-
Diabetes is a pervasive and serious global health issue. According to the International Diabetes Federation report, 463 million adults worldwide were living with diabetes in 2019, and this number is projected to reach 700 million in 2045[1].
Selenium (Se), a crucial trace element, plays a significant role in the mechanism of oxidative stress. Certain studies have indicated that elevated serum Se concentrations correlate with a higher incidence of diabetes mellitus. Moreover, the available evidence suggests that Se compounds may interfere with insulin-regulated molecular pathways, particularly the phosphoinositide-3-kinase/protein kinase β-signaling cascade. This interference may underpin some of the prodiabetic effects of Se[2].
Vitamin D is a pleiotropic steroid hormone and possesses immunoregulatory properties that can cause the alleviation of adipose tissue inflammation. Research indicates that vitamin D can significantly influence the development of diabetes by modulating critical processes related to the condition and associated complications. These processes include reducing the secretion of pancreatic insulin secretion, promoting the resistance to peripheral insulin, downregulating the expression of insulin receptor genes, inducing systemic “sterile” inflammation, and activating the immune system. In addition, vitamin D may fight oxidative stress and reduce insulin resistance through the antioxidant activity[3].
Exposure to Se and vitamin D can affect diabetes independently of each other. However, limited research has addressed the mediating role of vitamin D in the relationship between Se and diabetes mellitus. Therefore, this study used data from US adults to examine the link between Se exposure and the prevalence of type-2 diabetes, along with diabetes indicators, and investigated the potential influence of serum vitamin D.
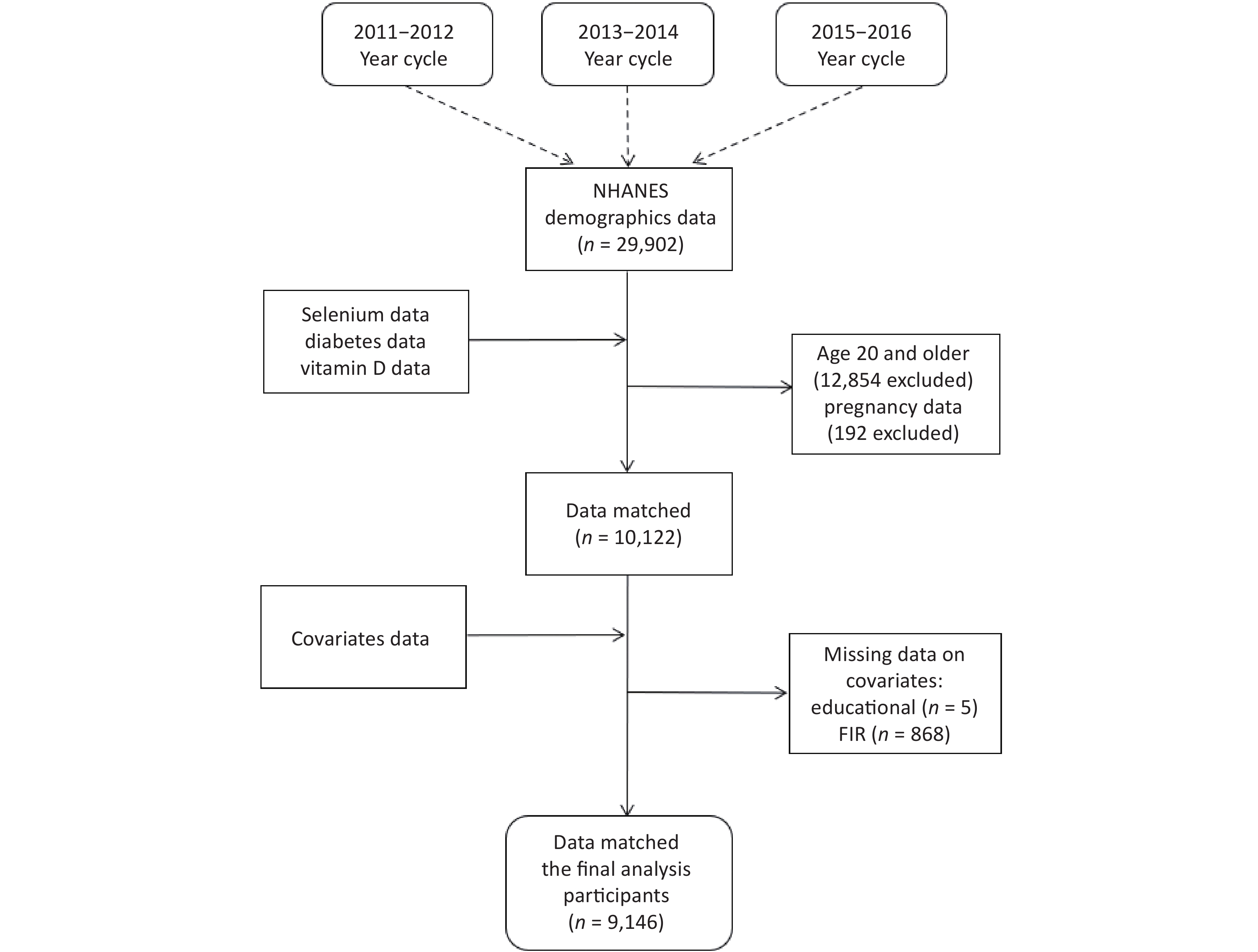
NHANES (The National Health and Nutrition Examination Survey https://www.cdc.gov/nchs/nhanes/index.htm) is a cross-sectional population-based survey that is used to assess the health and nutrition status of the institutionalized civilian population in the United States. Initially, we included a total of 29,902 study participants who participated in the NHANES 2011–2012, 2013–2014, and 2015–2016 cycles. We excluded individuals who were < 20 years of age (n = 12,854), pregnant at the time of the survey (n = 192), and missing data (n = 7,710), resulting in the inclusion of 9,146 study participants. The detailed inclusion and exclusion procedure is depicted in Supplementary Figure S1 (available in www.besjournal.com). The NHANES study was granted approval by the NCHS Research Ethics Review Committee, and all enrolled participants provided written informed consent during the recruitment process.
Se levels (μg/L) in whole blood samples were directly assessed via mass spectrometry. Type-2 diabetes was diagnosed as fasting blood glucose (FPG) ≥ 7.0 mmol/L, glycated hemoglobin (HbA1C) ≥ 6.5%, oral glucose tolerance test (OGTT) ≥ 11.1 mmol/L, or physician notification of type-2 diabetes. The vitamin D for this study comprised the total amount of D3 (25OHD3) and D2 (25OHD2) in human serum (nmol/L).
Demographic and social information was collected from household interviews through standardized questionnaires, including information on gender, age, race, smoking, alcohol consumption, education, exercise, and family income poverty ratio (FIR). Weight (kg) and height (m) information was obtained through physical examination, and the body mass index (BMI, kg/m2) was calculated as weight divided by the square of height. Smoking status was categorized as current regular and current occasional smoker and current nonsmoker. Alcohol consumption was defined as having had at least 12 drinks in a lifetime. Educational attainment was categorized as less than 9th grade, 9th–11th grade, and above 9th grade. Exercise status was categorized as having vigorous exercise per week, only moderate exercise per week, and other.
To explore whether a nonlinear association was present between Se and vitamin D and FPG, HbA1C, and OGTT, we performed restricted cubic splines (RCS) of Se and vitamin D with FPG, HbA1C, and OGTT levels. Furthermore, mediation analysis was conducted to examine the role of vitamin D in mediating the connection between Se and diabetes prevalence, FPG, HbA1C, and OGTT. All statistical analyses were performed using SPSS 22.0 (version 22.0; IBM Corp, Chicago, IL, USA) and R 4.2.2. Each reported P value was two-tailed and achieved significance with a threshold of P < 0.05.
As shown in Supplementary Table S1 (available in www.besjournal.com), a total of 9,146 participants (male: 4,515; female: 4,631) were included in the final study, of whom 2,958 (32.30%) were considered to have vitamin D deficiency (serum levels < 50 nmol/L). In total, 3,092 (33.80%) participants had type-2 diabetes in the study population. Furthermore, significant differences emerged between vitamin D deficient and nondeficient participants in terms of age, BMI, race, education, FIR, smoking status, and alcohol consumption. Significant differences were also found in the levels of FPG and Se.
Variable Total (9,146) Vitamin D (nmol/L) P < 50 (n = 2,958, 32.30%) ≥ 50 (n = 6,188, 67.70%) Age [M (P25, P75)] 42 (30, 56) 52 (37, 65) < 0.01 ** Gender (%) 0.036 * Male 4,515 (49.40) 1,501 (50.70) 3,014 (48.70) Female 4,631 (50.60) 1,457 (49.30) 3,174 (51.70) BMI [M (P25, P75)] 29.30 (24.90, 34.80) 27.40 (23.90, 31.70) < 0.01 ** Ethnicity (%) < 0.01 ** Mexican American 1,120 (12.20) 480 (16.80) 640 (10.30) Other Hispanic 962 (10.50) 295 (10.00) 667 (10.80) Non-Hispanic White 3,610 (39.50) 563 (19.00) 3,047 (49.20) Non-Hispanic Black 2,041(22.30) 1,095 (37.00) 946 (15.30) Other Race 1,413 (15.40) 525 (17.70) 888 (14.40) Education level (%) < 0.01 ** Less than 9th grade 1,986 (21.70) 685 (23.20) 1,301 (21.00) 9–11th grade 1,977 (21.60) 692 (23.40) 1,285 (20.80) More than High school graduate 5,183 (56.70) 1,581 (53.40) 3,602 (58.20) FIR (%) < 0.01 ** ≤ 1.3 3,096 (33.90) 1,151 (38.90) 1,945 (31.40) 1.31–3.5 3,288 (36.00) 1,110 (37.50) 2,178 (35.20) > 3.5 2,762 (30.20) 697 (23.60) 2,065 (33.40) Smoke (%) < 0.01 ** Every day 2,167 (28.60) 1,054 (35.60) 1,563 (25.30) Some days 8,26 (9.00) 334 (11.30) 492 (8.00) Not at all 5,730 (62.40) 1,570 (53.10) 4,133 (66.80) Drinking (%) < 0.01 ** Yes 4,287 (46.90) 1,303 (44.10) 2,984 (48.20) No 4,859 (53.10) 1,655 (55.90) 3,204 (51.80) Activity (%) 0.072 Vigorous 1,266 (13.80) 369 (13.40) 870 (14.10) Moderate 2,185 (23.90) 671 (22.70) 1,514 (24.50) Other 5,695 (62.30) 1,891 (63.90) 3,804 (61.50) FPG [M (P25, P75)] 5.551 (5.162, 6.217) 5.551 (5.162, 6.106) 0.036 * HbA1C [M (P25, P75)] 5.5 (5.2, 5.9) 5.5 (5.2, 5.9) 0.400 OGTT [M (P25, P75)] 8.0495 (6.051, 11.324) 8.049 (5.94, 11.324) 0.057 Diabetes (%) 0.247 Yes 3,092 (33.80) 975 (32.90) 2,117 (34.20) No 6,054 (66.20) 1,983 (67.10) 4,071 (65.70) Se[M(P25, P75)] 192.13 (176.85, 207.43) 193.31 (178.87, 208.76) 0.003 ** Note. *P < 0.05,**P < 0.01,***P < 0.001. FPG, fasting blood glucose (mmol/L); HbA1C, glycated hemoglobin (%); OGTT, oral glucose tolerance test (mmol/L); Se, Selenium (μg/L). Table S1. Baseline characteristics of included participants
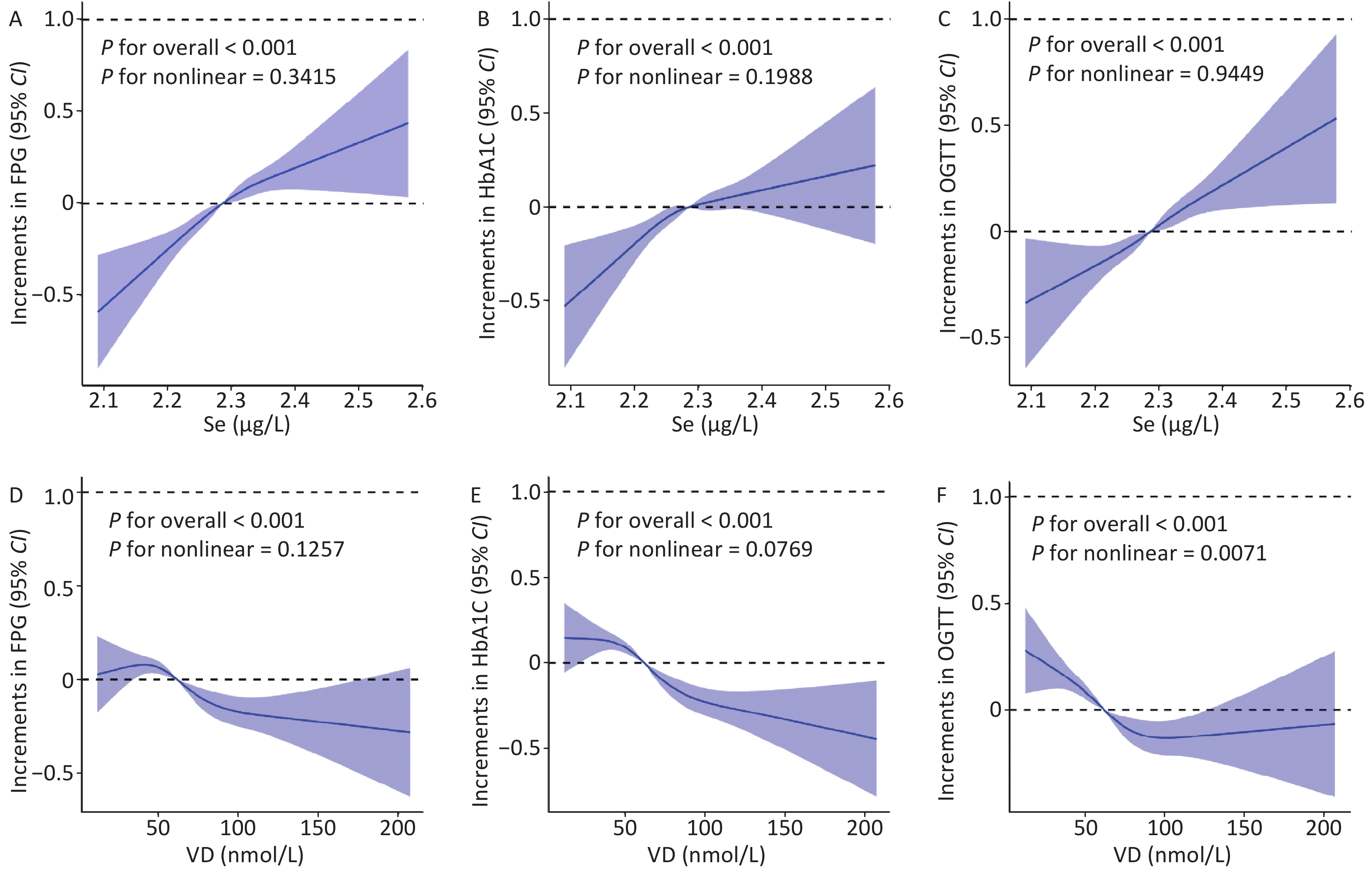
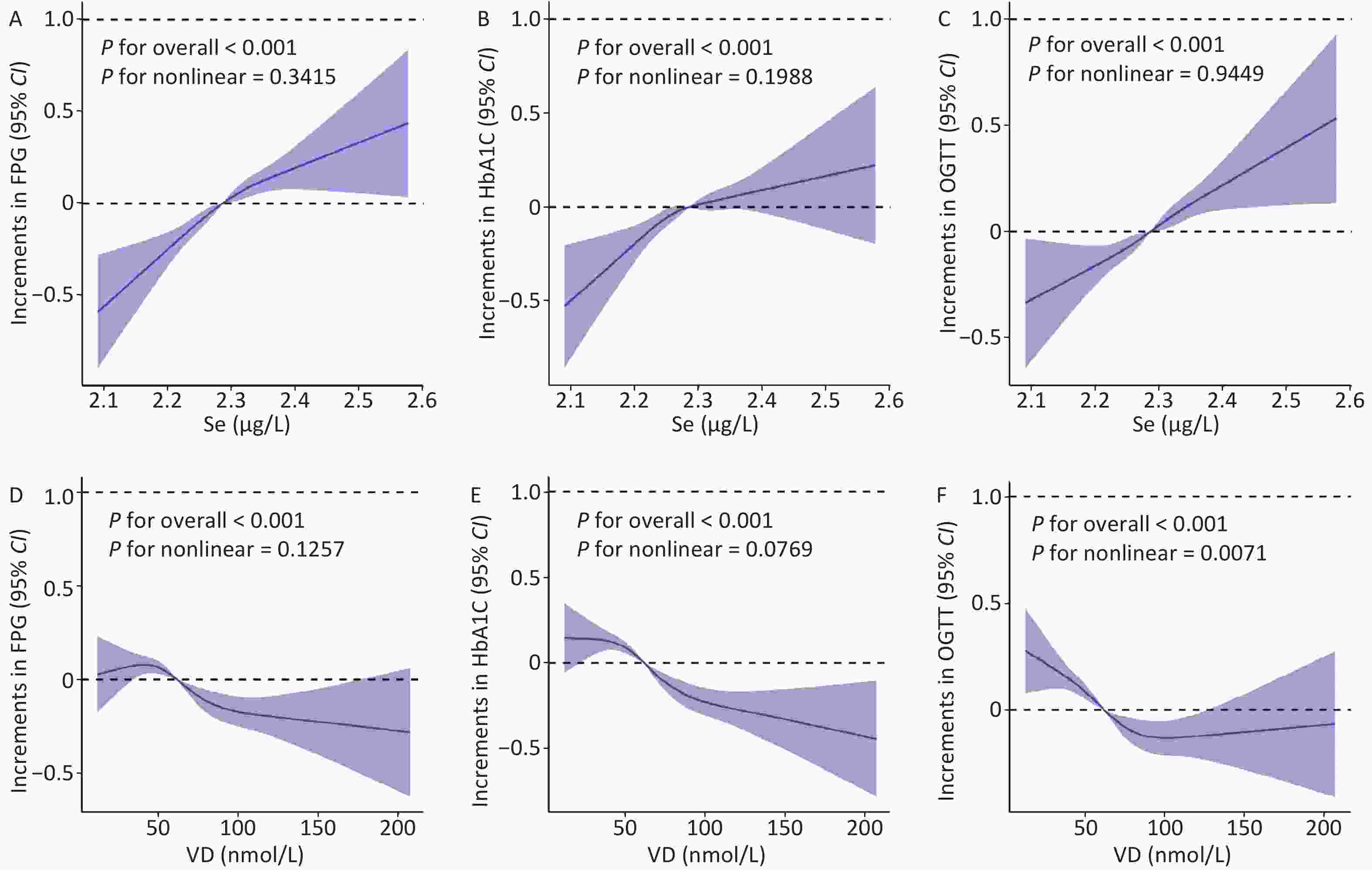
Figure 1A–C shows the RCS of Se (after log-transformation treatment) with FPG, HbA1C, and OGTT. FPG, HbA1C, and OGTT showed linear increasing trends with increasing Se concentration (P nonlinear = 0.342, 0.199, and 0.945). We also determined that OGTT showed a nonlinear change with increasing vitamin D concentration (P nonlinear = 0.007). FPG and HbA1C exhibited a decreasing trend with increasing vitamin D concentration (P nonlinear = 0.126, 0.077) (Figure 1D–F).
Table 1 shows the logistic results of Se and prevalence of type-2 diabetes. Both models were well fitted by the HL-test model (P > 0.05). Model 1 was the crude model, and the results showed that the OR of Se with diabetes was 2.521 (95% CI: 1.177, 5.400). In model 2, after adjusting for gender, age, BMI, ethnicity, education level, FIR, smoke, drinking, and activity, the results showed that the OR of Se with diabetes was 6.242 (95% CI: 2.708, 14.389).
Variables Model 1a (HL-testc: 0.062) Model 2b (HL-testc: 0.056) OR 95% CI P OR 95% CI P Se 2.521 1.177, 5.400 0.017 6.242 2.708, 14.389 < 0.001*** Gender − − − 1.055 0.958, 1.163 0.276 Age − − − 1.046 1.043, 1.050 < 0.001*** BMI − − − 1.062 1.055, 1.070 < 0.001*** Ethnicity − − − − − < 0.001*** Education level − − − − − < 0.001*** FIR − − − − − 0.001** Smoke − − − 0.920 0.815, 1.040 0.225 Drinking − − − 0.840 0.763, 0.925 < 0.001*** Activity − − − 1.008 0.852, 1.191 0.001*** Note. Se (ug/L). *P < 0.05, **P < 0.01, ***P < 0.001. Model 1a: crude model. Model 2b: Adjusted for the inclusion of the covariates gender, age, BMI, ethnicity, education level, FIR, smoke, drinking, and activity. HL-testc: The Hosmer-Lemeshow test is a model fit indicator. BMI: body mass index; FIR: family income poverty ratio. Table 1. Logistic regression of Se and prevalence of type-2 diabetes
The logistic regression analysis in this study consistently indicated a potential positive correlation between Se and the risk of type-2 diabetes. Although, most studies have found a significant positive associations between serum or plasma Se levels and diabetes, numerous studies have revealed an intricate nonlinear relationship with elevated Se status and diabetes prevalence, as demonstrated in a comprehensive five-study meta-analysis in NHANES. In NHANES 2011–2014, an increase of 10 μg/L in Se increased the prevalence of diabetes by 12% (OR: 1.12; 95% CI: 1.06, 1.18)[4]. The high Se exposure may affect the expression of key regulators of glycolysis and gluconeogenesis. This action might potentially be mediated by the selenoprotein glutathione peroxidase 1 (GPx1)[5] as demonstrated by a in vivo study[6] that showed that the overexpression of GPx1 caused obesity and insulin resistance.
Table 2 presents the findings concerning the mediating effect of serum levels of vitamin D in the associations between Se and type-2 diabetes prevalence, FPG, HbA1C, and OGTT, respectively. This shows that the direct effects (ADE) and total effects of Se on type-2 diabetes prevalence, FPG, HbA1C, and OGTT were positive and that the mediating effect (ACME) was negative. However serum levels of vitamin D mediated the effects of Se in the effects of FPG, HbA1C, and OGTT (P < 0.001), and the mediating ratios were all negative. The mediating ratio (Prop. Mediated) of vitamin D in the effect of Se on FPG, HbA1C, and OGTT was −0.361%, −0.169%, and −0.885%, respectively. However, the mediating influence of vitamin D did not reach significance in the relationship between Se and the prevalence of type-2 diabetes (P = 0.27).
Variables Diabetes FPG HbA1C OGTT Estimate 95% CI P Estimate 95% CI P Estimate 95% CI P Estimate 95% CI P ACME < −0.001 −0.001,
0.0000.270 −0.010 −0.016,
−0.010< 0.001 *** −0.002 −0.004,
0.000< 0.001 *** −0.053 −0.083,
−0.030< 0.001 *** ADE 0.051 0.019,
0.080< 0.001 *** 2.836 2.090,
3.720< 0.001 *** 1.392 0.964,
1.880< 0.001 *** 6.059 4.465,
7.790< 0.001 *** Total Effect 0.051 0.019,
0.080< 0.001 *** 2.826 2.082,
3.710< 0.001 *** 1.390 0.962,
1.870< 0.001 *** 6.005 4.413,
7.720< 0.001 *** Prop. Mediated −0.006% −0.001,
0.0000.270 −0.361% −0.005,
0.000< 0.001 *** −0.169% −0.002,
0.000< 0.001 *** −0.885% −0.012,
−0.010< 0.001 *** Note. *P < 0.05,**P < 0.01,***P < 0.001. FPG, fasting blood glucose (mmol/L); HbA1C, glycated hemoglobin (%); OGTT, oral glucose tolerance test (mmol/L); Se, Selenium (ug/L). ACME, the mediating effect; ADE, the direct effects; Prop. Mediated, the mediating ratio. Table 2. Mediating effect of serum vitamin D in the relationship between Se and type-2 diabetes
New evidence has identified vitamin D as a potential factor intervening in diabetes. This is consistent with the mediating results of this study, where vitamin D reduced the effects of Se on diabetes indicators. In a prospective cohort from the Irish Longitudinal Study of Aging, higher vitamin D concentrations were associated with a greater degree of reduction in the probability of developing prodromal diabetes[7]. In a meta-analysis, when contrasted with a control group administered placebos, the administration of Se, vitamin D, and select trace elements (either individually or in combination) demonstrated a substantial enhancement in glycemic control among women with gestational diabetes mellitus: FPG (MD = −9.02; 95% CI: −12.09, −5.96; P < 0.01), serum insulin (MD = −4.33; 95% CI: −5.35, −3.32; P < 0.01)[8]. Type-2 diabetes, in addition to hyperinsulinemia and hyperglycemia, exhibits heightened free radical generation and a decline in antioxidant capacity. Several experimental investigations have proposed that vitamin D could offer antioxidant properties, potentially curbing the generation of free radicals and consequently mitigating lipid peroxidation and other oxidative alterations in biomolecules[9]. The potential mechanism of vitamin D for diabetes prevention has been explored in a study that found that pancreatic iron overload may contribute to the development of type-2 diabetes in rats and revealed a potential protective effect of vitamin D against iron deposition in the diabetic pancreas via the NF-κB-DMT1–signaling pathway[10].
Notably, in the mediating effect results obtained in this study, vitamin D did not mediate the effect of Se on the prevalence of diabetes. For the three individual diagnostic indicators, all results were significant. This may be because the study defined diabetes according to the WHO-defined criteria and the NHANES database itself, which contains the diagnoses of diabetes by a physician. Therefore, diabetes is only a diagnostic result, and FPG, HbA1C, and OGTT are indicators that must reach a threshold to support a diagnosis of diabetes. Although many studies have reported the relationship between trace elements and diabetes, trace elements are not the main influencing factor, i.e., they are not absolute factors and may simply promote the process of diabetes but not necessarily determine the development of diabetes. As for specific blood glucose indicators, they may not necessarily meet the diagnostic criteria for diabetes, although they are susceptible to the subtle effects of trace elements exposure.
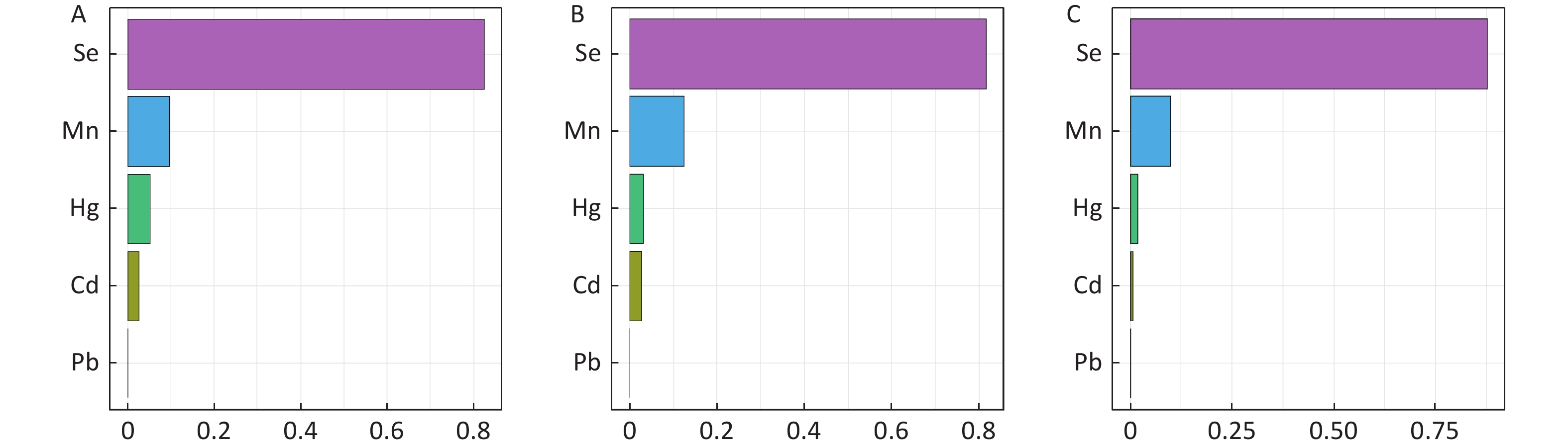
The strengths of this study include the large sample size of the study, which included participants from all three cycles of NHANES; we also controlled sufficient covariates in the analysis. The study used the WQS model for the assessment of mixed trace element exposure (Supplementary Figure S2, available in www.besjournal.com), and the most contributing trace element Se in the WQS model results was then selected for the next step of logistic regression and mediation analysis. In addition, our study helps fills the gap of epidemiological studies on the mediating role of serum vitamin D in the effect of Se on type-2 diabetes. Furthermore, our study provides references for future mechanistic studies.
Figure S2. Estimated risk and weighted values of heavy metals for fasting blood glucose (FPG), glycated hemoglobin (HbA1C), and oral glucose tolerance test (OGTT) by Weighted Quantile Sum (WQS) regression models. A: FPG; B: HbA1C; C: OGTT.
Our study has some limitations. First, the study was a cross-sectional study, and the causality argument was insufficient. Elevated biomarkers of Se, usually measured in patients with type-2 diabetes, are more likely to be the result than the cause. Second, since only Se was included in the study, the other trace elements need to be coanalyzed. Third, we only included internal exposure data of blood, and urinary trace elements also deserve further investigation. Fourth, the study did not take dietary supplementation of vitamin D into account. Fifth, the specific blood glucose indicators are susceptible to the subtle effects of trace elements exposure. Sixth, more studies are needed to discover the mediating effect of serum vitamin D and the mechanism of interaction.
Based on data from 9,146 US adults from NHANES (2011–2012, 2013–2014, and 2015–2016), we evaluated the association between Se and type-2 diabetes and three important indicators and explored the possible role of vitamin D. We found that Se was positively associated with type-2 diabetes and these indicators, and we further found that vitamin D moderated the relationship between Se and FPG, HbA1C, and OGTT. These results may offer fresh perspectives on comprehending the impacts of Se reduction through vitamin D on type-2 diabetes, opening new avenues for diabetes prevention.
In conclusion, our study indicates that the Se exposure may be a risk factor for diabetes. Exposure to Se in the environment and the burden of diabetes disease remains a public health challenge, and serum vitamin D may play an important mediating role in alleviating the type-2 diabetes risk of Se. Therefore, the focus on exposure to trace elements such as Se should be strengthened in measures to control type-2 diabetes; vitamin D should also be included as an important intervening influential factor. Subsequent cohort investigations and animal studies are needed to explore the direction and the specific underlying mechanisms of these relationships.
Selenium, Type-2 Diabetes, and the Possible Protective Role of Vitamin D
doi: 10.3967/bes2024.072
- Received Date: 2023-12-28
- Accepted Date: 2024-04-07
The authors declare no conflict of interest.
| Citation: | Runxue Ma, Ce Liu, Ling Zhang, Jingzhe Guo, Erkai Zhou, Ling Zheng, Li He, Xiaobing Shan, Yunhui Yu, Bin Luo. Selenium, Type-2 Diabetes, and the Possible Protective Role of Vitamin D[J]. Biomedical and Environmental Sciences, 2024, 37(6): 661-665. doi: 10.3967/bes2024.072 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: