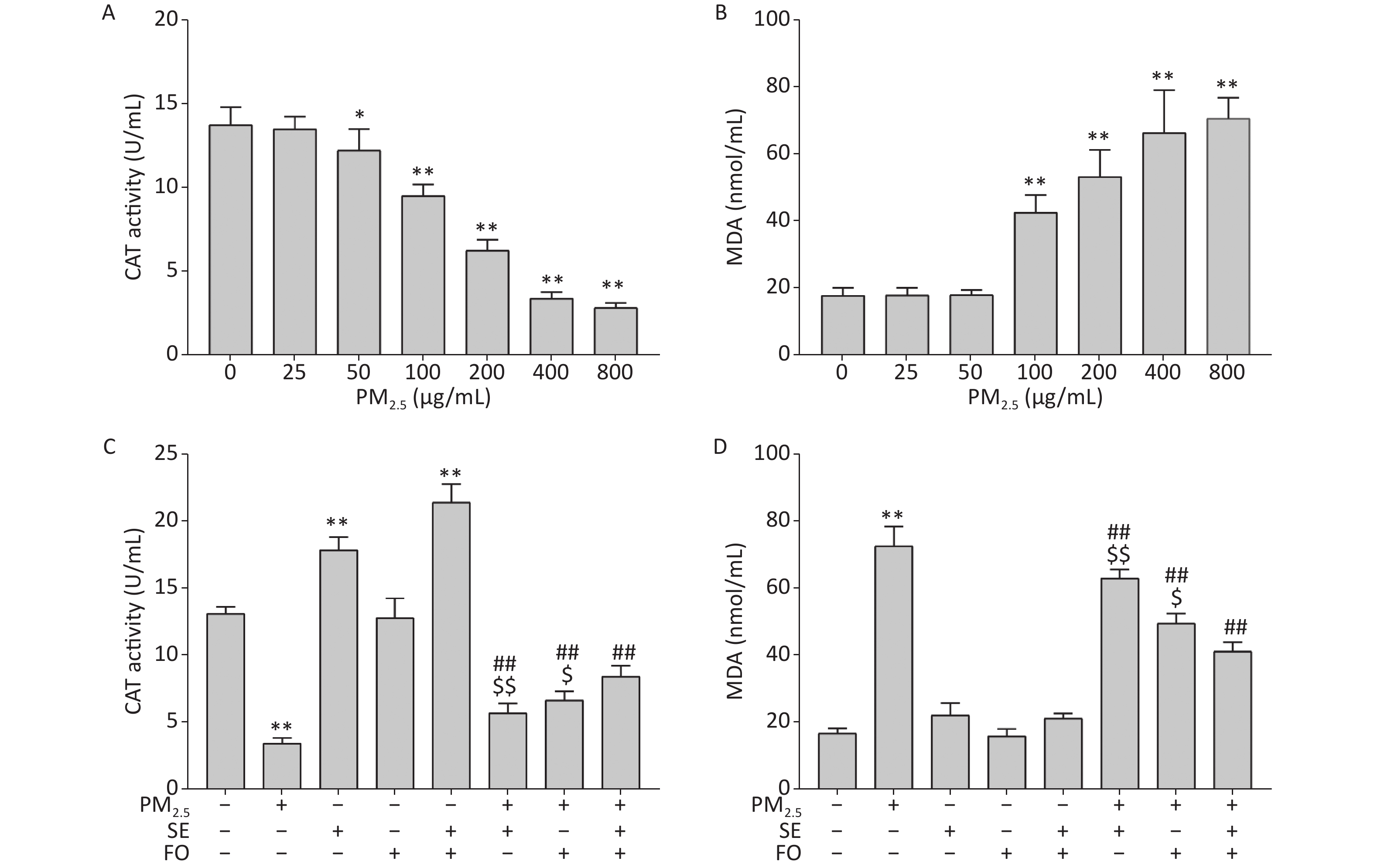
Previous studies have reported that CAT is the most adaptive antioxidant enzyme in the presence of oxidative stress and that linoleic acid, EPA, or DHA alone could enhance intracellular CAT enzyme activity after H2O2-induced oxidative damage in cells. However, these works did not investigate the effects of combined ω-6 and ω-3 fatty acids on CAT enzyme activity. We first examined the effects of PM2.5 on CAT activity in A549 cells to address this research gap. CAT activity started to decline at the PM2.5 concentration of 50 μg/mL and further decreased as the PM2.5 concentration was increased to 400 μg/mL (Supplementary Figure S1A, available in www.besjournal.com). This result demonstrated that PM2.5 can suppress antioxidant enzyme activity in A549 cells at high concentrations. Accordingly, oxidative damages, measured in the form of MDA contents, were observed in A549 cells exposed to high concentrations of PM2.5 (Supplementary Figure S1B). MDA is the end product of lipid peroxidation and oxidative stress. Therefore, it serves as an important indicator of oxidative damage. MDA contents started to increase at a PM2.5 concentration of 100 μg/mL and further increased at higher PM2.5 concentrations.
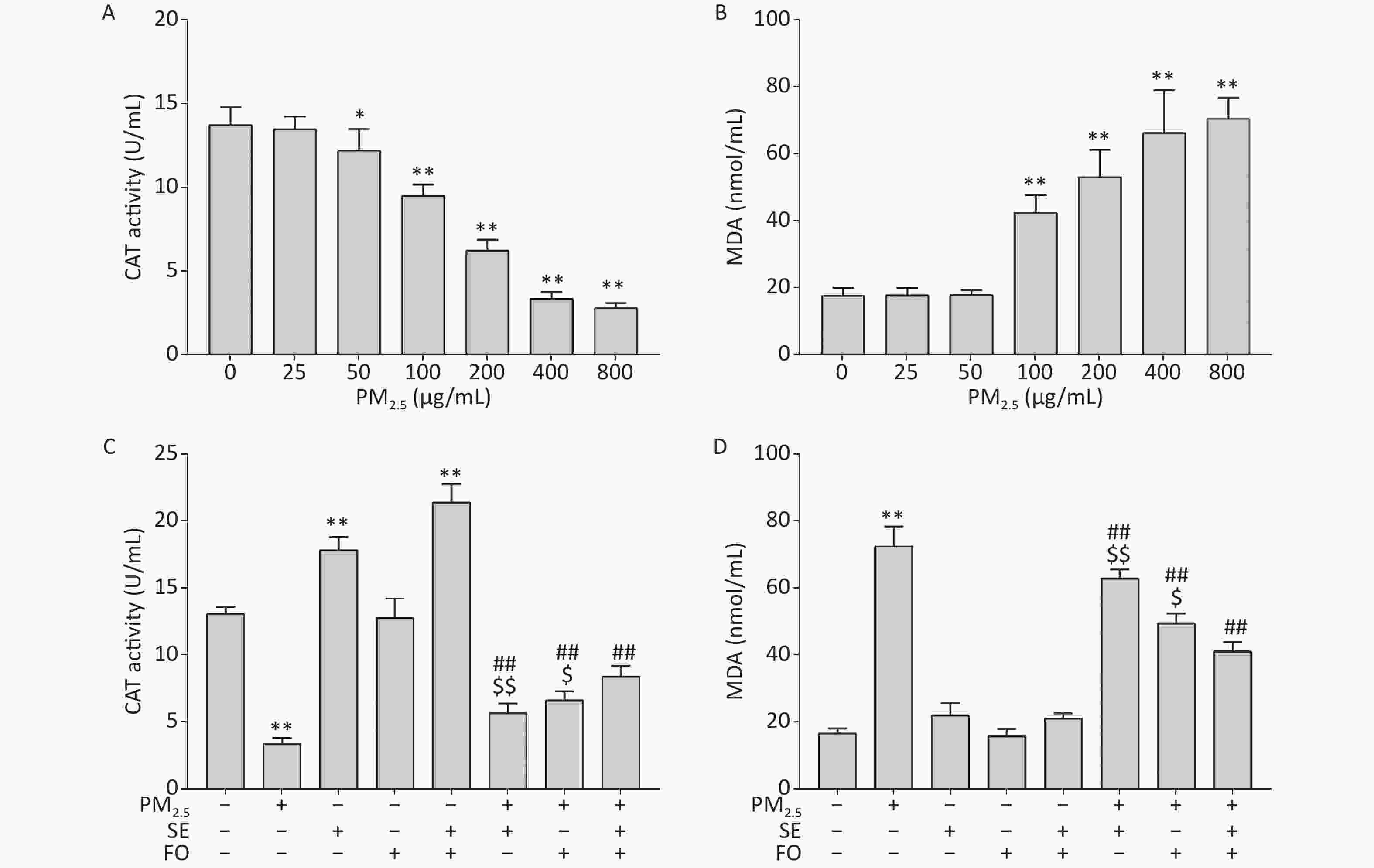
Figure S1. Changes in CAT activities (A, C) and MDA contents (B, D) in A549 cells. Cells were exposed to various concentrations of PM2.5 for 24 h with or without SE, FO, or SE+FO pretreatments for 24 h. Means ± SD, n = 3. *P < 0.05, **P < 0.01 vs. normal control; ##P < 0.01 vs. PM2.5 control. $P < 0.05, $$P < 0.01 vs. SE+FO treatment.
We pretreated the cells with SE, FO, or SE+FO for 24 h before we exposed them to 400 μg/mL PM2.5 to examine the protective effects of SE, FO, or SE+FO on the oxidative damages caused by PM2.5 to A549 cells. Intracellular CAT activity was significantly reduced in the cells exposed to PM2.5 without SE, FO, or SE+FO pretreatment (Supplementary Figure S1C). However, the reduction in CAT activities caused by exposure to PM2.5 was significantly alleviated when the cells were pretreated with SE, FO, or SE+FO. Interestingly, the cells treated with SE and SE+FO without PM2.5 exposure had higher CAT activities than the control, and treatments with FO did not induce higher CAT activity. This result indicated that SE might be more effective than FO for increasing CAT activity. Similar patterns were observed when MDA was measured as an indicator of oxidative damage (Supplementary Figure S1D). MDA content was significantly increased in PM2.5-exposed cells without any pretreatment. Pretreatments with FO or SE+FO resulted in significant decreases in MDA contents compared with PM2.5 exposure alone. SE+FO appeared to be more effective for reducing MDA contents in the cells exposed to PM2.5 than SE and FO alone. These results demonstrated that SE, FO, or SE+FO played a protective role against PM2.5-induced oxidative damage.
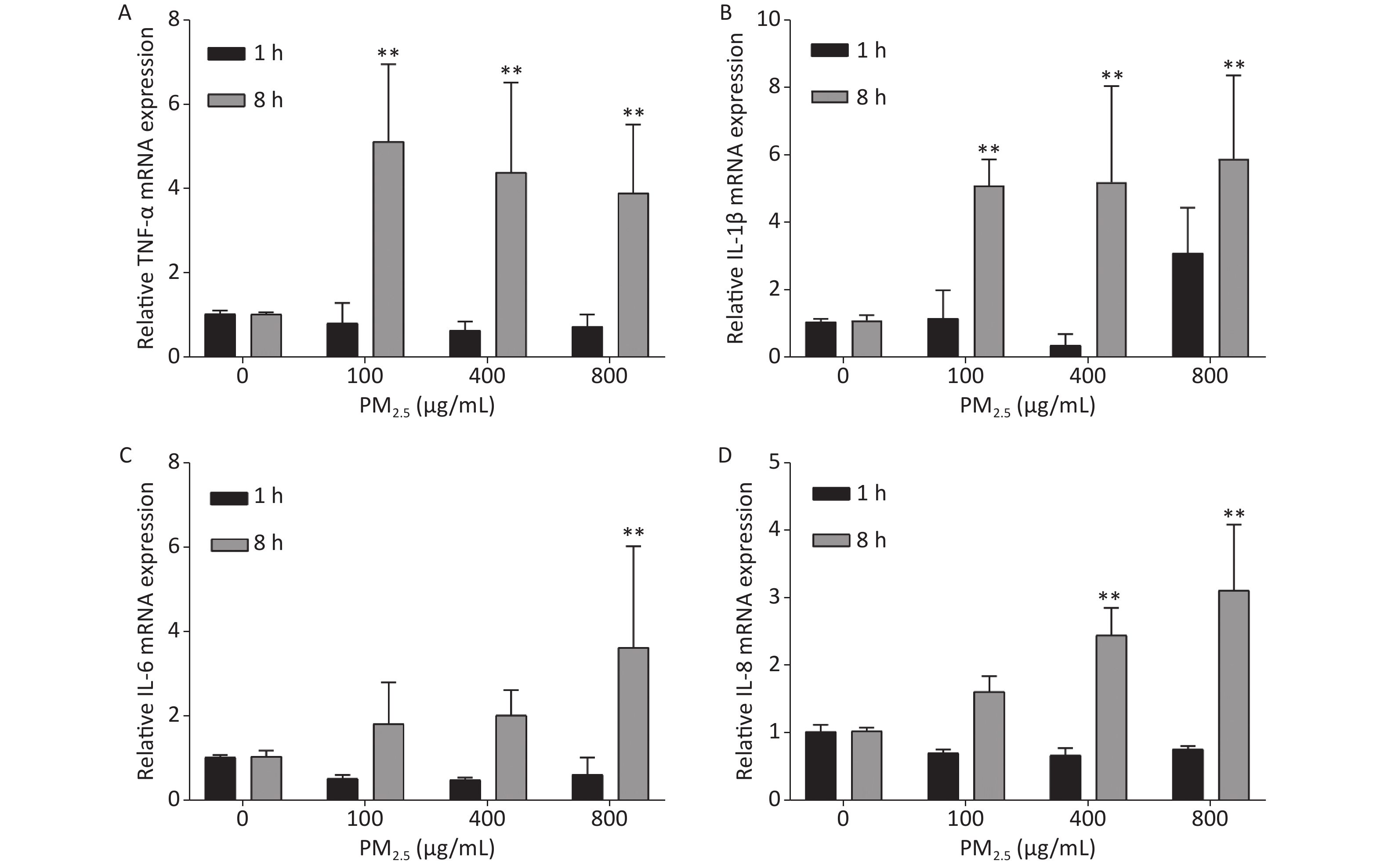
Several studies have found that the development of PM2.5-associated cardiovascular disease is linked to inflammatory responses, which in turn are closely related to the levels of inflammatory factors, such as TNF-α, IL-1β, IL-6, and IL-8. We used qPCR to detect the mRNA levels of inflammatory factors at different concentrations of PM2.5 [(the procedure is described in the Supplementary Materials and Methods, the primer sequences was showed in the Supplementary Table S2 (available in www.besjournal.com)] to investigate the changes in the gene expression of these factors in A549 cells exposed to PM2.5. Supplementary Figure S2 (available in www.besjournal.com) illustrates that no significant changes in the mRNA levels of all four factors, except for IL-1β, which exhibited elevated expression at a PM2.5 concentration of 800 μg/mL (Supplementary Figure S2B), were observed during the first hour. However, sharp increases in the mRNA levels of all inflammatory factors were observed 8 h after exposure to PM2.5. The mRNA levels of TNF-α and IL-1β showed similar increases at different PM2.5 concentrations (Supplementary Figure S2A, B). The increases in the mRNA levels of IL-6 and IL-8 were dependent on the concentration of PM2.5: higher PM2.5 concentrations resulted in higher mRNA levels (Supplementary Figure S2C, D). These results demonstrated that PM2.5 can induce inflammatory responses in A549 cells within 8 h.
Genes Forward (5’→3’) Reverse (5’→3’) β-Actin CTCCATCCTGGCCTCGCTGT GCTGTCACCTTCACCGTTCC TNF-α CTGCACTTTGGAGTGATCGG AACATGGGCTACAGGCTTGT IL-1β GCTCGCCAGTGAAATGATGG AGATTCGTAGCTGGATGCCG IL-6 ACTCACCTCTTCAGAACGAATTG CCATCTTTGGAAGGTTCAGGTTG IL-8 ACTGAGAGTGATTGAGAGTGGAC AACCCTCTGCACCCAGTTTTC Table S2. Primer sequences for qPCR
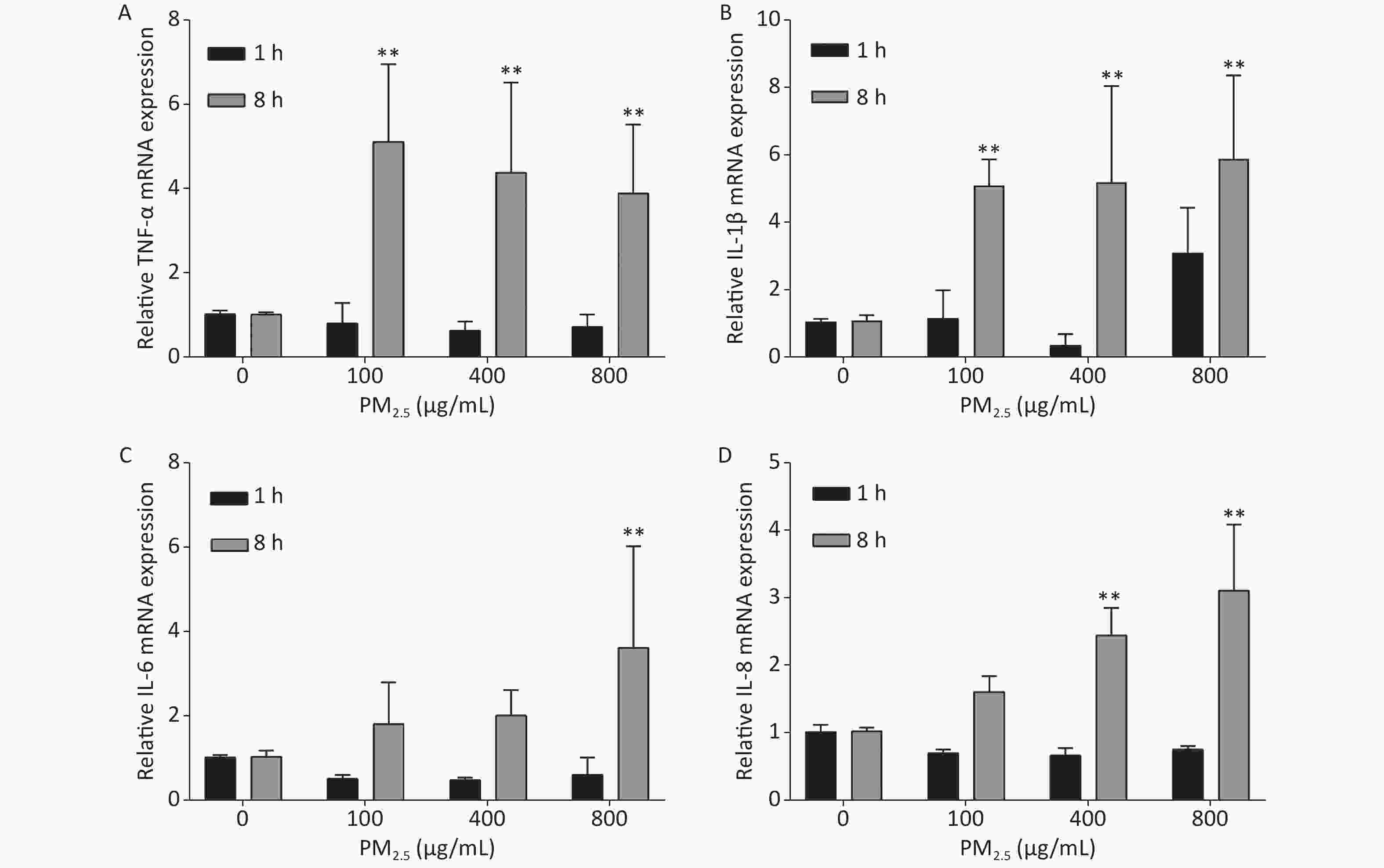
Figure S2. Inflammatory responses of A549 cells exposed to various concentrations of PM2.5. Means ± S.D., n = 3. *P < 0.05, **P < 0.01 vs. normal control.
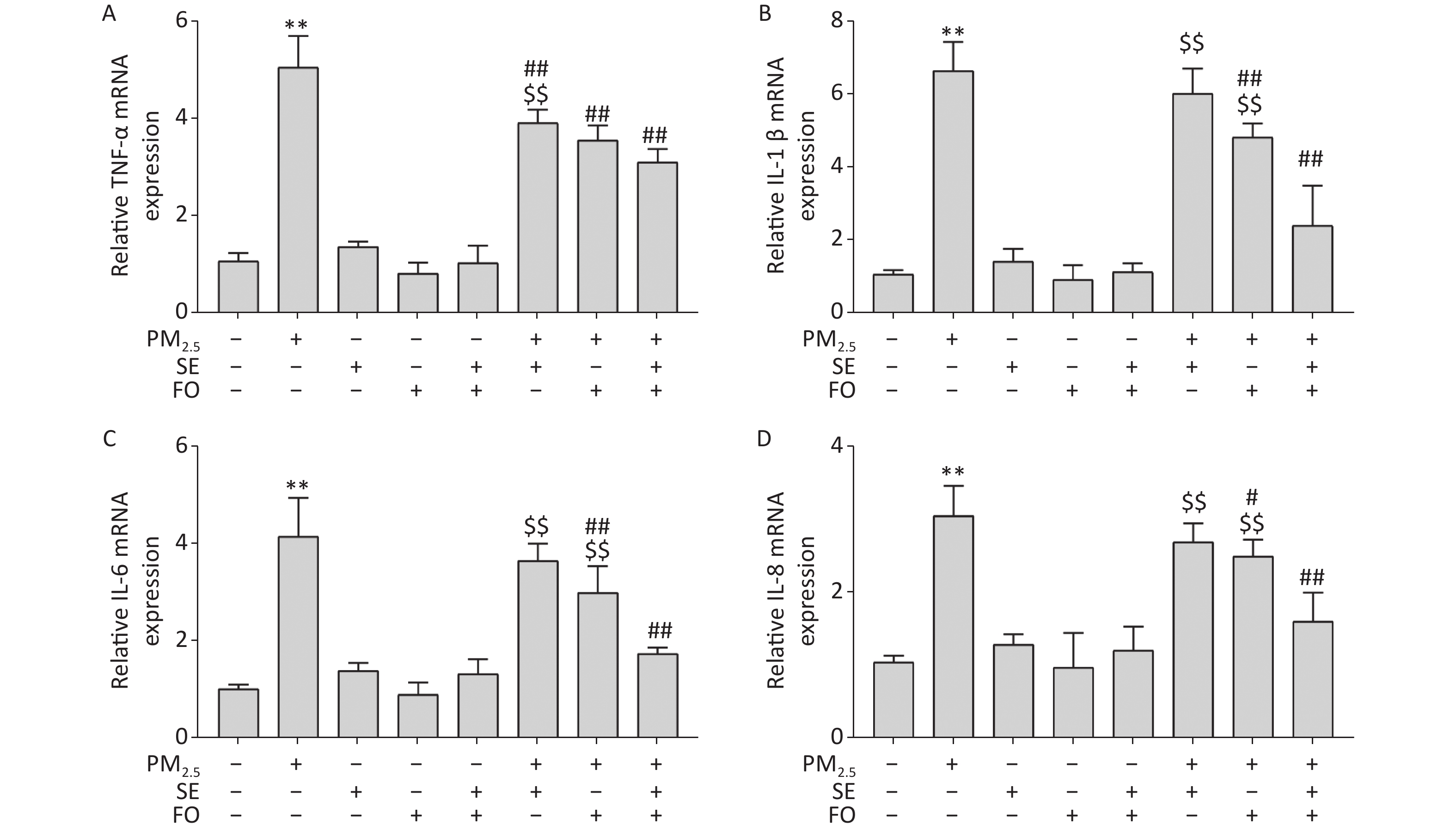
A549 cells were pretreated with SE, FO, or SE+FO for 24 h before exposure to 400 μg/mL PM2.5. The changes in the gene expression of TNF-α, IL-1β, IL-6, and IL-8 were monitored to test the effects of SE and FO on the inflammatory responses induced by exposure to PM2.5. Figure 3 shows that the mRNA levels of all inflammatory factors in the PM2.5-exposed cells had significantly increased compared with those in the blank control, and no significant change was observed in the cells treated with SE, FO, or SE+FO without exposure to PM2.5 (Figure 3A–D). However, when the cells were pretreated with SE, FO, or SE+FO for 24 h before exposure to 400 μg·mL−1 PM2.5, the increases in mRNA levels caused by exposure to PM2.5 were significantly reduced. The reduction in the mRNA levels of the inflammatory factors was most obvious in the cells pretreated with SE+FO, followed by that in the cells pretreated with FO and SE. These results demonstrated that SE, FO, and SE+FO had inhibitory effects on the gene expression of inflammatory factors induced by exposure to PM2.5.
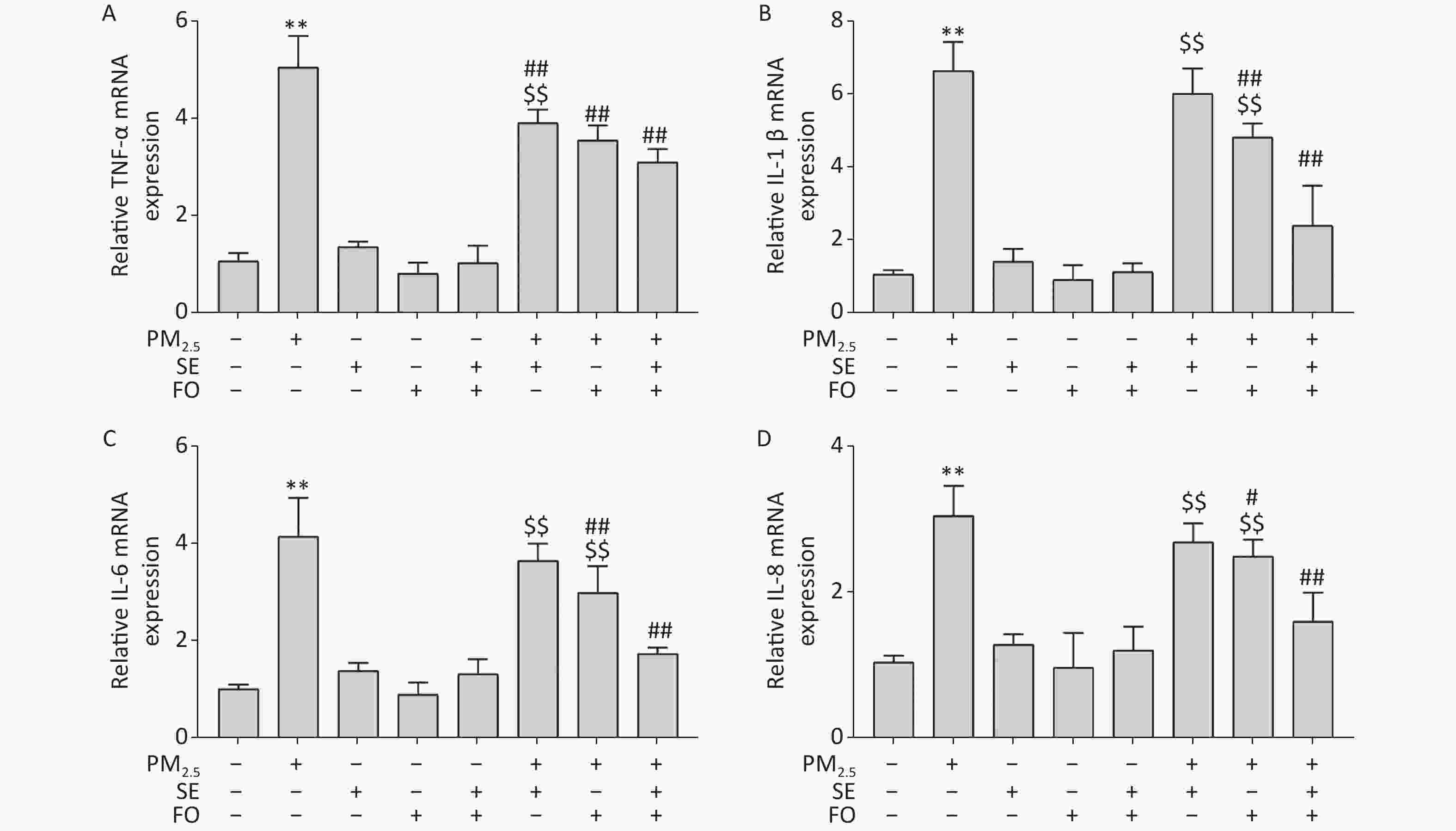
Figure 3. Relative expression levels of cytokine genes detected by qPCR: (A) TNF-α, (B) IL-1β, (C) IL-6, and (D) IL-8. A549 cells were pretreated with SE/FO for 24 h, then exposed to PM2.5 for 24 h. Means ± S.D., n = 3. **P < 0.01 vs. normal control; ##P < 0.01 vs. PM2.5 control. $$P < 0.01 vs. SE+FO treatment.
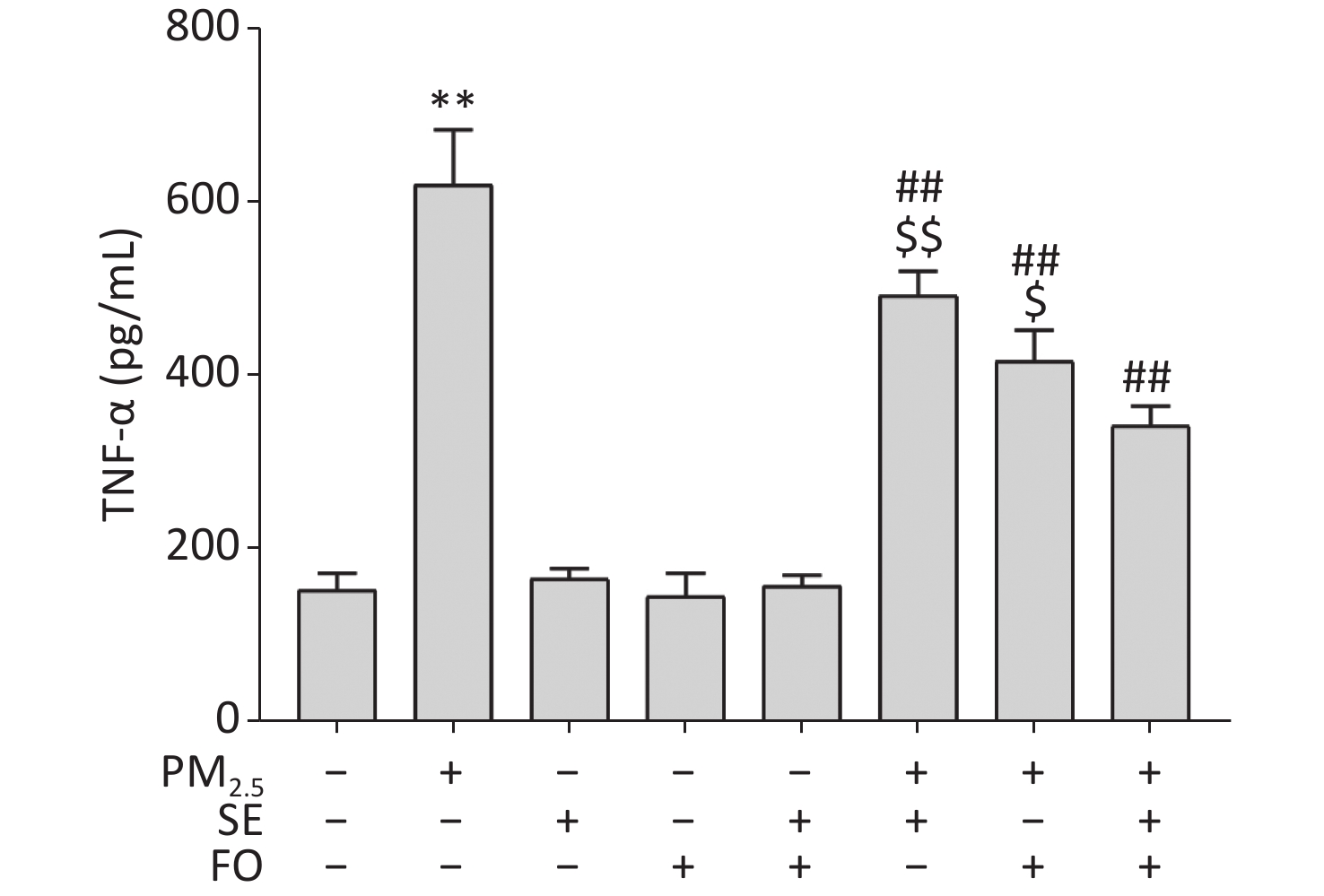
EPA and DHA have been suggested to be effective in reducing the LPS-induced activation of the NF-κB inflammatory pathway. Although several studies have shown that the NF-κB signaling pathway is activated in PM2.5-exposed cells, resulting in elevated levels of TNF-α in cells, no study has indicated that the combination of ω-6 and ω-3 fatty acids is effective in reducing the inflammatory factors induced by PM2.5 exposure. We measured TNF-α contents by ELISA to investigate the effect of SE, FO, and SE+FO on the PM2.5-induced production of the inflammatory factor TNF-α in A549 cells.
The results showed that the TNF-α level was significantly elevated in the cells exposed to PM2.5 only and did not change significantly in SE, FO or SE+FO treated cells without exposure to PM2.5 (Supplementary Figure S3, available in www.besjournal.com). However, in the cells pretreated with SE, FO, or SE+FO before exposure to PM2.5, the increases in TNF-α levels caused by exposure to PM2.5 were significantly reduced, demonstrating the inhibitory role of SE/FO in surges of TNF-α production. In addition, FO exerted stronger inhibitory effects on the increases in TNF-α production than SE, and SE+FO presented the highest inhibitory effects compared with SE or FO alone, suggesting that SE and FO have potential synergetic effects on PM2.5-induced increases in TNF-α production.
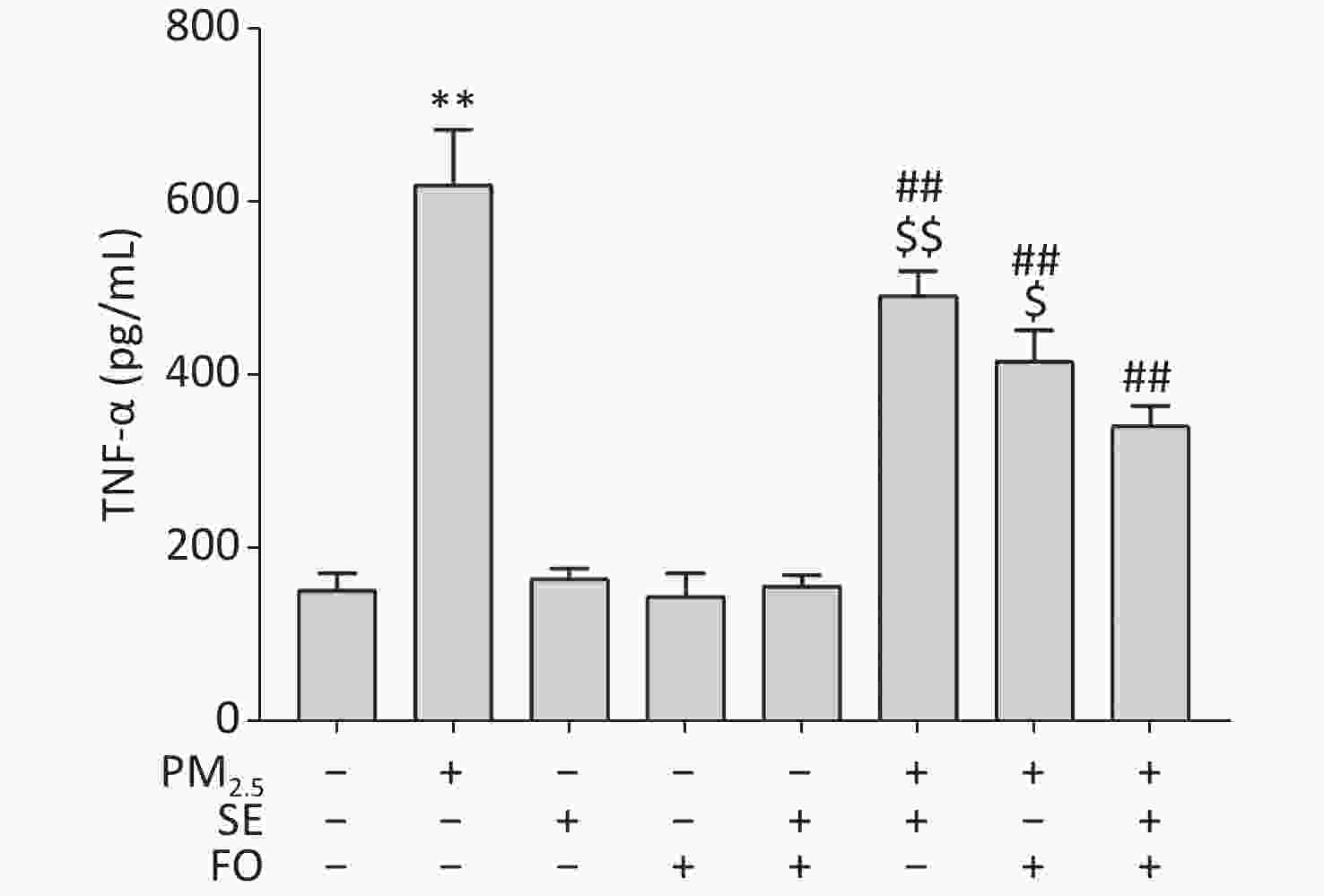
Figure S3. TNF-α contents in A549 cells pretreated with SE, FO, or SE+FO 24 h before exposure to PM2.5 for 24 h. Means ± S.D., n = 3. **P < 0.01 vs. normal control; ##P < 0.01 vs. PM2.5 control. $P < 0.05, $$P < 0.01 vs. SE+FO treatment.
Several reports have demonstrated the mitigating effects of FO alone or FO plus vitamin E on the toxicity of PM2.5 in cells, rat models, and human trials. However, the mechanism of these mitigating effects is still unclear at this stage. Our results demonstrated that PUFAs from SE and FO attenuated PM2.5 toxicity in three aspects: cell viability, oxidative stress, and inflammatory reactions caused by exposure to PM2.5. Thus, the alleviation of oxidative damages caused by PM2.5 exposure was postulated to be due to the protective roles of SE and FO in the antioxidative system in cells. For example, although the activity of the antioxidant enzyme CAT was suppressed by PM2.5, the suppression of CAT activity was alleviated by SE and FO pretreatments. Further research is needed to elucidate how GLA from SE and EPA/DHA from FO enhanced the antioxidant activity of cells.
The anti-inflammatory activities of EPA/DHA from FO have been well documented [9], whereas the effects of GLA on inflammatory responses are controversial[10]. GLA is one of the most important PUFAs derived from linoleic acid and is considered the precursor of several bioactive signaling molecules with proinflammatory and anti-inflammatory characteristics through the reactions of cyclooxygenase, lipoxygenase, and epoxygenase. GLA has been reported to act as a proinflammatory factor via conversion into arachidonic acid, which leads to a series of inflammatory reactions by forming eicosanoid mediators, such as prostaglandins. GLA has also been reported to function as an anti-inflammatory factor through conversion into dihomo-γ-linolenic acid, which stimulates a series of suppressive effects on inflammatory reactions through the production of prostaglandin E1. These phenomena might explain why early studies found that GLA could ameliorate chronic inflammatory diseases, whereas recent meta-analyses and reviews raised doubts about the effectiveness of GLA-rich supplementation. Our results here clearly demonstrated the anti-inflammatory role of GLA from SE in cells exposed to PM2.5. Notably, other fatty acids from SE, such as 16:0 and 18:2, might also participate in alleviating the harmful effects of PM2.5. Therefore, more detailed studies on the mechanism of the protective effect of GLA against PM2.5 damage should be carried out by using more concentrated or purified GLA to further validate the mode of action of GLA.
In conclusion, the harmful effects of PM2.5 on A549 cells manifested as the suppression of cell viability, induction of oxidative damage, and promotion of inflammatory reactions in a concentration-dependent manner. In A549 cells, the ethanolic extract of Spirulina (SE) executed a protective role against PM2.5 damages. Additive, but not synergistic, protective effects were observed when SE was combined with FO for the pretreatment of A549 cells before exposure to PM2.5. These data indicated that GLA might attenuate the damages induced by PM2.5 exposure to A549 cells. This article is the first report on the potential mitigating effect of GLA on PM2.5 toxicity.
Conflicts of Interest The authors declare no conflict of interest.
Author Contributions XIONG Jie: Conceptualization, Methodology, Supervision, Data curating, Writing–Reviewing, and Editing; CHEN Jin Xuan: Investigation, Visualization, Writing- Original draft preparation; WANG Tian Xi: Investigation, Visualization; LU Fan: Conceptualization, Data curating, Writing–Reviewing. All authors read and approved the final manuscript.
-
Fatty acid/total fatty acids (%) NO Fatty acid SE FO SE+FO 1 14:0 — 0.0538 0.0207 2 16:1 8.1126 14.6822 9.6212 3 16:0 53.7063 29.2863 44.9114 4 18:3 17.0647 0.5320 10.8998 5 18:2 18.3551 0.8918 13.2803 6 18:1 2.2616 12.2659 6.2254 7 20:5 — 22.6366 7.9112 8 20:4 — 1.9399 0.4883 9 20:2 0.3548 0.1704 0.1001 10 20:1 — 1.4662 0.4735 11 20:0 0.0618 0.5033 0.2094 12 22:6 — 12.6997 5.0835 13 22:1 — 1.8172 0.6692 14 22:0 — 0.1707 0.0724 Note. —:not detected. Table S1. Profiles of fatty acids in SE, FO, and SE+FO
HTML
 22315Supplementary Materials.pdf
22315Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: