-
Endometriosis (EMS) is one of the most common disorders among women of reproductive age and affects approximately 6%–10% of the world total population[1]. Women with EMS often experience symptoms such as dysmenorrhea, infertility, or difficulties in conceiving. Recent studies focusing on amino acids (AAs) metabolism have shed light on the mechanisms underlying the development and progression of EMS[2]. However, causal relationships remain unclear owing to the inherent limitations of observational studies. Mendelian randomization (MR) utilizes genetic variations identified in genome-wide association studies (GWASs) as instrumental variables (IVs) to ascertain the causal effects of risk factors (or exposures) on outcomes, thereby considerably reducing the biases arising from confounding factors and reverse causation[3]. Based on the exposed, this study aims to investigate the causal association between 20 AAs and EMS using MR analysis. Furthermore, considering that changes in AAs metabolism might be a consequence rather than a cause of EMS, we conducted a reverse MR analysis after EMS exposure.
Alanine, glutamine, glycine, histidine, isoleucine, leucine, phenylalanine, tyrosine, and valine levels were analyzed using the Nightingale Health metabolic biomarker platform based on high-throughput nuclear magnetic resonance. This analysis was conducted using approximately 118,000 EDTA plasma samples and 5,000 repeated assessments from baseline recruitment in the UK Biobank[4]. Data for the remaining 11 AAs were derived from an analysis conducted by Shin et al. using an ultra-high-performance liquid chromatography-tandem mass spectrometry platform involving approximately 7,800 participants[5]. EMS data were obtained from a GWAS meta-analysis of 24 cohorts encompassing 21,779 cases and 449,087 controls[6] (Table 1).
Phenotype Ref Ieu id Consortium Ancestry Participants, number Alanine 36402876 met-d-Ala UKB EUR 115,074 Glutamine 36402876 met-d-Gln UKB EUR 114,750 Glycine 36402876 met-d-Gly UKB EUR 114,972 Histidine 36402876 met-d-His UKB EUR 114,895 Isoleucine 36402876 met-d-Ile UKB EUR 115,075 Leucine 36402876 met-d-Leu UKB EUR 115,074 Phenylalanine 36402876 met-d-Phe UKB EUR 115,025 Tyrosine 36402876 met-d-Tyr UKB EUR 114,911 Valine 36402876 met-d-Val UKB EUR 115,048 Arginine 24816252 met-a-347 MRC-IEU EUR 7,528 Asparagine 24816252 met-a-638 MRC-IEU EUR 7,761 Aspartic acid 24816252 met-a-388 MRC-IEU EUR 7,721 Cysteine 24816252 met-a-455 MRC-IEU EUR 7,692 Glutamic acid 24816252 met-a-466 MRC-IEU EUR 7804 Lysine 24816252 met-a-326 MRC-IEU EUR 7,812 Methionine 24816252 met-a-327 MRC-IEU EUR 7,795 Proline 24816252 met-a-355 MRC-IEU EUR 7,816 Serine 24816252 met-a-464 MRC-IEU EUR 7,796 Threonine 24816252 met-a-324 MRC-IEU EUR 6,020 Tryptophan 24816252 met-a-304 MRC-IEU EUR 7,804 EMS 36914876 NA Rahmioglu N EUR 21,779 Note. EUR, European; MRC-IEU, MRC integrative epidemiology unit; UKB, UK biobank; EMS, endometriosis; Ref, reference (pubmed id); Ieu id, integrative epidemiology unit identification; NA, not applicable. Table 1. Detailed information of data sources
Single-nucleotide polymorphisms (SNPs) were used as IVs in the MR analysis with genome-wide significant associations (P < 0.001) and strict linkage disequilibrium parameter sets (r2 < 0.001, clumping distance = 10,000 kb). Owing to the absence of genome-wide significant SNPs for the 11 AAs from Shin et al.’s GWAS study, a more lenient threshold of 5 × 10-6 was adopted. Weak IVs were assessed using the F-statistic (F = beta²/se²), which exceeded 10. In the absence of an SNP in the resulting dataset, an alternative SNP strongly linked to disequilibrium (r2 > 0.8) with the primary SNP was selected. The MR-Steiger filter was applied to eliminate variations that were more strongly correlated with the outcome than with the exposure. The MR study methods employed the inverse-variance weighted approach[7], with Cochrane’s Q test to check for heterogeneity, and MR-Egger and MR pleiotropy residual sum and outlier (MR-PRESSO) tests for pleiotropy. Considering multiple comparisons, Bonferroni correction was applied, setting a significance threshold for causal association at P < 0.002 (0.05/20). Associations with P-values between 0.002 and 0.05 were considered suggestive of causal evidence.
Bidirectional MR analyses (Figure 1) were conducted using IVs ranging from 2 to 44. All IVs passed the MR-Steiger filter and outliers identified by MR-PRESSO were excluded from the final analyses. The average F-statistics of the IVs varied from 22 to 522, indicating a significant reduction in bias due to weak IVs. In the forward MR analysis, a significant causal association was found; specifically, genetically predicted higher circulating levels of tyrosine were associated with an increased risk of EMS (odds ratio [OR] = 1.171, 95% confidence interval [CI] = 1.073–1.279, P < 0.001). Additionally, two suggestive pieces of evidence were identified: a protective causal effect of leucine on EMS (OR = 0.836, 95% CI = 0.704–0.993, P = 0.04) and a harmful causal effect of aspartic acid on EMS (OR = 2.011, 95% CI = 1.152–3.509, P = 0.01). No causal evidence was found for other AAs in the EMS. In the reverse MR analysis, two suggestive pieces of evidence were found: genetically predicted EMS, per one standard deviation increase, was associated with higher circulating levels of phenylalanine (β = 0.027, 95% CI = 0.005–0.049, P = 0.01) and tyrosine (β = 0.027, 95% CI = 0.004–0.051, P = 0.02). No causal evidence was found for the effect of EMS on the other AAs. The sensitivity analyses further corroborated the robustness of the results (Figure 1).
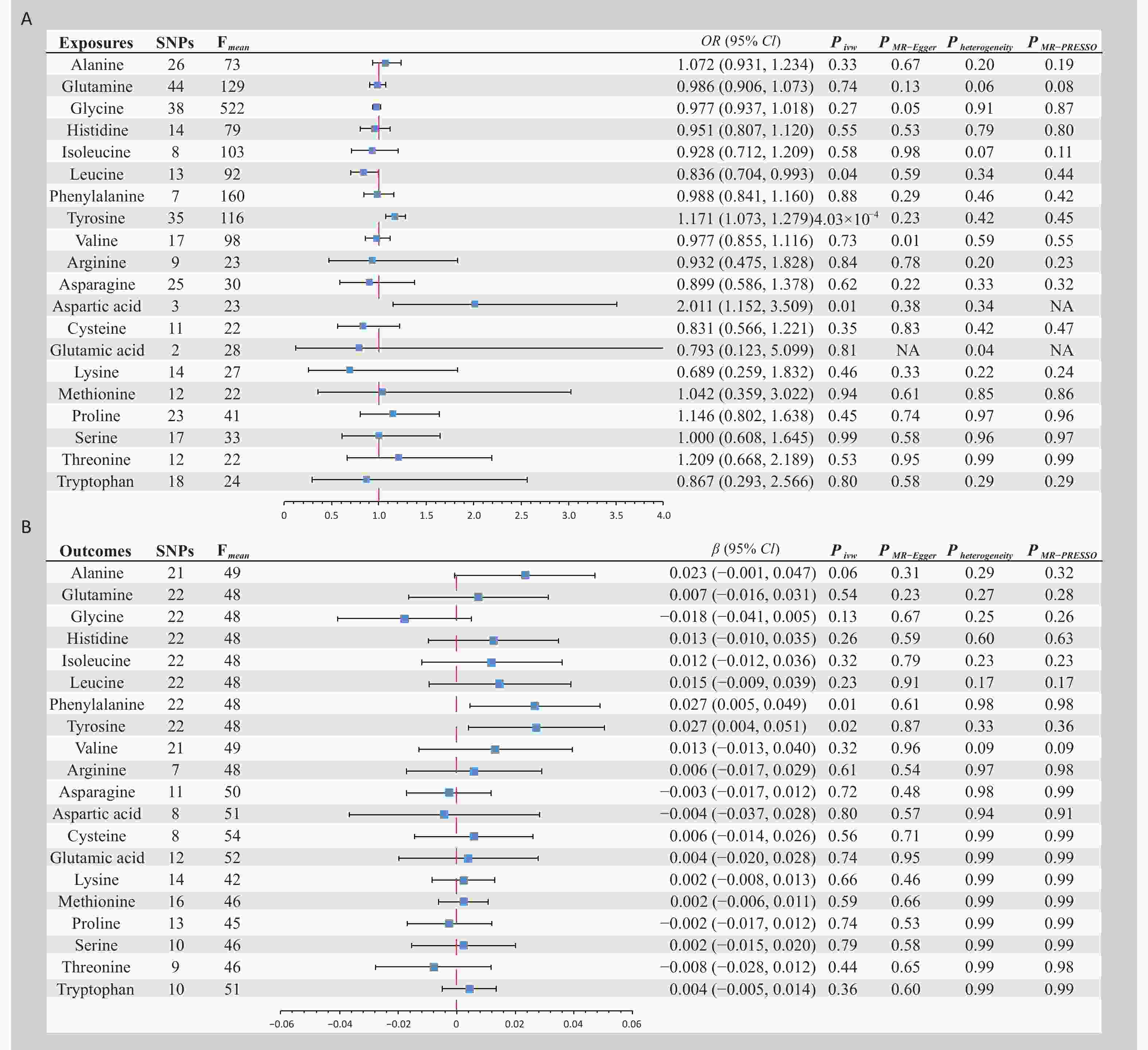
Figure 1. Bidirectional Mendelian randomization analysis of plasma amino acid levels and endometriosis. (A) Plasma amino acid levels as exposure phenotype and endometriosis as outcome. (B) Endometriosis as exposure phenotype and plasma amino acid levels as outcome phenotype. OR, Odds ratio; CI, Confidence interval; IVW, Inverse variance weighted; SNPs, Single nucleotide polymorphisms; MR-PRESSO, Mendelian randomization -pleiotropy residual sum and outlier.
We performed an extensive MR analysis, revealing a bidirectional causal relationship between tyrosine levels and EMS risk, along with other suggestive evidence. Tyrosine, a precursor of key neurotransmitters, including dopamine, norepinephrine, and thyroid hormones, may affect neuroendocrine system function when in high levels[8], thus impacting hormonal balance and the physiological state of the endometrium. In addition, tyrosine may exacerbate oxidative stress and inflammatory responses, which have key roles in the pathogenesis[9]. EMS can also indirectly elevate tyrosine levels by affecting metabolic pathways such as chronic inflammation, liver function, and hormonal balance[10]. Therefore, regulating tyrosine levels or the associated metabolic pathways may help control EMS development. Overall, this MR study suggests a role for AA metabolism in EMS and could guide future research and treatment strategies. Further validation of the suggestive causal evidence is required in future studies.
HTML
-
This study was based solely on publicly accessible, de-identified data from published studies and summary databases. No ethical approval was required for this study.
&These authors contributed equally to this work.
Reference







 Quick Links
Quick Links
 DownLoad:
DownLoad: