-
Di (2-ethylhexyl) phthalate (DEHP) is a widely used plasticizer known for its reproductive developmental and immune system toxicity, mainly through esophagal, dermal, and respiratory exposure[1-3]. Maternal exposure to DEHP during pregnancy can lead to adverse birth outcomes in offspring, including impacts on the thyroid system of adolescent offspring[2-4].
Autoimmune diseases (ADs) are chronic diseases characterized by immune intolerance to self-antigens, affecting various organ systems, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), ankylosing spondylitis (AS), and ulcerative colitis (UC)[5-6]. The global incidence of ADs has significantly risen in recent years, with exposure to environmental pollutants such as DEHP being considered a potential contributor[5]. Given that the inflammatory process in ADs alters the number, shape, and size of peripheral blood cells, blood markers are widely used as simple indicators. Lymphocytes (LYM), platelets (PLT) and mean platelet volume (MPV) exhibit significant effects among peripheral blood markers in patients with ADs[7]. However, the mechanism through which human exposure to DEHP leads to susceptibility to ADs remains unknown. Therefore, we aimed to explore whether blood biomarkers could establish a link between DEHP and ADs.
Despite limited data on DEHP’s immunotoxic effects, our data provide a theoretical basis for studying its impact on the immune system and support for future studies exploring the mechanisms by which DEHP affects AD susceptibility.
Two-week-old ICR mice were obtained from Beijing Life River Biotechnology Co. Ltd. (license no. SCXK (Beijing) 2016-0011): SCXK (Beijing, China) 2016-0011). The mice were acclimatized to the environment under specific pathogen-free conditions for one week before being assigned to experimental groups based on body weight. Following the dose selection of our previous study[4], mice in the DEHP group were orally administered 0, 5, 50, and 200 mg/kg DEHP (Sigma-Aldrich, USA, D201154, 1% body weight) daily during the third through fourth weeks (total 7 days). The mice were euthanized at the end of the fourth week. The use of animals and protocols were approved by the Animal Care and Use Committee of Anhui Medical University (20170394, LLSC20200694). The number and morphology of peripheral blood cells such as erythrocytes (RBC), leukocytes (WBC), and PLT were assessed using Wright-Giemsa staining using a fully automated blood cell analyzer (XN-351, Sysmex Europe, Japan).
The primers were designed according to the sequences obtained from GenBank, synthesized, and purified by Shanghai Biotechnology Co.. Total RNA of the thymus tissues was extracted using TRIzol Reagent (Invitrogen Life Technologies, USA). The RNA quality was measured using a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA). Subsequently, cDNA was synthesized using the fragmented mRNA as a template and random oligonucleotides as primers. After configuring the amplification reaction system, the cDNA was added. The qPCR amplification procedure was then performed using a fluorescent quantitative PCR instrument (Roche LightCycler®, Switzerland). Relative quantitative and solubility curve analyses were conducted after setting the gene names. The primers were listed below: Fcgr2b (F 5’-AGGGCCTCCATCTGGACTG3,’ R 5’-GTGGTTCTGGTAATCATGCTCTG3’); Il10 (F 5’-GCTCTTACTGACTGGCATGAG3,’ R 5’-CGCAGCTCTAGGAGCATGTG3’); Irf5 (F 5’-GGTCAACGGGGAAAAGAAACT3,’ R 5’-CATCCACCCCTTCAGTGTACT3’). All curated interactions between DEHP genes and DEHP-related diseases in the Comparative Toxicogenomics Database (CTD) are publicly available. This analysis was based on data downloaded on Mar 1, 2023 (https://ctdbase.org). The CTD cross-species gene vocabulary (symbols, names, and synonyms) was derived from the gene database of the National Center for Biotechnology Information (NCBI), a division of the U.S. National Library of Medicine. Gene information was obtained from Genecards (http://www.genecards.org). Gene-Phenotype Relationships from OMIM (http://www.omim.org). Gene-gene interaction networks were obtained from GeneMANIA (http://genemania.org/).
All statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software, USA). Statistical significance was determined using either an independent t-test or Mann-Whitney U-test. Statistical significance was set at P < 0.05 (two-tailed).
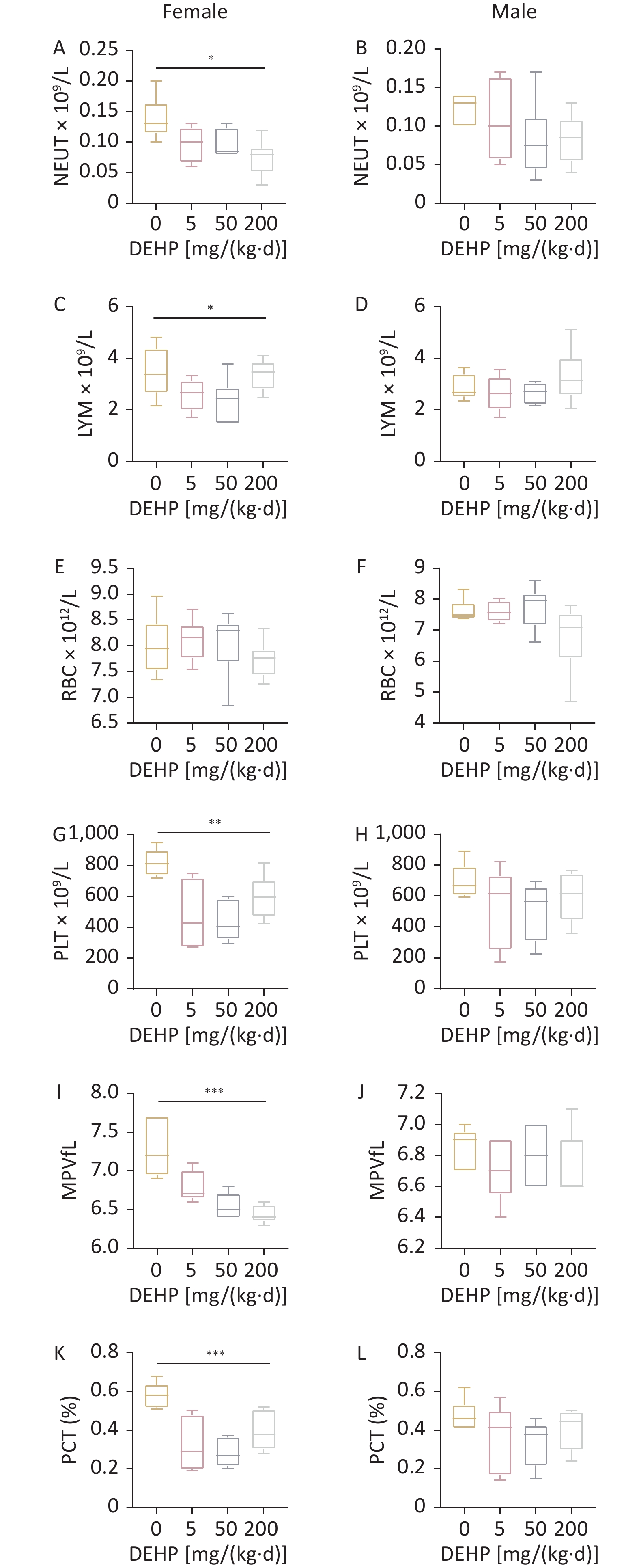
To investigate whether exposure to DEHP affects the blood cells of adolescent mice, we performed complete blood count. As shown in Figure 1, there was a significant decrease in neutrophil (NEUT), LYM, PLT, MPV, and platelet crit (PCT) levels in female adolescent mice as the dose increased. However, the RBC counts in female mice and the indicators in male mice were not significantly different. The observed results suggests that DEHP has a more significant effect on blood cells in female adolescent mice than in male adolescent mice. NEUT, LYM, and PLT levels play significant roles in controlling inflammation and indirectly affect the immune system, leading to susceptibility to ADs[7]. The reason that DEHP has a more significant effect on blood cells in female adolescent mice may be related to the fact that sex hormones that cause ADs, such as SLE and RA, have a greater effect on females.
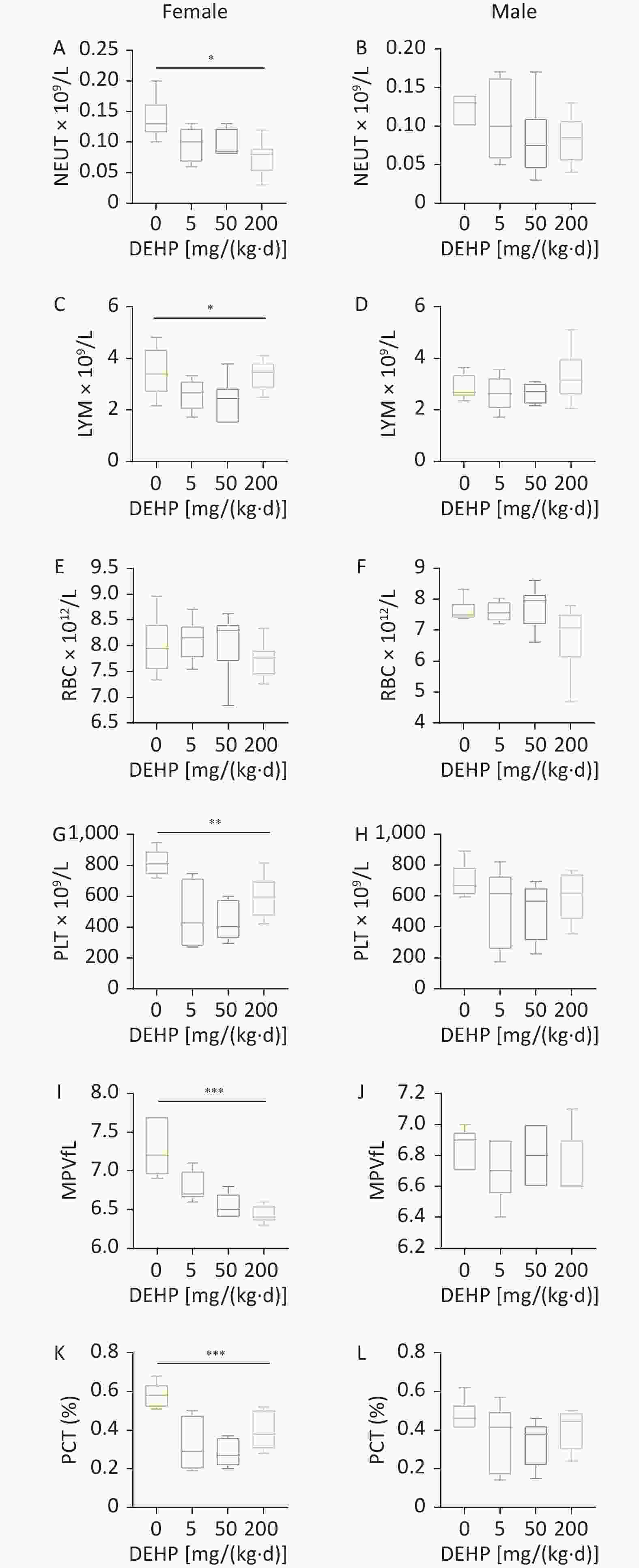
Figure 1. Effect of DEHP exposure on blood routines in adolescent mice. Number of (A–B) NEUT, (C–D) LYM, (E–F) RBC, (G–H) PLT, (I–J) MPV in routine blood. (K–L) Percentage of PCT in blood count. N = 6. Data are represented by mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. DEPH, Di(2-ethylhexyl) phthalate; NEUT, neutrophils; LYM, lymphocytes; RBC, red blood cells; PLT, platelet; MPV, mean platelet volume; PCT, platelet pressure product.
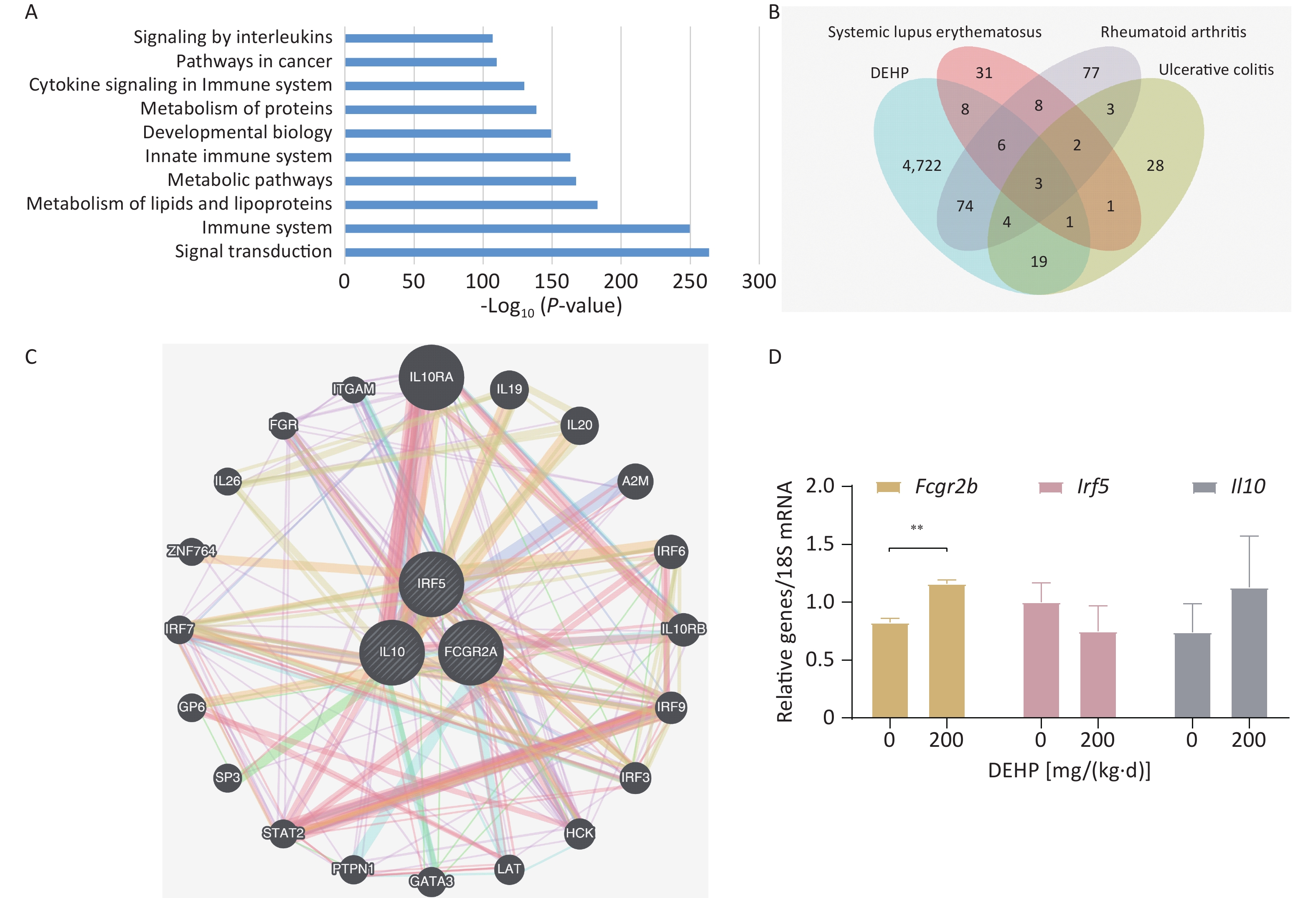
To gain insight into the toxic effects of DEHP, 4,837 genes related to DEHP were identified through the CTD database, and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was performed. The pathways associated with the DEHP-affected genes were elucidated using KEGG pathway enrichment analysis. The top 10 representative pathways, based on the number of enriched genes and significant P values, are shown in Figure 2.
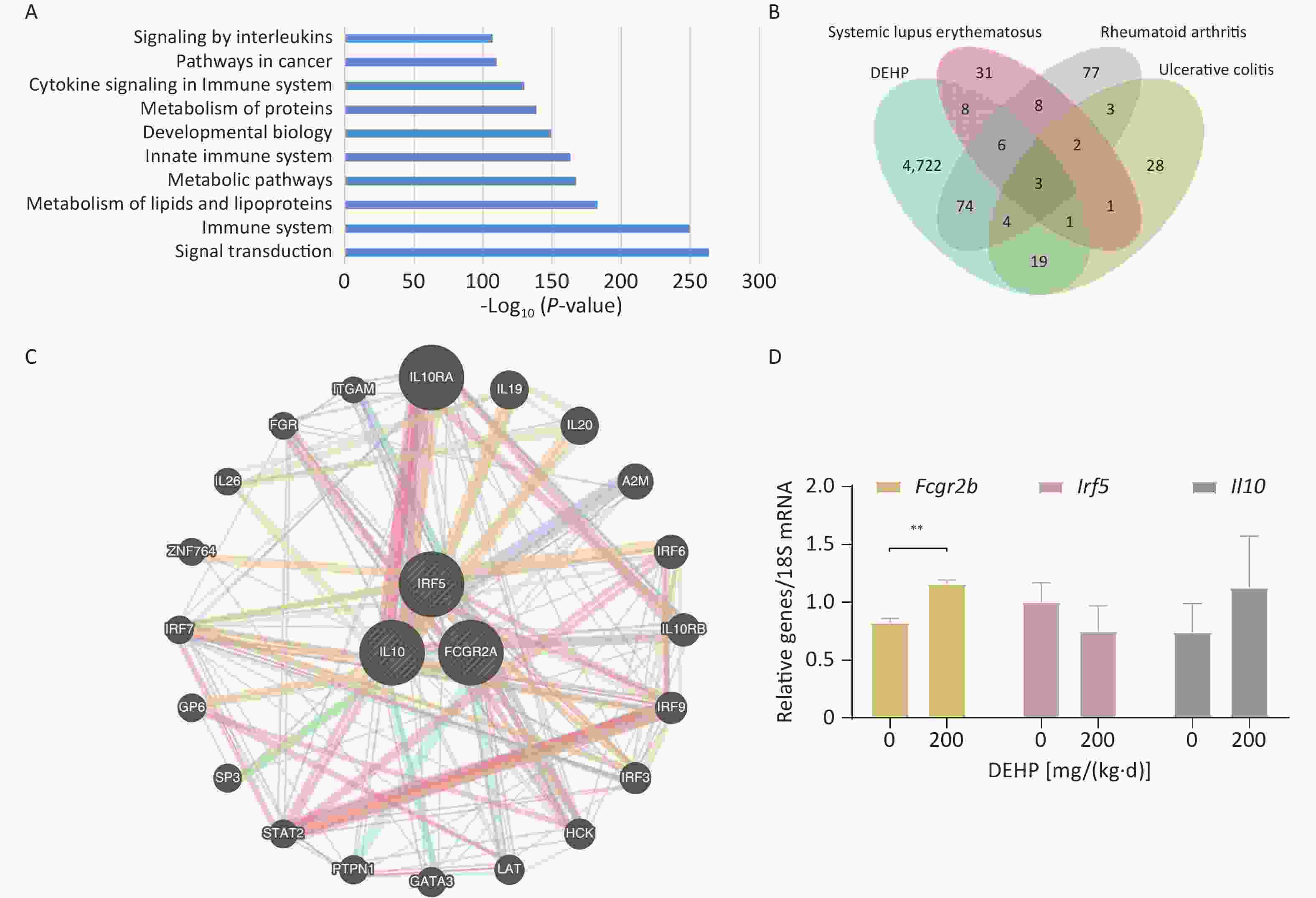
Figure 2. Toxicity and disease associations. (A) The top 10 KEGG pathway enrichment analyses. (P < 0.05). Venn diagram of (B) Venn diagrams of DEHP, SLE, RA and UC. (C) Construction of a Gene-gene interaction network using the three screened genes. (D) Fcgr2b, Il10, and Irf5 mRNA expression levels in peripheral blood of adolescent mice. Values are means ± SEM with N = 4. **P < 0.01. DEPH, Di(2-ethylhexyl) phthalate; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; UC, ulcerative colitis
The immune system plays a major role; therefore, we decided to determine the relationship between DEHP and disease. The top 10 diseases associated with DEHP were Rheumatoid arthritis, HIV infection, Diabetes mellitus (type 1), Asthma, Lymphoma, Autoimmune Diseases, Multiple Sclerosis, Allergic Contact Dermatitis, Respiratory Hypersensitivity, Hypersensitivity based on the number of reference counts and inference scores (Supplementary Table S1, available in www.besjournal.com).
# Disease name Inference score Reference count 1 Arthritis, Rheumatoid 74.14 34 2 HIV Infections 33.12 11 3 Diabetes Mellitus, Type 1 25.32 19 4 Asthma 19.58 53 5 Lymphoma, Large B-Cell, Diffuse 16.43 8 6 Autoimmune Diseases 16.17 17 7 Multiple Sclerosis 13.45 13 8 Dermatitis, Allergic Contact 12.45 12 9 Respiratory Hypersensitivity 10.99 8 10 Hypersensitivity 9.37 15 11 Lupus Erythematosus, Systemic 8.78 18 12 Precursor Cell Lymphoblastic Leukemia-Lymphoma 7.84 13 13 Immunologic Deficiency Syndromes 7.48 9 14 Leukemia, Lymphocytic, Chronic, B-Cell 7.25 10 15 Burkitt Lymphoma 6.11 5 16 Glomerulonephritis, IGA 6.05 7 17 Cryopyrin-Associated Periodic Syndromes 5.36 6 18 Lymphoma, Mantle-Cell 5.29 5 19 Precursor T-Cell Lymphoblastic Leukemia-Lymphoma 5.28 9 20 Dermatitis, Atopic 5.19 13 21 Common Variable Immunodeficiency 5.09 5 22 Mevalonate Kinase Deficiency 4.44 8 23 Multiple Myeloma 4.29 17 24 IMMUNODEFICIENCY-CENTROMERIC INSTABILITY-FACIAL ANOMALIES SYNDROME 1 4.13 6 25 Ataxia Telangiectasia 3.41 6 26 Angioedemas, Hereditary 3.31 7 27 Encephalomyelitis, Autoimmune, Experimental 3.24 10 28 Urticaria 3.15 7 29 Lymphoma 3.09 8 30 Rhinitis, Allergic 3.01 5 31 Hodgkin Disease 2.51 12 32 Lymphoma, Non-Hodgkin 2 9 Table S1. Diseases are associated with Diethylhexyl Phthalate or its descendants, while Reference Count > 5 was used as cut-odd criterion. (CTD - https://ctdbase.org)
Therefore, we foucused on ADs and investigated the association of DEHP with ADs. The genetic Venn diagrams of DEHP and ADs identified three diseases: SLE, RA, and UC. Moreover, five genes were identified at these intersections. After intersecting the five genes with DEHP, we screened three candidate gene targets: FCGR2A, IL10 and IRF5 (Figure 2B). To investigate these three genes, we searched the OMIM database for gene-phenotype relationships. The gene-phenotype Relationships are listed in Supplementary Table S2 (available in www.besjournal.com). FCGR2A is a crucial gene that encodes a cell surface receptor found on immune cells such as macrophages and neutrophils. These receptors play vital roles in the phagocytosis and clearance of immune complexes. IL10 is a cytokine produced by monocytes and lymphocytes. It is a major immunomodulatory cytokine that promotes RA progression. IRF5 is a member of the family of interferon regulatory factors that regulates cell growth, differentiation, apoptosis, and immune system activity, predisposing humans to inflammatory bowel disease.
Gene Phenotype Phenotype number FCGR2A {Lupus nephritis, susceptibility to} 152700 {Malaria, severe, susceptibility to} 611162 {Pseudomonas aeruginosa, susceptibility to chronic infection by, in cystic fibrosis} 219700 IL10 {Graft-versus-host disease, protection against} 614395 {HIV-1, susceptibility to} 609423 {Rheumatoid arthritis, progression of} 180300 IRF5 {Inflammatory bowel disease 14} 612245 {Systemic lupus erythematosus, susceptibility to, 10} 612251 Table S2. Gene-Phenotype Relationships of FCGR2A, IL10 and IRF5. (OMIM - http://www.omim.org)
The gene-gene interaction network diagram is shown in Figure 2-C. The functions of the three intersecting genes, with P < 0.05, are presented in Supplementary Table S3 (available inwww.besjournal.com). The top ten are listed: response to type I interferon, cellular response to type I interferon, cellular response to interferon-gamma, receptor signaling pathway via STAT, response to interferon-gamma, interferon-alpha production, regulation of interferon-alpha production, positive regulation of receptor signaling pathway via STAT, receptor signaling pathway via JAK-STAT, and regulation of receptor signaling pathway via JAK-STAT.
# Function FDR Genes in network 1 response to type I interferon 6.33E-08 7 2 cellular response to type I interferon 6.33E-08 7 3 cellular response to interferon-gamma 3.88316E-06 6 4 receptor signaling pathway via STAT 3.98617E-05 6 5 response to interferon-gamma 3.98617E-05 6 6 interferon-alpha production 4.94182E-05 4 7 regulation of interferon-alpha production 4.94182E-05 4 8 positive regulation of receptor signaling pathway via STAT 0.000278132 4 9 receptor signaling pathway via JAK-STAT 0.000438946 5 10 regulation of receptor signaling pathway via JAK-STAT 0.003197879 4 11 regulation of receptor signaling pathway via STAT 0.004805696 4 12 positive regulation of type I interferon production 0.010427851 3 13 regulation of cytokine secretion 0.010427851 3 14 regulation of type I interferon production 0.010427851 4 15 type I interferon production 0.011757103 4 16 regulation of interferon-beta production 0.014054377 3 17 cytokine secretion 0.014319046 3 18 interferon-beta production 0.015750258 3 19 Fc receptor signaling pathway 0.044216246 4 Table S3. Functions of the three intersecting genes, while P < 0.05 was used as cut-odd criterion. (GeneMANIA - http://genemania.org/)
We used RT-qPCR to validate three possible key genes. RT-qPCR validation showed that the Fcgr2b gene expressed in the peripheral blood of the mice increased significantly with increasing doses (Figure 2D). Il10 showed an upward trend compared to the control group and Irf5 exhibited a downward trend; however, these changes were not significant, consistent with previous studies[8]. Therefore, we speculated that DEHP might influence ADs susceptibility by affecting the key gene Fcgr2b in adolescent mice. The FCGR gene family encodes a protein known as FcγRII, a cell surface receptor linked to various human autoimmune diseases due to its polymorphisms[9]. The FCGR2A gene is expressed only in humans, whereas the Fcgr2b gene is expressed only in mice. The protein encoded by Fcgr2b gene acts as a low-affinity receptor for the Fc region of the immunoglobulin gamma complex. Abnormalities in Fcgr2b gene may heighten susceptibility to autoimmune conditions such as SLE, arthritis, and lupus nephritis. Although our results showed elevated expression of Fcgr2b in the peripheral blood of mice, the increased translation of FcγRIIb resulted in heightened degree of immune system suppression. This may potentially affect the expression of the FCGR2A gene because of their antagonistic relationship[10]. Therefore, the FCGR gene holds promise as a potential blood biomarker for DEHP that affects susceptibility to ADs.
Owing to the lack of population samples, we solely assessed the Fcgr2b gene in the mouse model, omitting the examination of other members of the FCGR family. Furthermore, we did not extend our analysis to test serum levels of IgG using simple statistical methods, a shortfall we aim to rectify in future experiments. Importantly, our study exclusively investigated the effects of one-week DEHP exposure in adolescent mice and its potential impact on AD susceptibility. Although our findings provide valuable insights into the relationship between DEHP exposure and immune system function, further studies are needed to fully understand the mechanisms by which DEHP affects the susceptibility to AD via FCGR.
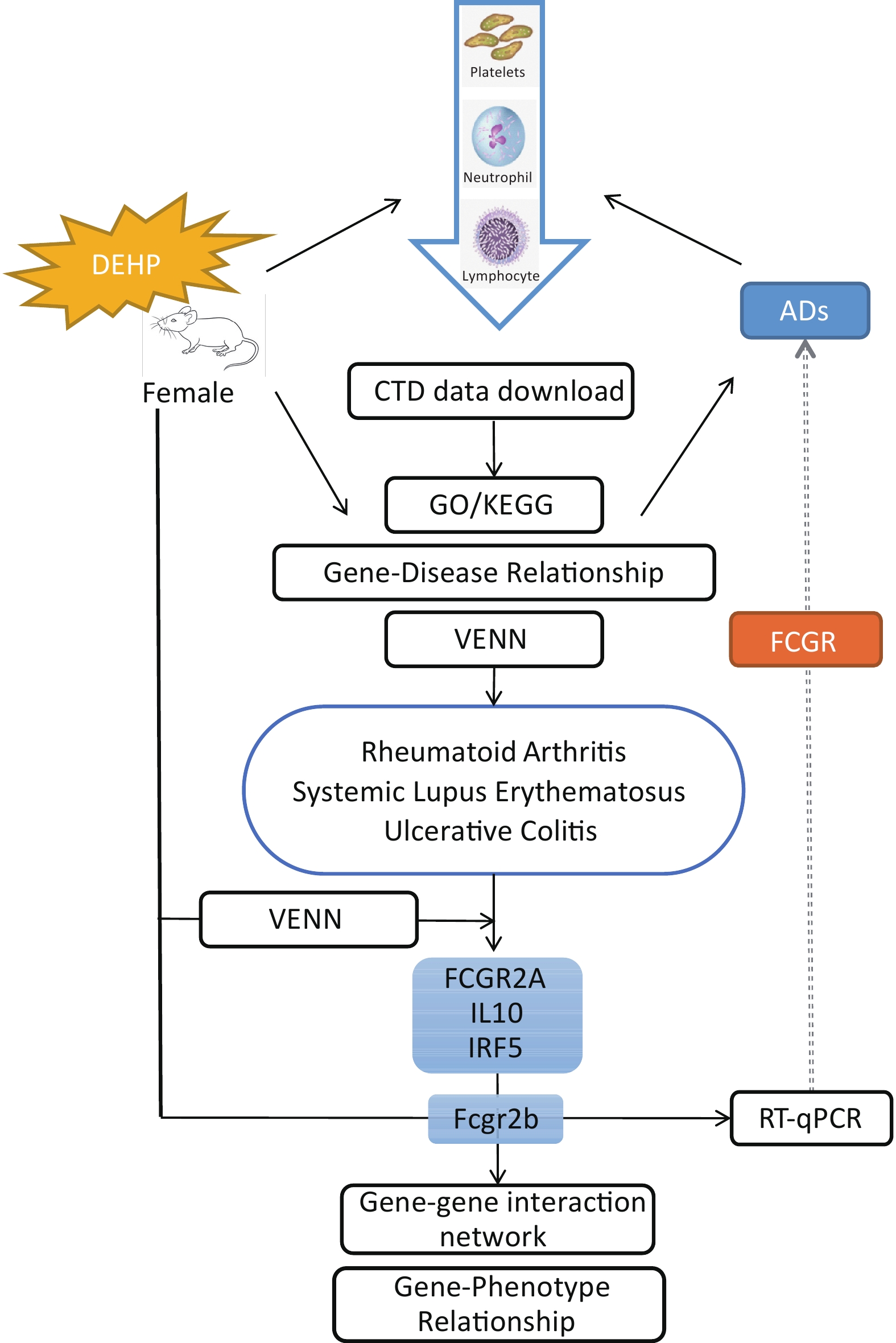
In summary, exposure to DEHP decreased the number of blood cells in female adolescent mice, particularly NEUT, LYM, and PLT levels. DEHP may influence ADs susceptibility by affecting the key gene FCGR. Our results elucidate the immune system effects of DEHP exposure in mice and provide a new perspective for assessing the effects of environmental pollutants, particularly on Ads (Figure 3).
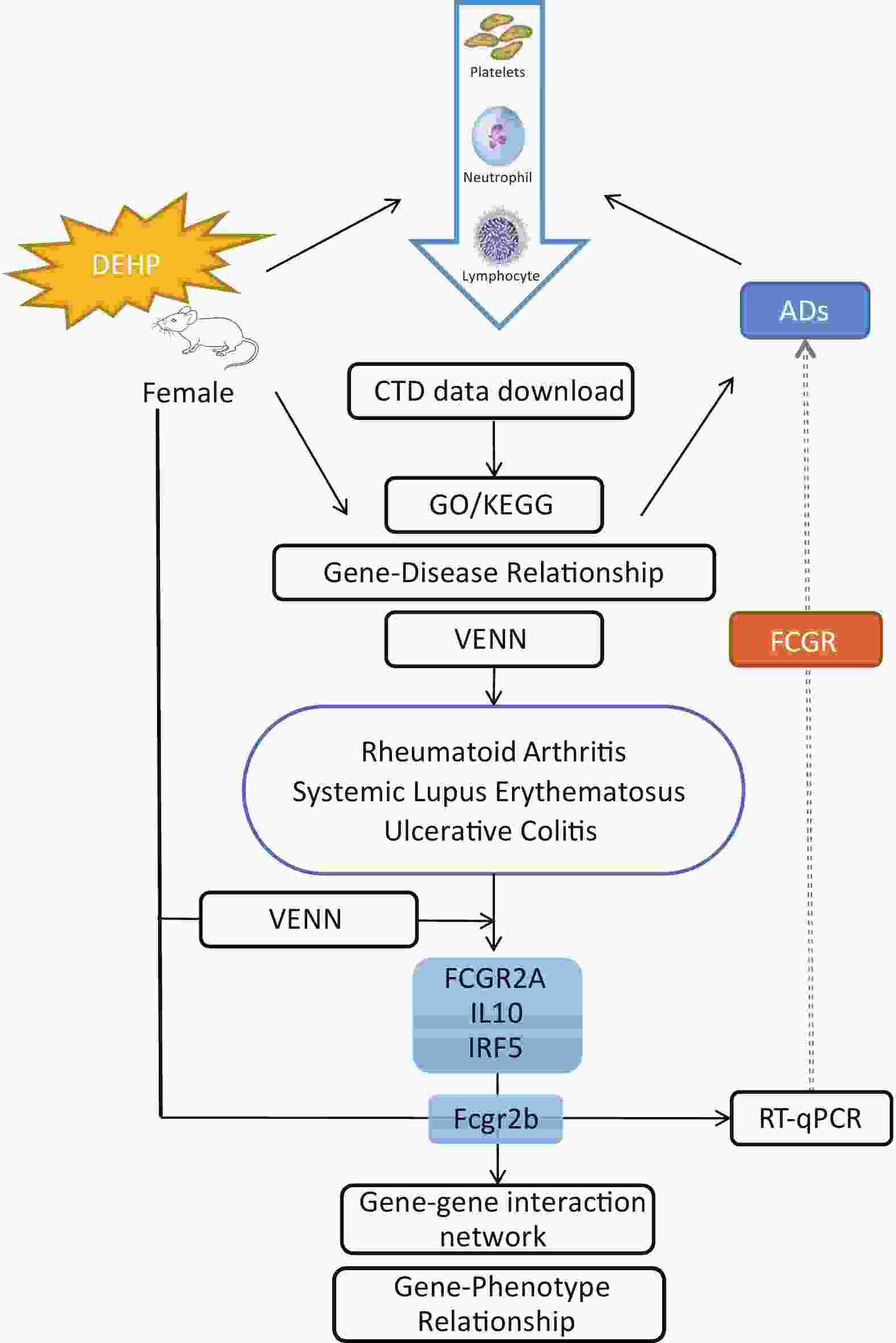
Figure 3. Schematic diagram illustrating that DEHP may increase the risk of ADs in women by affecting FCGR. DEHP’s own GO/KEGG analysis, gene-disease relationship table and routine peripheral blood analysis in mice demonstrate that DEHP is associated with Autoimmune Diseases. FCGR2A, IL10 and IRF5 were identified as potential target genes for DEHP and three ADs based on intersection analysis of Venn diagram. Fcgr2b was subsequently confirmed as a target gene through RT validation in adolescent mice. DEHP may influence ADs susceptibility by affecting the key gene FCGR. DEHP:Di(2-ethylhexyl) phthalate, ADs: Autoimmune diseases, CTD: Comparative Toxicogenomics Database, GO: Gene Ontology, KEGG: Kyoto Encyclopedia of Genes and Genomes.
HTML
 23381-S.pdf
23381-S.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: