-
Pertussis, also known as the whooping cough, is a highly contagious and vaccine-preventable respiratory tract disease caused by Bordetella pertussis. Since the 1950s, large-scale vaccination with the whole-cell pertussis vaccine (wP), made from inactivated whole-cell bacteria[1], has resulted in a marked reduction in the morbidity and mortality of this disease worldwide[2]. In the 1990s, acellular pertussis (aP) vaccines replaced wP vaccines in many countries, especially in developed countries[3], owing to their positive serological response and reduced side-effects[4-5]. However, in the past decades, pertussis has seen a resurgence in developed countries despite the high vaccination coverage of aP vaccines[6-8]. Previous studies have demonstrated that two characteristics of aP vaccines play a crucial role in this resurgence: (1) the rapid waning of immunity induced by aP vaccines[9-10], and (2) the inability of aP vaccines to prevent the colonization and transmission of B. pertussis in nonhuman primates[11]. In 2018, the World Health Organization reported that 151,000 pertussis cases were recorded worldwide, despite 81% of the target population receiving three doses of pertussis vaccine during infancy[12]. In China, diphtheria and tetanus toxoids and whole-cell pertussis vaccines (DTwPs) were included in the Expanded Program on Immunization (EPI) in 1978. Since 2007, diphtheria and tetanus toxoids and acellular pertussis vaccines (DTaps) have gradually replaced DTwP, and only DTaPs have been used since 2013. The vaccination schedule for infants in China includes three primary doses of the DTaP vaccine administered at 3, 4, and 5 months of age, and one booster dose administered at 18–24 months of age. According to Chinese official statistics, the coverage of the three primary doses has been greater than 99% since 2009[13]. Under these circumstances, the annual incidence of pertussis declined to 0.2/100,000 between 2006 and 2010[14]. However, it has been increasing in recent years, reaching 2.15/100,000 in 2019[13,15].
The Guangxi Zhuang Autonomous Region (Guangxi) is located in southern China and has 56 million residents. Guangxi has 14 prefectural cities, as shown in Table 1, of which Baise, Fangchenggang, and Chongzuo border Vietnam to the southwest. In recent years, Guangxi has experienced a resurgence of pertussis. The number of reported cases of pertussis between 2009 and 2017 was < 10 per year, with an annual incidence of less than 0.02/100,000. However, the incidence increased markedly to 159 cases in 2018 and 553 cases in 2019 with annual incidences of 0.2/100,000 and 1.0/100,000, respectively. We speculate that improvements in diagnostic techniques, increased physician awareness, and the rapid waning of vaccine-induced immunity likely contributed to this resurgence. However, unlike the United States and some European countries, where pertussis cases resurged mainly in older children, adolescents, and adults[16-17], pertussis cases in Guangxi mainly occurred in young children, especially infants under 1 year of age in 2018–2019. The clinical presentation of pertussis in adolescents and adults is usually a mild cough or even asymptomatic[18-19], adolescents and adults have thus become the reservoir of pertussis and the main source of transmission to unvaccinated infants[20-21]. Therefore, estimating the status of pertussis in these populations is crucial for the control and prevention of pertussis in children. However, the status of pertussis in Guangxi remains unclear.
Characteristics No. of subjects No. of positivity Positivity rate (%) P Median (Q1, Q3)a (IU/mL) P Total 10,215 1,833 17.94 16.06 (5.00b, 37.91) Regions Nanning 762 172 22.57 < 0.001 19.13 (5.00, 45.38) < 0.001 Liuzhou 765 130 16.99 16.77 (5.00, 37.84) Guilin 777 134 17.25 17.20 (5.00, 38.44) Laibin 757 120 15.85 16.26 (5.00, 35.50) Hechi 763 149 19.53 15.26 (5.00, 38.60) Baise 771 132 17.12 13.73 (5.00, 35.91) Guigang 787 144 18.3 16.07 (5.00, 38.93) Yulin 754 129 17.11 15.8 (5.00, 36.84) Beihai 531 64 12.05 12.82 (5.00, 31.39) Fangchenggang 493 82 16.63 16.54 (5.00, 37.04) Qingzhou 754 138 18.30 15.38 (5.00, 37.49) Wuzhou 776 170 21.91 19.38 (5.00, 44.49) Hezhou 767 156 20.34 16.99 (5.00, 39.98) Chongzuo 758 113 14.91 14.71 (5.00, 33.24) Age groups (years) < 2 765 71 9.28 < 0.001 5.00 (5.00, 17.59) < 0.001 2 753 48 6.37 5.00 (5.00, 17.39) 3 773 55 7.12 5.00 (5.00, 11.47) 4 736 63 8.56 5.00 (5.00, 15.06) 5 289 32 11.07 5.00 (5.00, 26.42) 6 312 44 14.1 11.64 (5.00, 29.42) 7–10 1,086 194 17.86 14.92 (5.00, 36.42) 11–20 1,855 467 25.18 22.91 (5.00, 50.72) 21–30 841 174 20.69 23.45 (10.94, 42.99) 31–40 1,226 261 21.29 24.35 (12.30, 45.38) 41–50 868 226 26.04 28.57 (15.80, 51.02) ≥ 51 711 198 27.85 30.40 (18.11, 54.37) Sexc Male 4,549 732 16.09 < 0.0001 13.75 (5.00, 34.71) < 0.0001 Female 5,666 1,101 19.43 18.17 (5.00, 40.32) Vaccination histories Yes 5,947 810 13.62 < 0.0001 5.00 (5.00, 27.54) < 0.0001 No 482 113 23.44 26.17 (14.07, 47.75) Unknown 3,786 910 24.04 25.67 (12.82, 48.77) Occupationc Farmer 2,651 626 23.61 0.364 26.68 (14.00, 48.27) 0.034 Worker 189 39 20.63 24.50 (11.94, 41.66) Government Staff 143 32 22.38 22.21 (13.33, 47.44) Student 178 52 29.21 24.35 (11.33, 57.35) Teacher 155 45 29.03 30.20 (17.97, 60.85) Healthcare Worker 320 70 21.88 22.81 (11.97, 44.64) Public service staff 64 13 20.31 25.31 (11.51, 44.16) Others 202 50 24.75 24.37 (11.40, 50.02) Note. The Chi-square test was used to compare the rates; a non-parametric test (Mann-Whitney U tor Kruskal-Wallis H test) was performed to compare antibody concentration between two or more groups; P < 0.05 indicated that the difference was statistically significant. aQ1 and Q3 represent the 25th and 75th percentile, respectively; bThe median concentration equal to 5.00 IU/mL indicated that it was below the lower detection limit of the pertussis IgG kit; cMann-Whitney U test was used to compare the concentrations by sex. Table 1. Distribution of anti-pertussis IgG antibody levels among healthy population in Guangxi
This survey had two aims: (1) to evaluate anti-pertussis IgG levels in a population of all ages, and (2) to reveal the infection status of pertussis in the population, especially in older children, adolescents, and adults. The data obtained from this survey will contribute to improving measures for controlling and preventing pertussis, adjusting immunization strategies, and predicting the incidence levels in Guangxi.
-
A cross-sectional survey was conducted from August to November 2018 in 14 prefecture-level cities in Guangxi, China. The survey participants were local residents who had been living in the area for more than 6 months. A multistage stratified random sampling method was used. In each prefecture-level city, 2–3 districts/counties were randomly selected. A township/street was randomly selected from each district/county. Finally, one village/community was randomly selected from each township or street. A total of 41 villages/communities were involved in the survey, with approximately 250 residents participating from each village and community. Information on age, sex, vaccination history, and occupation of each resident was obtained by asking the resident or their guardian to check the vaccination certificate. All participants were divided into 12 age groups (< 2, 2, 3, 4, 5, 6, 7–10, 11–20, 21–30, 31–40, 41–50, ≥ 51). Serum samples used in this survey were stored at −40 °C before testing.
-
Anti-pertussis IgG and anti-pertussis-toxin IgG (anti-PT IgG) levels were measured using two commercially available enzyme-linked immunosorbent assay (ELISA) kits, the Pertussis IgG kit and the Pertussis Toxin IgG kit (Institute Virion/Serion GmbH, Germany). Antibody concentrations are expressed in International Units (IU) for both kits. Anti-pertussis IgG was used to assess the level of anti-pertussis antibodies in the population, which reflects the population's resistance to pertussis. Anti-PT IgG was used to determine the pertussis infection status. The concentration of anti-PT IgG ≥ 100 IU/mL indicates recent infection or vaccination, which can decrease to 40 IU/mL within 1 year after the onset of the disease or vaccination[22].
According to the manufacturer’s instructions, the quantitative detection range of pertussis IgG kit was 10–1,000 IU/mL, and the concentration of anti-pertussis IgG ≥ 50 IU/mL was interpreted as positive, otherwise, it was interpreted as negative. The quantitative detection range of the Pertussis Toxin IgG kit was 5–600 IU/mL. The concentration of anti-PT IgG ≥ 100 IU/mL indicated recent infection or vaccination, and the concentration of anti-PT IgG ≥ 40 IU/mL was considered positive, whereas the concentration < 40 IU/mL was interpreted as negative.
-
Demographic information and test results were recorded and organized using Microsoft Office Excel 2010. All statistical analyses were performed using EPI-Info 7.0. The Chi-square test was used to compare the positivity rates of antibodies among the different groups based on age, sex, vaccination history, and occupation. Owing to the non-conformity of antibody concentrations to the normal distribution, non-parametric tests (Mann-Whitney U or Kruskal-Wallis H test) were performed to compare antibody concentrations between two or more groups. Concentrations above the upper limit of the kit are expressed as the upper limit, whereas those below the lower limit of the kit are expressed as half the lower limit. Statistical significance was set at P < 0.05.
-
A total of 10,215 residents aged 10 months to 89 years (median age: 12 years) participated in the survey. The sex ratio was 1:1.25, with 4,549 males and 5,666 females. Of the total participants, 3,902 provided information about their occupations. The vaccination status of the entire survey population is shown in Table 1. The pertussis vaccination rate for children under 5 years in this survey was 98.91% (2,993/3,026). According to their vaccination certificates, 2,969 of these children (99.13%) received the domestic DTaP.
-
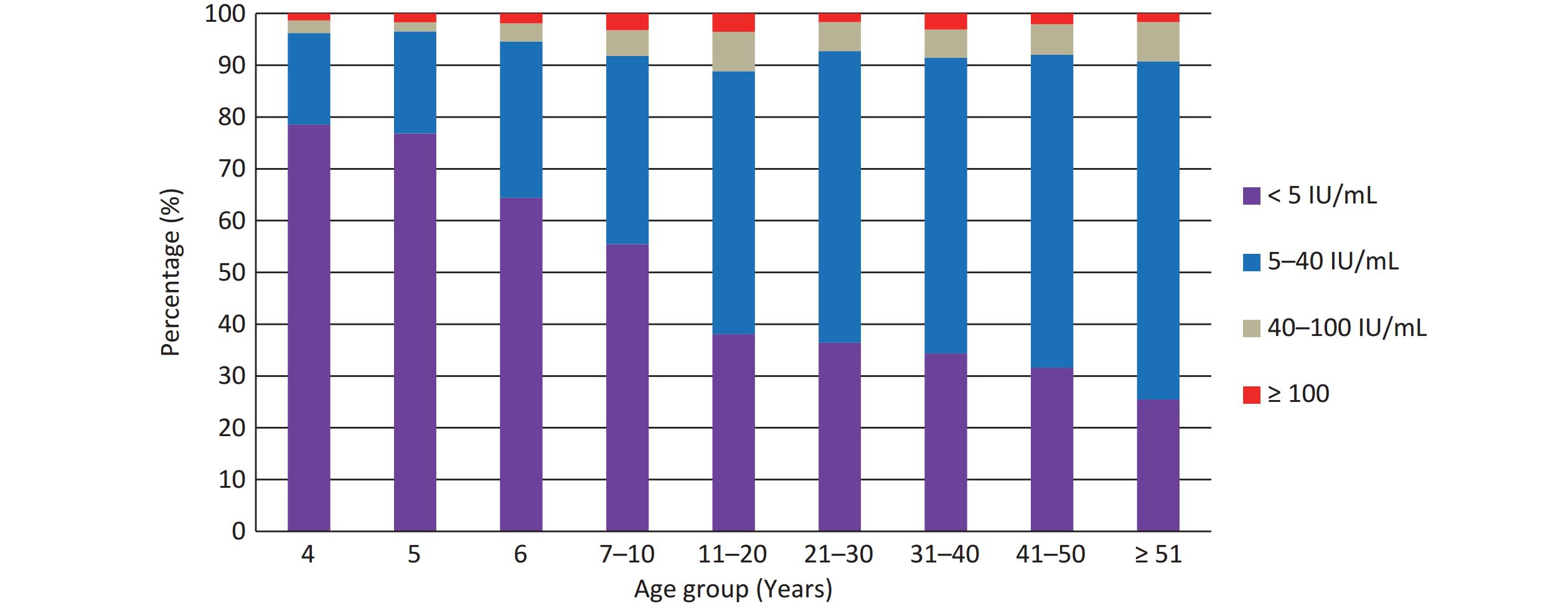
Among the study population, 1,833 participants (17.94%) tested positive for anti-pertussis IgG antibody, with the median concentration of 16.06 IU/mL. As shown in Table 1, among 14 prefecture-level cities, the highest positivity rate and median concentration were observed in Nanning (22.57%, P < 0.001) and Wuzhou (19.38 IU/mL, P < 0.001), respectively. Comparing the anti-pertussis IgG antibody levels in different age groups, the positivity rate increased with age, except for the age groups of < 2 years and 11–20 years (P < 0.001), and the median concentrations of ≤ 5 years groups were 5 IU/mL, which was below the lower limit of the kit, which then also exhibited an upward trend with age group (P < 0.001). Spearman rank correlation analysis also confirmed that both the positivity rate and median concentration were positively correlated with age (r = 0.937, P < 0.001; r = 0.943, P < 0.001). In addition, as shown in Figure 1, antibody level < 10 IU/mL accounted for more than 60% in children under 4 years of age but declined with age, whereas the percentages of the other three levels (10–40, 40–50, and ≥ 51 IU/mL) almost increased with age (P < 0.001). The positivity rate and median concentration of anti-pertussis IgG antibodies were significantly higher in females than in males (P < 0.001 and P < 0.001, respectively). Compared with populations with no or unknown vaccination history, the positivity rate and median concentration were lower in the vaccinated population (P < 0.001 and P < 0.001, respectively). No statistically significant difference in positivity rates was found among the occupational groups (P = 0.364); however, there was a statistically significant difference in the median concentrations (P = 0.034).
-
Majority of children (98.91%) in Guangxi were vaccinated against pertussis. The vaccination schedule for infants in China includes three primary doses of the DTaP vaccine administered at 3, 4, and 5 months of age, followed by one booster dose administered at 18–24 months of age. The concentration of anti-PT IgG antibody can drop to less than 40 IU/mL within 1 year of vaccination[22]. To assess the prevalence of pertussis infection in the population and reduce the impact of vaccination, participants aged ≥ 4 years were selected for the analysis.
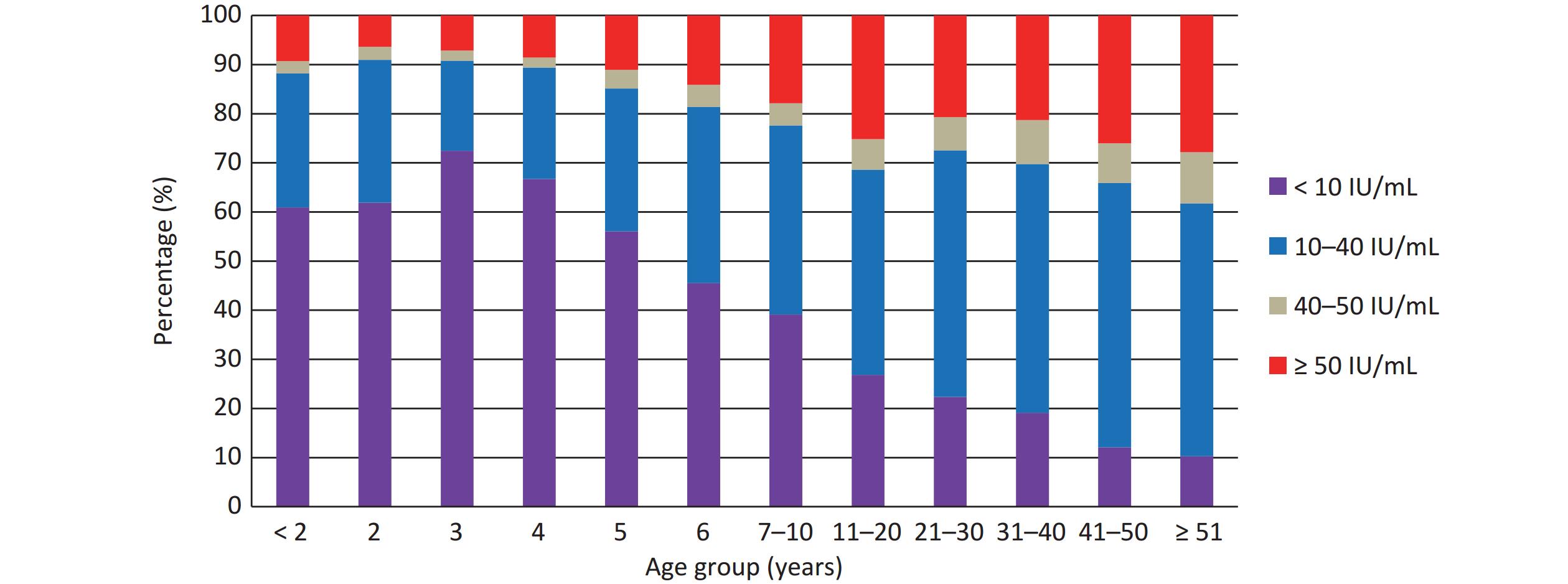
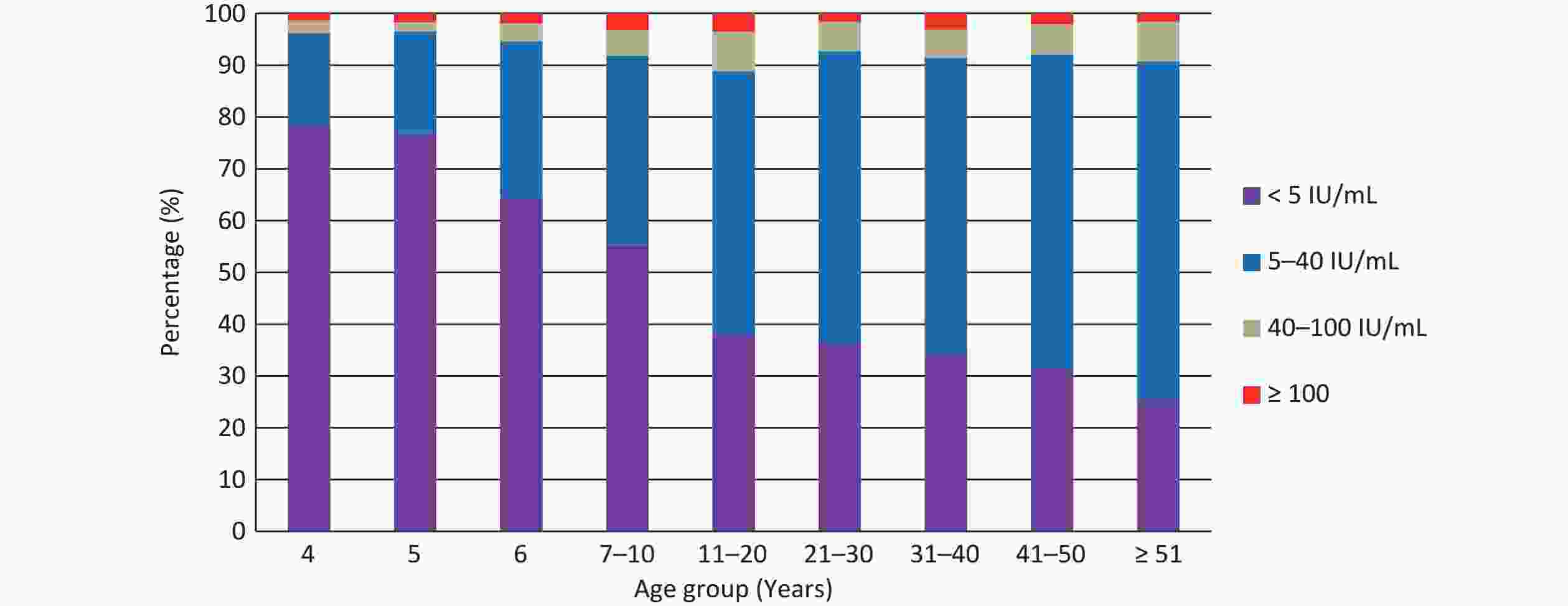
A total of 7,924 participants (≥ 4 years old) were tested for anti-PT IgG, of which 653 (8.24%) tested positive (≥ 40 IU/mL) with a median concentration of 5.89 IU/mL, and 204 participants (2.57%) had recent pertussis infection (≥ 100 IU/mL). As shown in Table 2, the positivity rates among different age groups ranged from 3.46% (5 years) to 11.16% (11–20 years), the recent infection rates from 1.36% (4 years) to 3.56% (11–20 years), and the median concentrations from 2.50 IU/mL (≤ 6 years) to 8.74 IU/mL (≥ 51 years). The differences in positivity rates, recent infection rates, and median concentrations among the age groups were statistically significant (P < 0.001, P = 0.005, and P < 0.001, respectively). In addition, as shown in Figure 2, the 5 IU/mL concentration decreased with age, whereas 5–40 IU/mL increased with age. The percentage of 40–100 IU/mL concentration also increased with age and peaked in the age group of 11–20 years old, remaining relatively stable thereafter. The percentage of ≥ 100 IU/mL exhibited a “bimodal” distribution with age, with one peak occurring in the 11–20 years age group, and the other peak appearing in the 31–40 years age group. Among occupational groups, teachers had higher rates of positivity (14.19%), recent infections (4.52%), and median concentration (9.29 IU/mL). The difference in positivity rates was statistically significant (P = 0.049) but no significant difference in recent infection rates (P = 0.100) and median concentrations (P = 0.05) was observed among the occupational groups. In the comparison of positivity and recent infection rates, no statistically significant differences were observed among the groups based on region, sex, or vaccination history (P > 0.05).
Characteristics No. of subjects ≥ 40 IU/mL ≥ 100 IU/mL Median (Q1, Q3)
(IU/mL)P No. of
positivityPositivity
rate (%)P No. of Recent
InfectionRecent Infection
rate (%)P Total 7,924 653 8.24 204 2.57 5.89 (2.50, 11.65) Regions Nanning 591 66 11.17 0.089 26 4.40 0.118 7.19 (2.50e, 17.85) < 0.001 Liuzhou 588 43 7.31 17 2.89 5.64 (2.50, 11.98) Guilin 606 46 7.59 16 2.64 5.74 (2.50, 13.03) Laibin 585 42 7.18 10 1.71 5.84 (2.50, 12.49) Hechi 592 52 8.78 12 2.03 6.10 (2.50, 15.31) Baise 592 54 9.12 14 2.36 5.81 (2.50, 13.26) Guigang 616 61 9.90 23 3.73 5.85 (2.50, 14.59) Yulin 582 45 7.73 10 1.72 6.04 (2.50, 14.19) Beihai 418 23 5.50 5 1.20 2.50 (2.50, 10.03) Fangchenggang 390 28 7.18 9 2.31 5.87 (2.50, 12.15) Qingzhou 591 41 6.94 16 2.71 5.12 (2.50, 12.56) Wuzhou 604 58 9.60 17 2.81 6.55 (2.50, 14.34) Hezhou 595 52 8.74 15 2.52 6.58 (2.50, 15.35) Chongzuo 574 42 7.32 14 2.44 5.80 (2.50, 12.79) Age groups (years) 4 736 28 3.80 < 0.001 10 1.36 0.005 2.50 (2.50, 2.50) < 0.001 5 289 10 3.46 5 1.73 2.50 (2.50, 2.50) 6 312 17 5.45 6 1.92 2.50 (2.50, 6.98) 7–10 1,086 89 8.20 35 3.22 2.50 (2.50, 10.98) 11–20 1,855 207 11.16 66 3.56 7.06 (2.50, 17.81) 21–30 841 62 7.37 14 1.66 6.87 (2.50, 14.59) 3–40 1,226 105 8.56 38 3.10 7.16 (2.50, 14.56) 41–50 868 69 7.95 18 2.07 7.82 (2.50, 15.29) ≥ 51 711 66 9.28 12 1.69 8.74 (2.50, 17.38) Sex a Male 3,308 251 7.59 0.069 75 2.27 0.144 5.28 (2.50, 12.78 ) < 0.001 Female 4,616 402 8.71 129 2.79 6.35 (2.50, 14.16 ) Vaccination history Yes 3,682 287 7.79 0.280 100 2.72 0.274 2.50 (2.50, 10.48) < 0.001 No 475 36 7.58 7 1.47 7.71 (2.50, 14.63) Unknown 3,767 330 8.76 97 2.57 7.52 (2.50, 16.01) Occupationa Farmer 2,651 205 7.73 0.049 53 2.00 0.100 7.64 (2.50, 15.15) 0.050 Worker 189 13 6.88 5 2.65 6.68 (2.50, 13.69) Government Staff 143 11 7.69 5 3.50 7.24 (2.50, 13.52) Student 178 20 11.24 6 3.37 7.51 (2.50, 20.46) Teacher 155 22 14.19 7 4.52 9.29 (2.50, 22.66) Healthcare Worker 320 26 8.13 4 1.25 6.83 (2.50, 14.78) Public service staff 64 6 9.38 2 3.13 7.39 (2.50, 12.84) Others 202 24 11.88 8 3.96 7.09 (2.50, 20.46) Total 3,902 327 8.38 90 2.31 7.48 (2.50, 15.43) Note. The Chi-square test was used to compare the rates. Non-parametric tests (Mann–Whitney U or Kruskal–Wallis H test) were performed to compare antibody concentrations between two or more groups. Statistical significance was indicated by P < 0.05. aMann–Whitney U test was used to compare the concentrations by sex. eA median concentration of 2.50 IU/mL indicated that it was below the lower detection limit of the Pertussis Toxin IgG kit. Table 2. The infection status of pertussis among healthy population in Guangxi
-
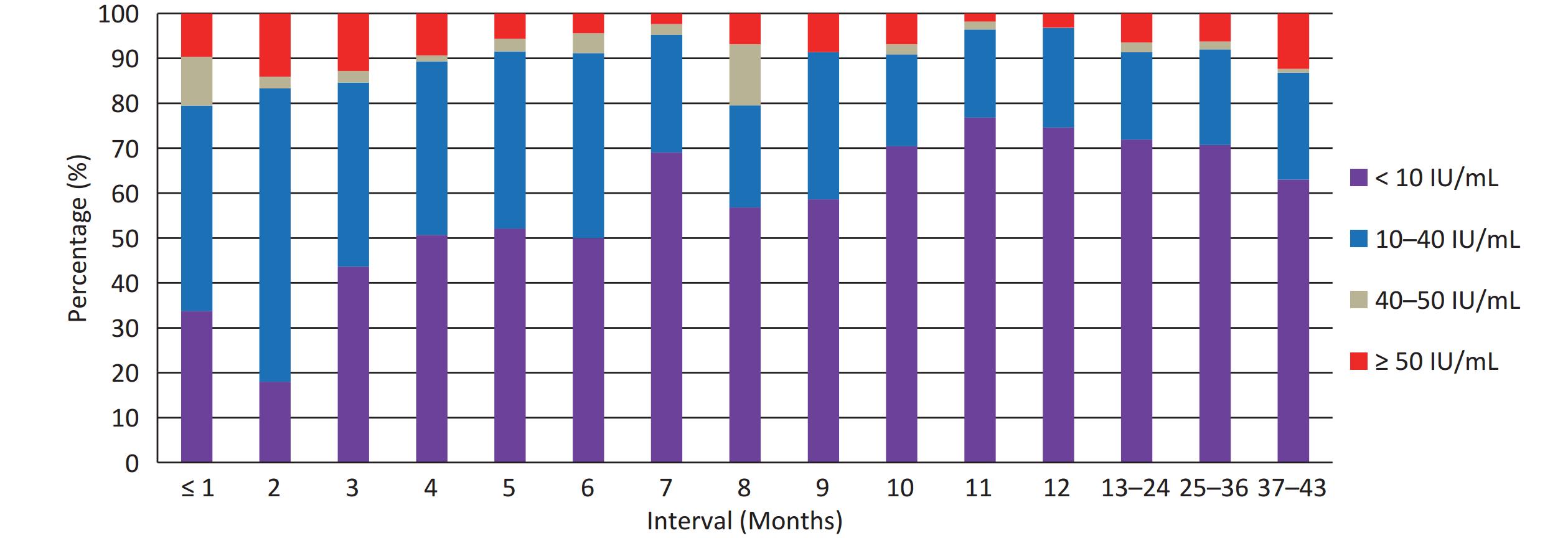
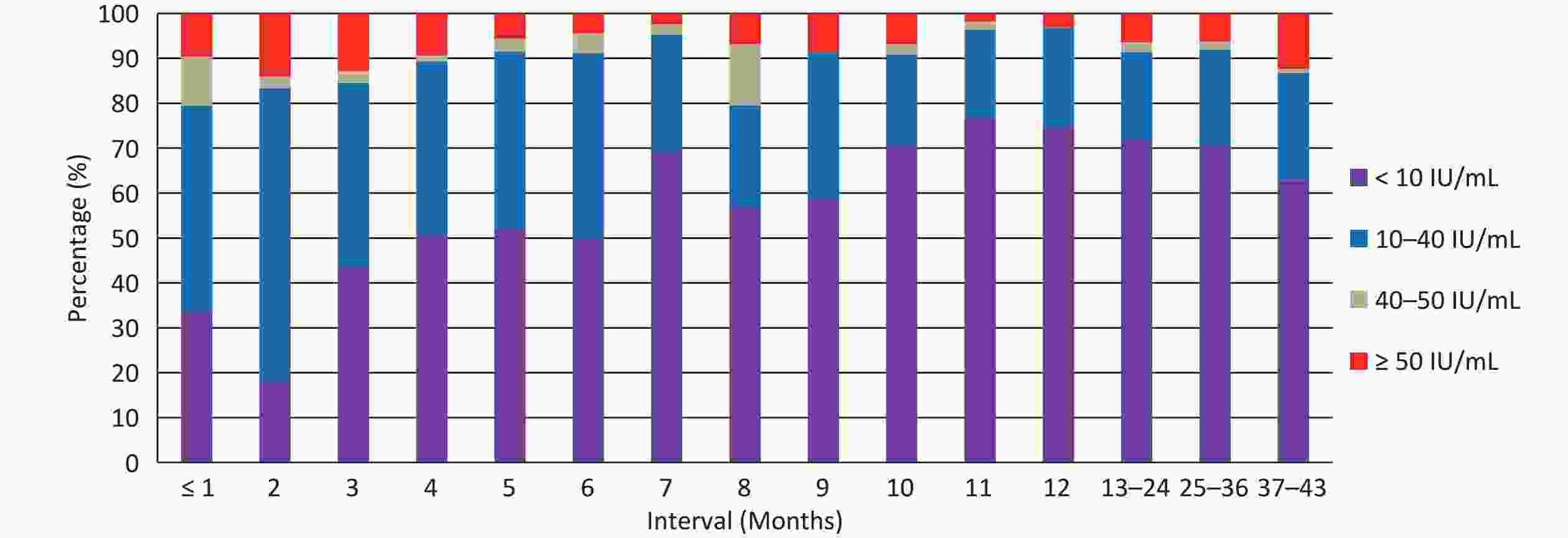
A total of 2,369 children under 5 years of age who had received four doses of domestic DTaP vaccine and provided a clear vaccination date for the fourth dose were selected to analyze the duration of anti-pertussis IgG. The post vaccination intervals ranged from 2 days to 43 months. The results showed that 174 (7.34%) children were tested positive for anti-pertussis IgG antibodies, with a median concentration of 5 IU/mL. In addition, as shown in Figure 3, the percentage of antibody level < 10 IU/mL initially decreased from 33.70% at 1 month to 17.95% at 2 months. However, thereafter, it almost increased with intervals except at 7-months, ranging from 43.59%–52.11% at 3–6 months, 56.82%–58.62% at 8–9 months, and 70.45%–76.79% at 10–36 months. However, it decreased to 63.00% at 37–43 months. The difference in antibody levels (< 10, 10–40, 40–50, and ≥ 50 IU/mL) was statistically significant at vaccination intervals (P < 0.001).
-
To the best of our knowledge, this is the first large-scale survey of pertussis that covers all 14 prefecture-level cities in Guangxi. A total of 10,215 healthy individuals aged 10 months to 89 years were included in this survey. Therefore, the results obtained from this survey reflect the antibody levels and infection status of pertussis in the entire population during the resurgence of pertussis in Guangxi in 2018.
Detection of serum antibody levels using ELISA plays an important role in the evaluation of protective antibodies after natural infection or vaccination. In this survey, the positivity rate of anti-pertussis IgG was 17.94%, and the median concentration was 16.06 IU/mL. Some cross-sectional studies conducted in different regions of China from 2015–2020 used the same commercial reagents and cutoff values. The positivity rates in these studies ranged from 10.41%–26.64%[23-25]. Such a low level of antibodies in the population indicates that a protective barrier against pertussis has not been effectively formed in Guangxi. This could be one of the primary factors contributing to the significant increase in the number of reported pertussis cases between 2018 and 2019.
As shown in Table 1, the distribution of anti-pertussis IgG antibody levels was mainly affected by three factors: age, region, and history of vaccination. First, significant differences were found in positivity rates and median concentrations of anti-pertussis IgG in different age groups, and Figure 1 accurately represents the distribution of antibody levels among age-groups. Antibody levels in young children initially decrease with age owing to the rapid waning of vaccine-induced immunity, reaching their lowest point at 3 years of age. However, in subsequent age groups, the antibody levels increased with age. The age distribution of antibody levels in our survey was generally consistent with studies conducted in different regions of China, in which the age group of 4–6 years had the lowest anti-pertussis antibody levels, whereas adolescents and adults had higher levels[26-27]. The duration of immunity is estimated to be 4–12 years after the last vaccination[28]. Although the “V” distribution of anti-pertussis IgG levels in different age groups was contrary to this viewpoint, considering there was no booster for old children, adolescents, and adults in China, we speculated that the increase in antibody levels with age was due to natural pertussis infection. Second, the positivity rate and median concentration were lower in the population with a confirmed vaccination record than in those with no or unknown vaccination history. This finding confirms the views of Campbell et al., who stated that natural pertussis infection contributes more to antibody levels compared with vaccination[29]. Among the 14 prefecture-level cities, Nanning exhibited the highest positivity rate. This is because the vaccination rates of acellular pertussis vaccine among children in Guangxi were generally higher than 99%. Nanning, the capital of Guangxi, has a large population, high population density, and frequent exchange of people throughout the country. These factors significantly increased the probability of natural pertussis in this region.
PT is the most specific antigen of B. pertussis, whereas other antigens such as filamentous hemagglutinin (FHA), pertactin, and fimbriae are less specific because of their cross-reactivity with other microbial species[30]. Determination of PT-IgG antibodies is widely recommended for diagnosing pertussis. However, a universally recommended cutoff level of PT-IgG, indicating a recent pertussis infection, did not exist. Internationally, the cutoff value ranges from 50–125 IU/mL[31]. A comparison of ROC curves analyzed with data from Denmark, the Netherlands, and the United Kingdom showed that the single cutoff value representing optimal sensitivity and specificity may be in the range of 60–75 IU/mL[32]. In this survey, the cutoff value of ≥100 IU/mL was used according to the manufacturer's instructions, which was also used in most of the relevant studies in China[13,33-37]. The positivity rates of anti-PT IgG (≥ 40 IU/mL) in those studies ranged from 1.70%–11.40%, and the recent infection rates (≥ 100 IU/mL) from 0.17%–2.90%. A study conducted in 14 European countries showed that the positivity rates of anti-PT IgG (≥ 50 IU/mL) ranged from 0.9% in Poland to 8.8% in Italy, and the recent infection rates (≥ 100 IU/mL) ranged from 0.2% in Hungary to 5.7% in Portugal[38]. In our survey, the overall positivity rate(≥ 40 IU/mL) among participants was 8.24%, and the recent infection rate (≥ 100 IU/mL) was 2.57%, suggesting that pertussis infection was relatively common in Guangxi. However, only 159 cases of pertussis were reported in 2018, most of which occurred in infants. As shown in Table 2, the infection rates of pertussis (≥ 40 IU/mL) in adolescents and adults were significantly higher than those in children under 5 years of age, but only fewer than 10 cases were reported in adolescents and adults in 2018, indicating that the annual incidence of pertussis in Guangxi was seriously underestimated, especially in adolescents and adults, which was consistent with previous studies in China[39-40]. We speculate that the main reason for this is that the diagnostic criteria for suspected cases of pertussis in China should include the typical clinical symptoms of pertussis, such as paroxysmal and spasmodic cough. However, symptoms in adolescents and adults are mostly prolonged and nonspecific coughs[18-19], which may lead to a misdiagnosis. In addition, recent pertussis infection rates exhibited a “bimodal distribution” by age, with the first peak occurring at 11–20 years and the second peak at 31–40 years. We speculate that the occurrence of the first peak was due to the waning of vaccine-induced immunity. Additionally, infection-acquired immunity against pertussis does not induce lifelong protection and wanes 7–20 years after the infection[41]. Consequently, pertussis reinfection has become common among adults, with a second peak occurring at 30–41 years of age. Among different occupational groups, teachers had higher positivity rate (14.19%), recent infection rate (4.52%), and median concentration (9.29 IU/mL) compared with other occupational groups. Therefore, we believe that the educational campuses are the main location for pertussis infection in Guangxi because of the large number of people gathering there. Previous studies have also indicated a high transmission rate of pertussis among primary schools in China[42-43].
Vaccination is the most effective and reliable method to prevent pertussis infection in young children[20,28]. In China, two types of acellular pertussis vaccine are currently in use. One is a free EPI vaccine produced by Chinese institutions containing two pertussis antigens (PT and FHA), and the other is a self-funded non-EPI vaccine produced by GSK containing three pertussis antigens (PT, FHA, and pertactin) or by Sanofi Pasteur containing two pertussis components (PT and FHA)[13,44]. Most children (99.13%) in Guangxi were vaccinated with free domestic DTap vaccine. To assess the efficacy of the domestic DTaP vaccine, children under 5 years of age who had received four doses of domestic DTaP vaccine were selected to analyze the duration of anti-pertussis IgG. As shown in Figure 3, the percentage of < 10 IU/mL increased from 17.95% at 1-month interval to between 70.45% and 76.79% at 10–12-month interval, implying that more than 50% of these children experienced a significant decay in anti-pertussis IgG at 10–12-month interval. Thereafter, the percentage of antibody levels < 10 IU/mL remained above 70% at 13–36-month interval but declined to 63.00% at 37–43 month interval due to natural pertussis infection. Therefore, we speculate that the vaccine-induced protective efficacy would be maintained for approximately 36 months after administering four doses of the domestic DTaP vaccine to children following the immunization schedule. The World Health Organization estimates that the duration of immunity wanes between 4 and 12 years after the last vaccination dose[29]. A previous study in China reported that the potency of domestic DTaP vaccines (PT and FHA) was lower than that of imported aP vaccines (PT, FHA, and pertactin). This study attributed these differences to the formulation and composition of the aP vaccines[44]. However, pertactin-deficient strains of B. pertussis were reported in 2009[45], and pertactin-deficient strains accounted for more than 50% of isolates collected in the United States in 2012[46]. Therefore, further investigation is necessary to improve the formulation and composition of domestic DTaP vaccines. Furthermore, a recent study in China found that component-purified pertussis vaccines demonstrated superior immunogenicity and a longer duration to copurified pertussis vaccines. In that study, domestic DTaP vaccines were produced using a copurification process, whereas imported aP vaccines were produced using a component purification process[47]. Therefore, it is essential to improve the production of domestic DTaP vaccines in order to achieve higher potency.
Although this survey covered all of Guangxi, it primarily focused on rural areas. The proportion of urban population was relatively low, and the subjects were not strictly registered based on urban and rural populations. Therefore, this survey was unable to analyze the antibody levels of pertussis and the infection status in urban and rural areas.
-
Anti-pertussis IgG antibody levels demonstrated that all age groups lacked immunity against pertussis. The antibody level first decreased with age in young children due to the rapid waning of vaccine-induced immunity, and then increased with age due to natural pertussis infection. Moreover, pertussis frequently occurs in Guangxi. The incidence of pertussis in Guangxi is significantly underestimated, particularly among adolescents and adults. Educational campuses were the main location of pertussis infection. To prevent pertussis outbreaks in primary schools, it is strongly recommended that children receive another booster dose of the pertussis vaccine when they start primary school at the age of 6 years.
-
This survey was approved by the Ethics Committee of the Guangxi Zhuang Autonomous Region Center for Disease Control and Prevention (GXIRB 2018-0005), and the participants signed informed consent forms.
Antibody Levels and Infection Status of Pertussis in the Population under Pertussis Resurgence in Guangxi in 2018: A Cross-Sectional Survey
doi: 10.3967/bes2024.069
- Received Date: 2023-09-20
- Accepted Date: 2024-01-17
-
Key words:
- Pertussis resurgence /
- Anti-pertussis IgG /
- Anti-pertussis toxin IgG /
- Antibody level /
- Infection status
Abstract:
| Citation: | Liang Liang, Qiuyun Deng, Lili Deng, Jinghang Wei, Shiyi Chen, Yizhi Wei, Yuyan Ma, Yue Qin, Wei Liu. Antibody Levels and Infection Status of Pertussis in the Population under Pertussis Resurgence in Guangxi in 2018: A Cross-Sectional Survey[J]. Biomedical and Environmental Sciences, 2024, 37(6): 628-638. doi: 10.3967/bes2024.069 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: