-
Currently, cigarette smoke (CS) remains a major contributor to disease morbidity and mortality. CS can be divided into cigarette mainstream smoke (CMS) and side-stream smoke, depending on where it is produced by burning tobacco[1]. CMS is inhaled by smokers from the filter end during cigarette combustion and is strongly associated with the development of several diseases[2-4]. CS is a mixture of thousands of gaseous and solid pollutants, many of which are considered risk factors for serious illnesses, typically toxic substances such as PM2.5 and nicotine. PM2.5 can penetrate deep into the lungs and eventually lead to lung injury by inducing inflammation and oxidative stress[5]. Nicotine, the primary addictive component of tobacco, is absorbed by the mucous membranes, skin, alveoli, and gastrointestinal system, resulting in lung injury[6].
Cumulative evidence has demonstrated that smoking can affect the composition and structure of gut microbiota. A population-based cross-sectional study of Korean smokers revealed that the composition of the intestinal microbiota of current smokers was altered compared to that of never-smokers[7]. Furthermore, smoking remarkably reduces the concentration of several organic acids (e.g., acetic acid, propionic acid, butyric acid, and valeric acid) and the population of Bifidobacteria in the cecum of rats[8]. In this study, we constructed a CMS exposure mouse model to further illustrate the influence of CMS and its main components (i.e., PM2.5 and nicotine) on the lungs and gut. We tried to explore the potential role of the nuclear factor kappa B (NF-κB) signaling pathway in modulating CMS-induced lung injury and changes in the gut. These results might contribute to the understanding of the health risks of CMS and provide a potential therapeutic strategy for treating CMS-induced pathological changes in both the lungs and gut.
PM2.5 in the CMS was prepared as described previously[9]. All the animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of Nanchang Royo Biotech Co., Ltd. (Nanchang, China) with an approval number of RYE2020091602. C57BL/6 mice (male, six-week-old, 18–20 g) were provided by Hunan SJA Laboratory Animal Co., Ltd (Changsha, China). They were housed in the laboratory animal center of the Institute of Translational Medicine, Nanchang University.
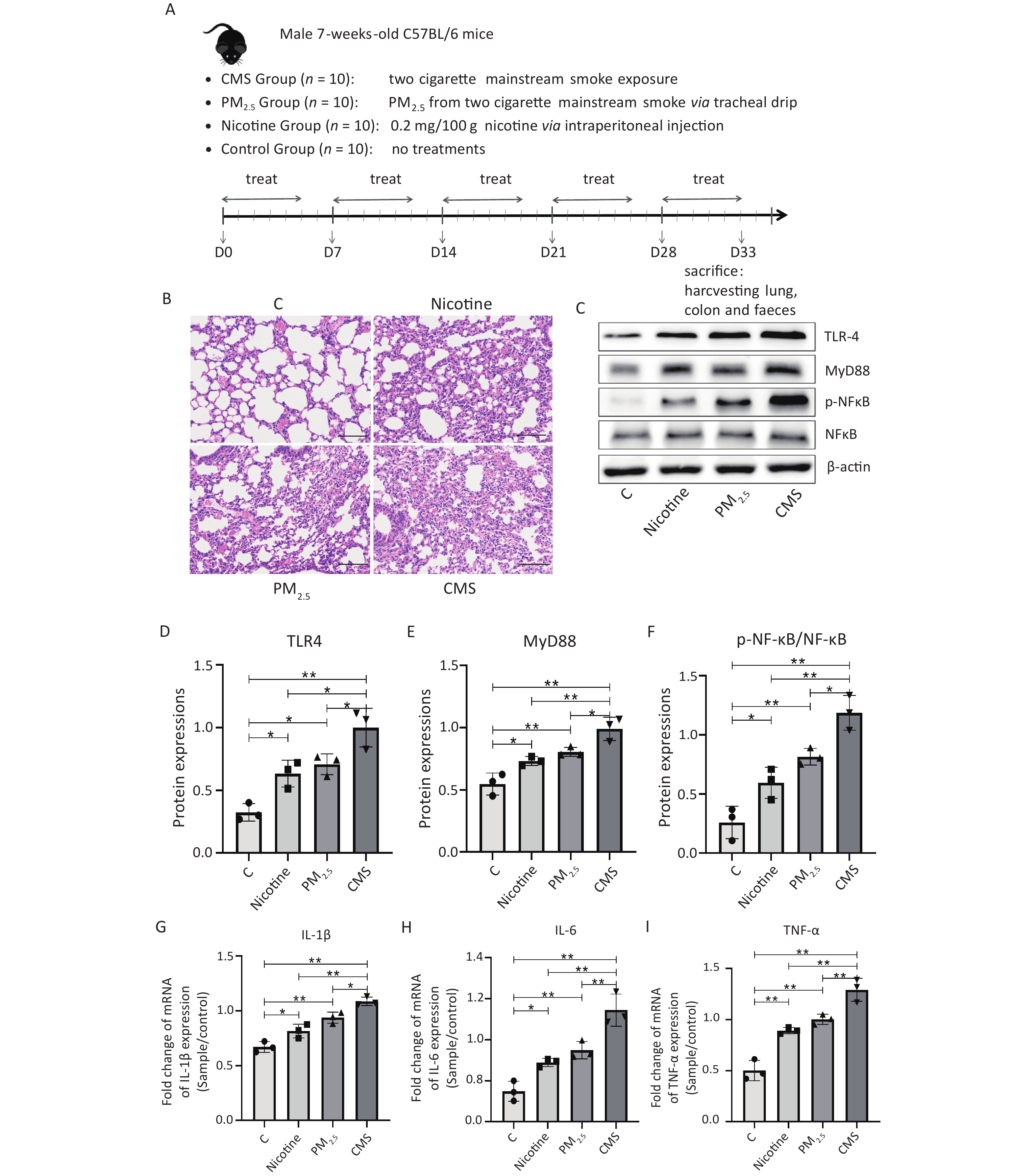
The schedule of animal experiments is shown in Figure 1A. Forty mice were randomly assigned into four groups (n = 10/group): control (C), nicotine (nicotine), PM2.5 (PM2.5), and CMS-exposed (CMS). The mice in group C did not receive any treatment and were assigned as normal controls (C). Mice in the nicotine group were treated with a total dose of 0.2 mg/100 g of free-radical nicotine by intraperitoneal injection[10]. Mice in the CMS-exposed group inhaled CMS generated from two cigarettes per day with a 1 h smoke-free interval, and each exposure lasted for 1 h. The CMS dose and time of exposure used in this study were associated with the good tolerance of mice to CMS sessions without visible pathology or mortality since our pre-experimental results showed that more CMS-exposed doses and times would cause coughing, loss of appetite, and lethargy in mice (data not shown). To maintain consistency with the CMS group, mice in the PM2.5 group were treated with a suspension of PM2.5 collected from two cigarettes per time by tracheal drip. For the CMS group, a homemade inhalation chamber (30 cm × 30 cm × 30 cm) was designed to simulate exposure to tobacco smoke, in which the cigarette filter tip was connected to the inlet end of a powered pump and the outlet end of the pump was extended into the inhalation chamber. When the cigarette was lit, mainstream smoke was drawn into the inhalation chamber through a pump. All treatments were administered five days per week for five weeks.
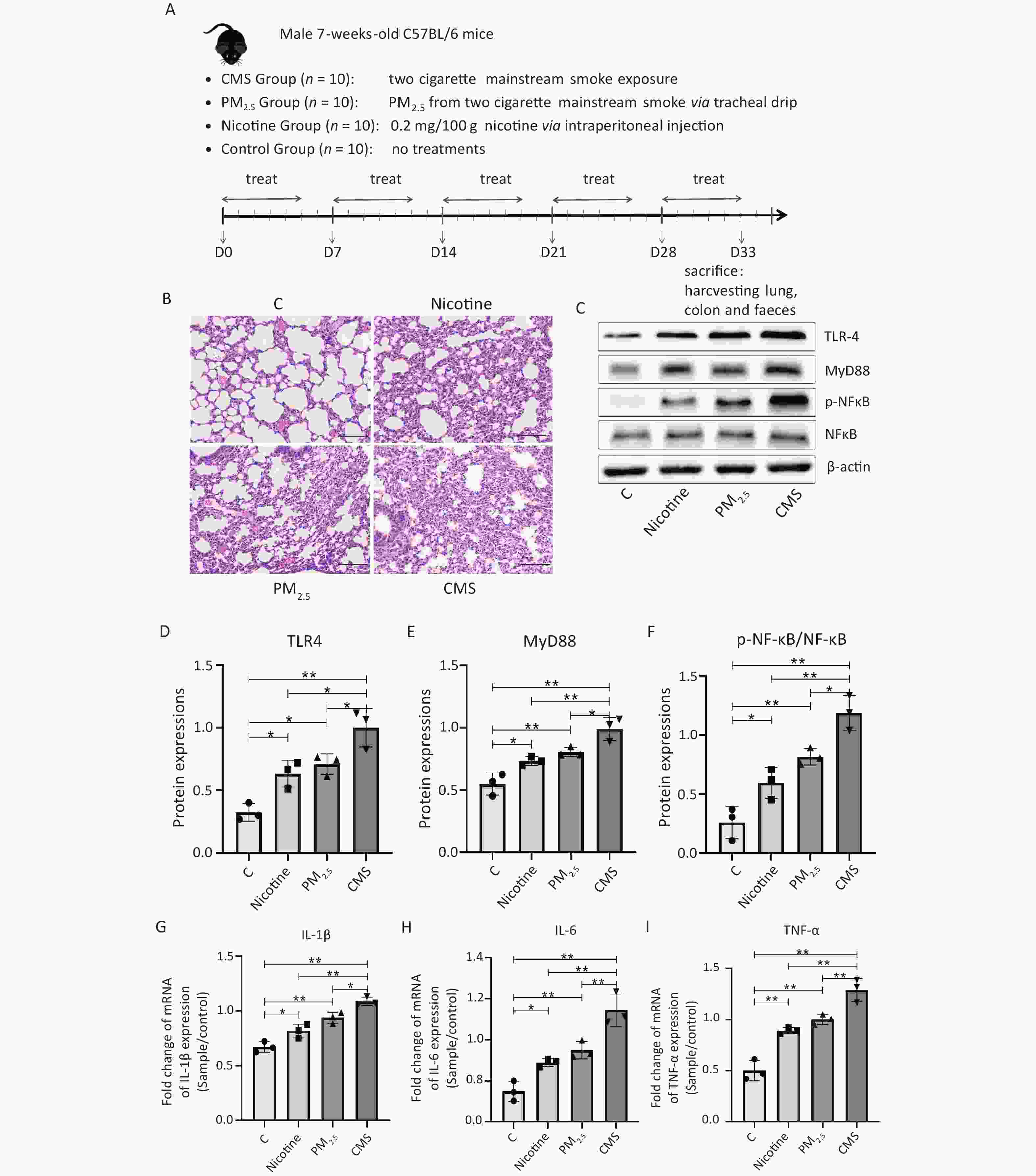
Figure 1. Cigarette mainstream smoke (CMS) and its components (PM2.5, nicotine) cause lung injury and inflammation in mice. (A) Flowchart of the animal experiments in this study. (B) Hematoxylin-eosin (HE) staining of the lung tissue (200×). (C) Western blot analysis of toll-like receptor 4 (TLR4), myeloid differentiation primary response 88 (MyD88), nuclear factor kappa B (NF-κB), and phosphor-NF-κB (p-NF-κB) expression in lung tissues. (D–F) The relative quantification of TLR4, MyD88, and p-NF-κB/NF-κB, referring to western blotting data using β-actin as the internal control. (G–I) The transcription level of pro-inflammatory cytokines interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) in lung tissues. C, normal control group; nicotine, nicotine-treated group; PM2.5, PM2.5-treated group; CMS, CMS-exposed group. The data were represented as means ± SD that were calculated from a random selection of three mice/group. *P < 0.05, **P < 0.01, no marker indicates no significant difference (P > 0.05).
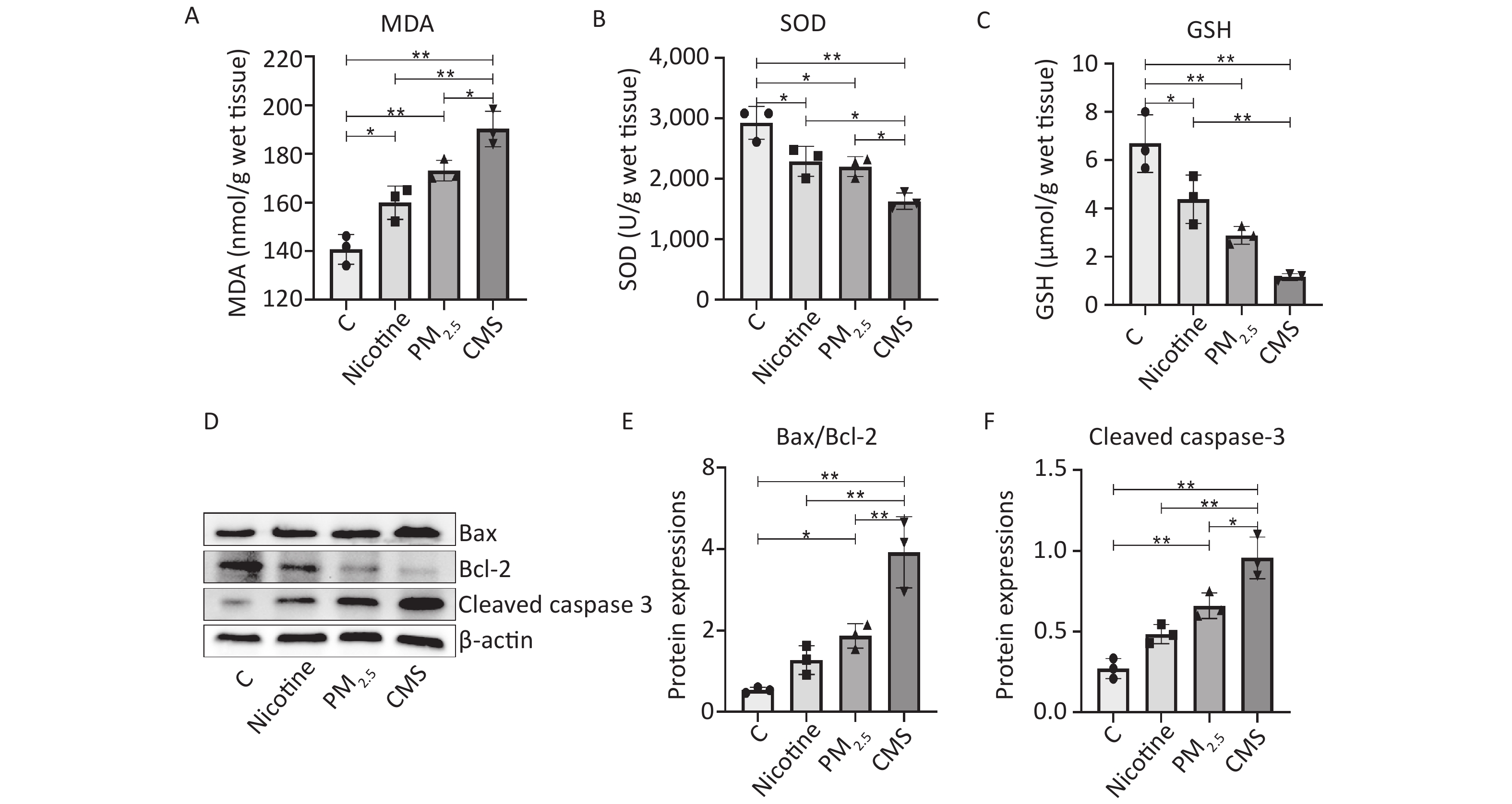
We demonstrated significant adverse histological changes in the lungs of the CMS, PM2.5, and nicotine groups, which were characterized by abnormal inflammatory infiltrates, loss of mucosal epithelial cells, structural changes in the crypt, and atrophy (Figure 1B). The expression of several key proteins in the toll-like receptor 4 (TLR-4) / myeloid differentiation primary response 88 (MyD88) /NF-κB signaling pathway was significantly increased in the lungs of mice exposed to CMS, PM2.5, and nicotine (Figure 1C−F), which was accompanied by higher transcriptional levels of inflammatory factors, including interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) (Figure 1G–I). Mice in the CMS group exhibited the most severe histological changes and inflammatory responses in their lungs, indicating the most severe lung damage, which was consistent with the findings of other studies[2]. We suggested that CMS, PM2.5, and nicotine exposure could cause oxidative stress and cell apoptosis in the lungs, characterized by changes in the levels of malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione (GSH) and the expression of B-cell lymphoma protein 2 (Bcl-2)-associated X (Bax) /Bcl-2 and cleaved caspase 3 in the lungs (Supplementary Figure S1, available in www.besjournal.com).
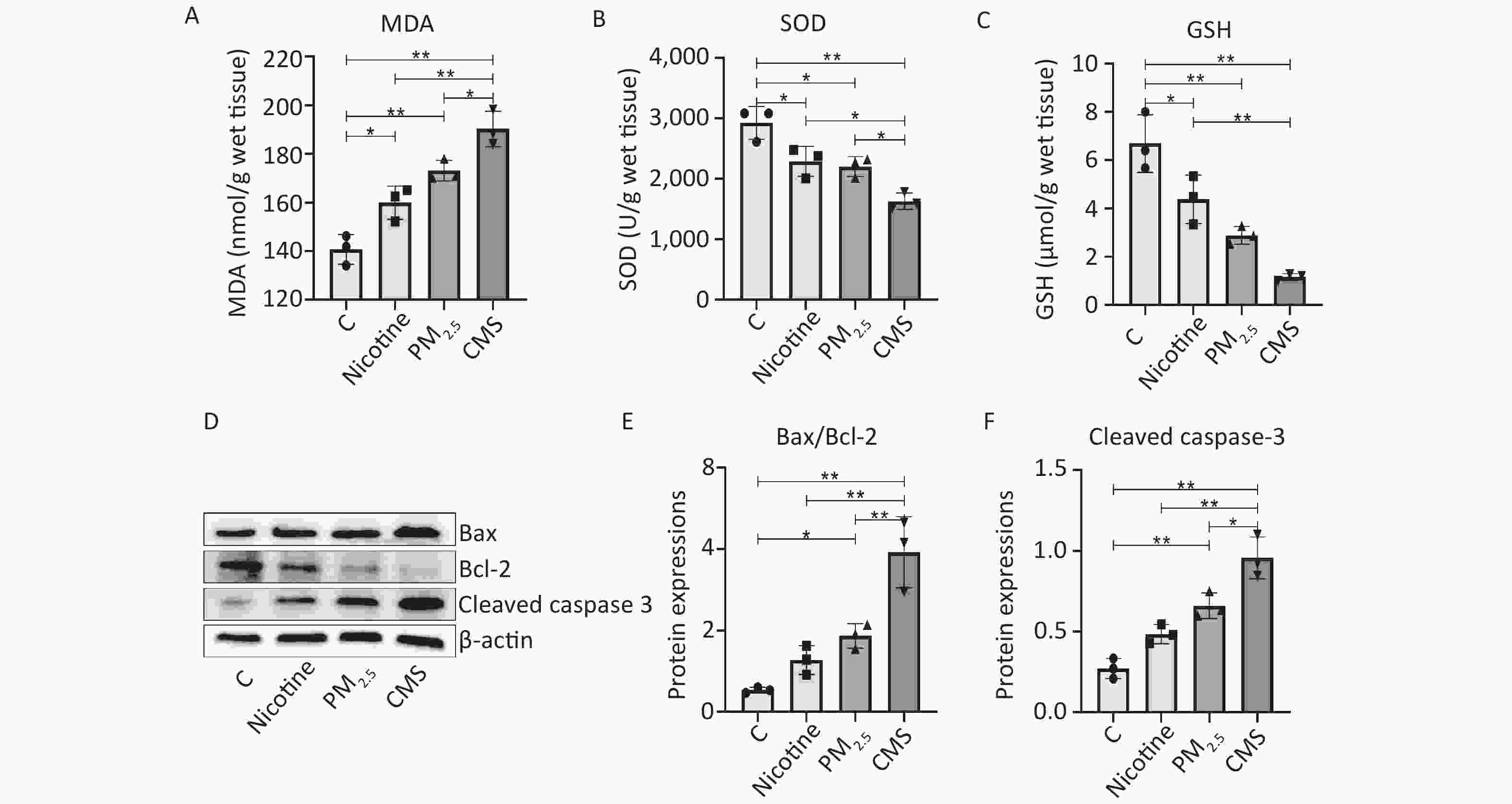
Figure S1. CMS and its components (PM2.5 and nicotine) exposure caused oxidative stress and apoptosis in the lungs. (A-C) The activity of MDA, SOD, and GSH in the lungs. (D) Western blotting analysis of Bax, Bcl-2 and Cleaved caspase 3 expression in lung tissues. (E-F) The quantification of Bax/Bcl-2 and Cleaved caspase 3 expression, referring to western blot results using β-actin as the internal control. C, the normal control group; Nicotine, the nicotine treated group; PM2.5, the PM2.5 treated group; CMS, the CMS-exposed group. Data were presented as means ± SD (n = 3 / group). *P < 0.05, **P < 0.01, no marker indicates no significant difference (P > 0.05).
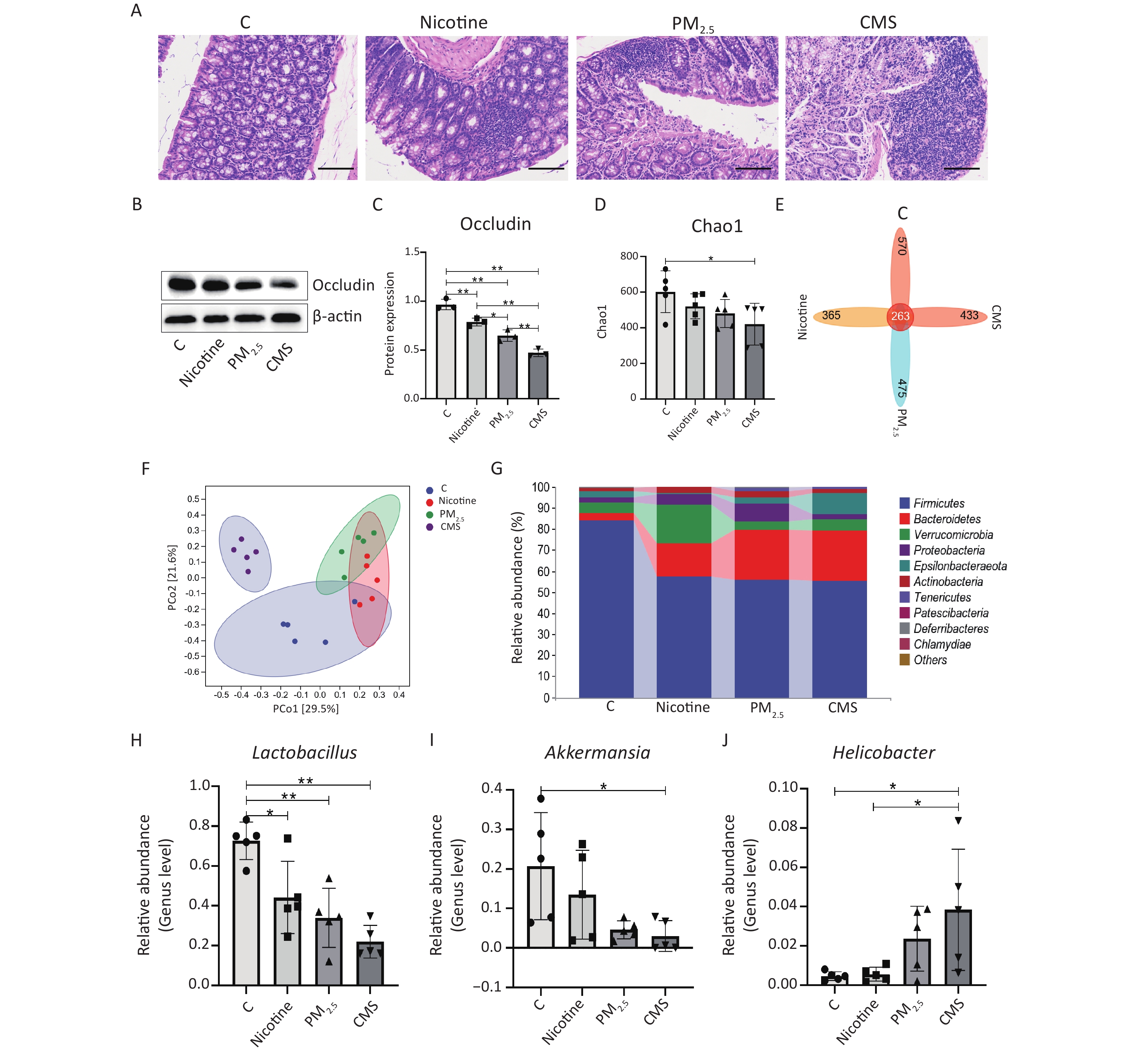
Furthermore, we observed different degrees of colonic histological changes in the CMS, PM2.5, and nicotine groups, which were characterized by a substantial influx of inflammatory cells and an increase in the thickness of the interstitial colons (Figure 2A). Occludin expression in the colon was inhibited after treatment with CMS, PM2.5, and nicotine (Figure 2B−C). Additionally, CMS appeared to have the strongest impact on intestinal barrier function, causing intestinal injury to a certain extent. As for the gut microbiota, we demonstrated that exposure to CMS induced a reduction in gut microbiota diversity, as indicated by a lower Chao1 index in the CMS group compared to that of the C group (Figure 2D). Moreover, there were 365, 475, 433, and 570 unique operational taxonomic units (OTUs) in the mouse gut microbiota among the nicotine, PM2.5, CMS, and C groups, respectively, and 263 shared OTUs among these groups (Figure 2E). Principal coordinate analysis (PCoA) analysis showed that the mice in each group clustered together, yet they were separated from each other, with the CMS group being farther away from the other groups (Figure 2F). Furthermore, at the phylum level, in comparison to the C group, the relative abundance of Firmicutes was decreased in the CMS-exposed groups, while the levels of Bacteroidetes were increased (Figure 2G). At the genus level, the relative abundance of Lactobacillus and Akkermansia was significantly decreased in the CMS group, accompanied by a higher abundance of Helicobacter (Figure 2H–J).
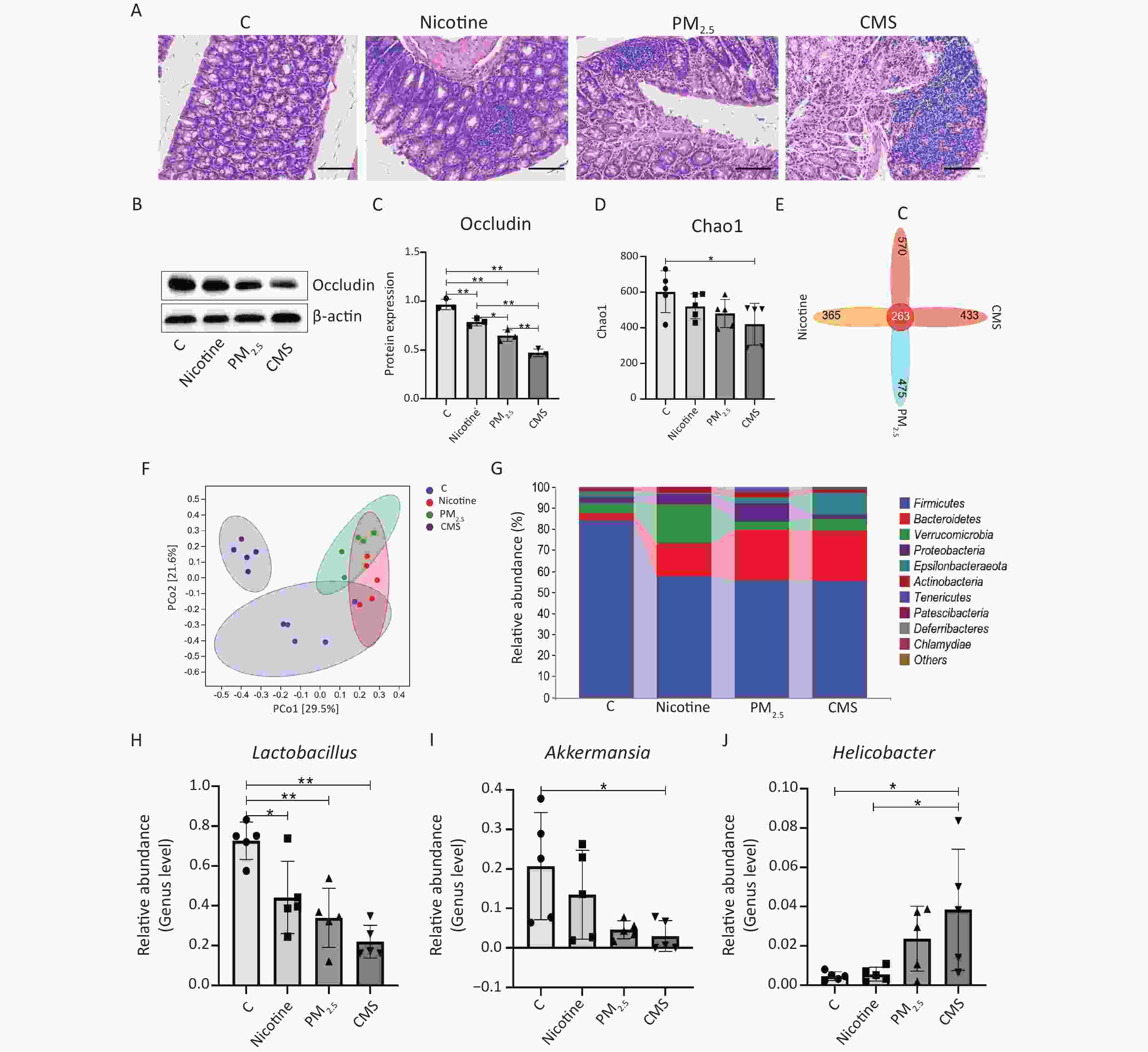
Figure 2. Effects of cigarette mainstream smoke (CMS) and its components (PM2.5, nicotine) on the intestinal and gut microbiota. (A) Hematoxylin-eosin (HE) staining of the lung tissue (200×). (B, C) Western blot analysis of occludin expression and its relative expression in lung tissues. (D) The Chao1 index of the gut microbiota. (E) Venn map representation of the operational taxonomic units (OTUs). (F) Principal coordinate analysis (PCoA) of the β diversity index. (G) Microbial composition at the phylum level. (H-J) Relative abundances of Lactobacillus, Akkermansia, and Helicobacter at the genus level. Data were represented as means ± SD (n = 3/group for intestinal injury analysis, n = 5/group for gut microbiota analysis). *P < 0.05, **P < 0.01, no marker indicates no significant difference (P > 0.05).
Another animal experiment was conducted to investigate the potential effects of blocking the NF-κB pathway on CMS-induced lung injury by inhibiting the expression of NF-κB with ammonium pyrrolidinedithiocarbamate (PDTC, a common inhibitor of NF-κB). Thirty mice were housed as described above and randomly assigned to three groups (n = 10/group): C, CMS, and CMS-exposed combined with PDTC (CMS+PDTC). The mice in the C and CMS groups were treated as described above. Mice in the CMS+PDTC group were treated with a single intraperitoneal injection of 50 mg/kg PDTC during CMS exposure.
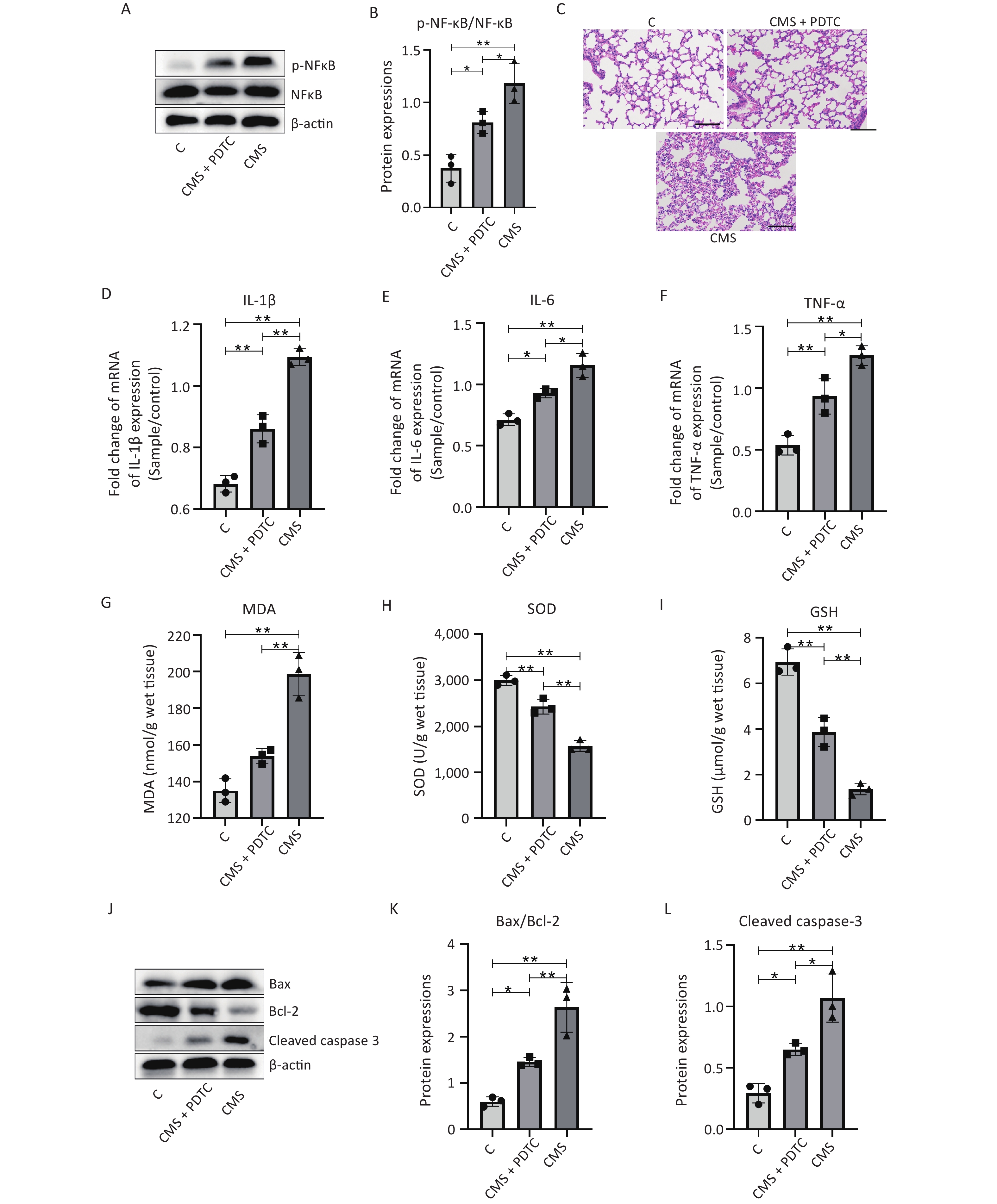
The expression of phosphor- NF-κB (p-NF-κB)/NF-κB was significantly reduced in the CMS+PDTC group (Figure 3A–B); moreover, PDTC treatment could mitigate CMS-induced lung pathological changes, characterized by the reduction of inflammatory cell infiltration and increase interstitial and intra-alveolar space thickness in the lungs (Figure 3C). PDTC injection significantly reduced the expression of inflammatory factors, including IL-1β, IL-6, and TNF-α, which were stimulated by CMS in vivo (Figure 3D–F). PDTC treatment effectively reversed the oxidative stress and cell apoptosis caused by CMS in the lungs, as indicated by increased MDA levels and decreased GSH and SOD levels (Figure 3G–I) and the downregulation of Bax/Bcl-2 and cleaved caspase 3 (Figure 3J–L). In addition, we confirmed that the inhibition of NF-κB signaling by PDTC effectively reinstated the CMS-induced gut microbiota disturbance (Supplementary Figure S2, available in www.besjournal.com). These results suggested that suppressing the expression of NF-κB by PDTC could effectively ameliorate CMS-induced lung injury, indicating the NF-κB signaling pathway might be a breakthrough point in the treatment of smoke exposure-mediated lung injury.

Figure 3. Inhibition of the nuclear factor kappa B (NF-κB) pathway ameliorates cigarette mainstream smoke (CMS)-mediated lung injury. (A–B) Western blot analysis of phosphor-NF-κB (p-NF-κB)/NF-κB expression and its relative quantification in lung tissues. (C) Hematoxylin-eosin (HE) staining of the lung tissue (200×). (D–F) The transcription level of interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) in lung tissues. (G–I) Activities of malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione (GSH) in the lung tissue. (J-I) Western blot analysis and relative quantification of B-cell lymphoma protein 2 (Bcl-2)-associated X (Bax), Bcl-2, and cleaved-caspase 3 expression in lung tissues. C, normal control group; CMS, CMS-exposed group; CMS+ ammonium pyrrolidinedithiocarbamate (PDTC), CMS-exposed group combined with PDTC treatment. Data were represented as means ± SD (n = 3/group). *P < 0.05, **P < 0.01, no marker indicates no significant difference (P > 0.05).
We conclude that exposure to CMS, PM2.5, and nicotine leads to severe lung injury, intestinal permeability, and alterations in the gut microbiota. Inhibition of NF-κB signaling seemed to effectively reverse these changes in both the lungs and the gut. However, there are a few limitations to this study that should be addressed in future work. The potential crosstalk mechanism between the lungs and gut should be elucidated. Although it is well established that CMS can directly affect the gut microbiota and cause lung and intestinal injury, whether these pathological changes are modulated by the gut microbiota requires further study. Lastly, the exact mechanism of the NF-κB signaling pathway in mediating the therapeutic action of CMS-induced lung injury should be explored.
-
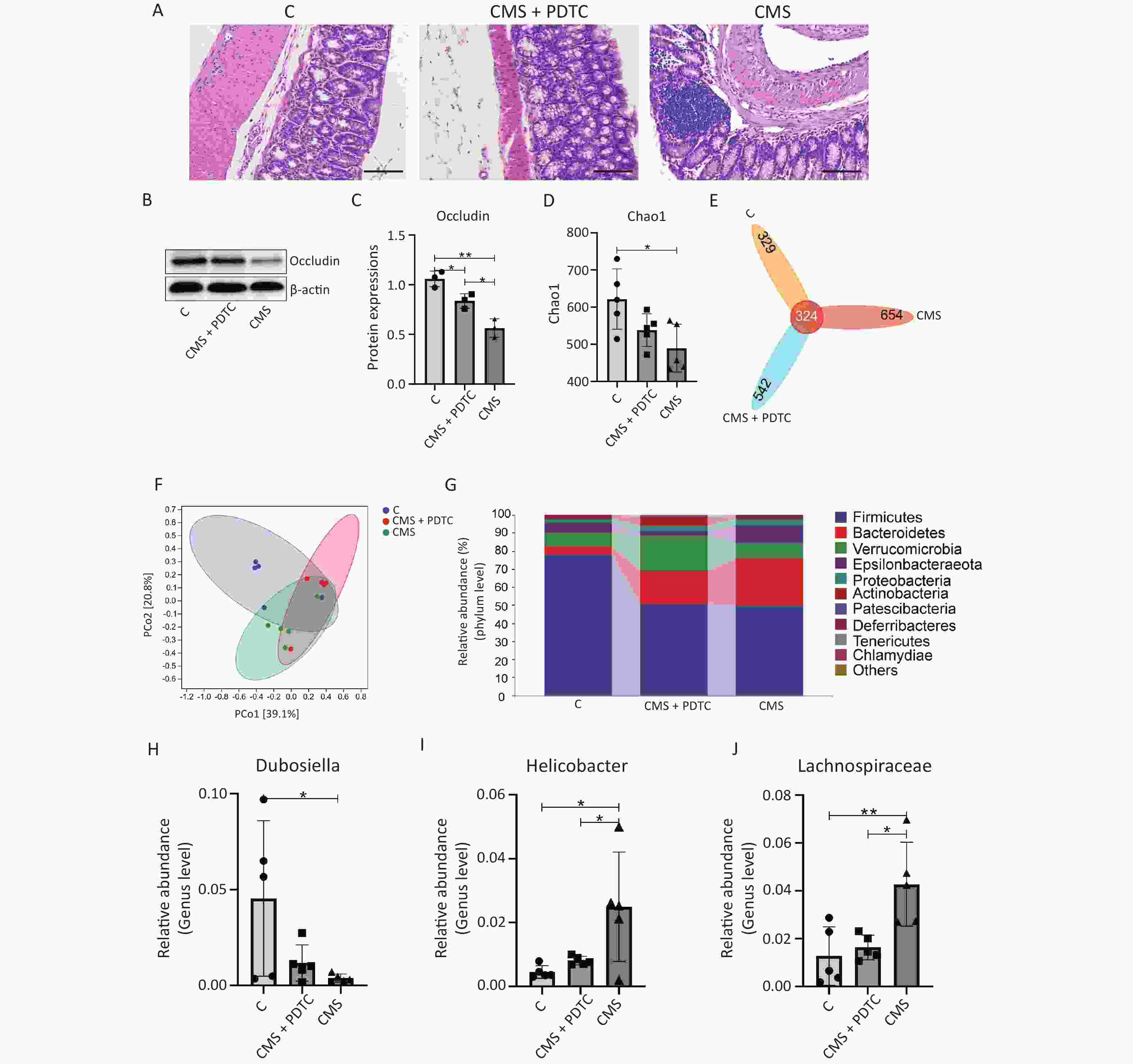
Figure S2. Inhibition of NF-κB pathway attenuated CMS-induced intestinal injury and the changes in gut microbiota. (A) HE staining image of colon tissues (200×). (B-C) Western blotting and the relative quantification analysis of Occludin expression in colon tissues. (D) The Chao1 index. (E) Venn map representation of OTUs. (F) PCoA of β diversity index. (G) Microbial composition at the phylum level. (H-J) The relative abundance of (H) Dubosiella, (I) Akkermansia, (J) Lachnospiraceae_NK4A136_group. C, the normal control group; CMS, the CMS-exposed group; CMS+PDTC, the CMS-exposed group combined the treatment with PDTC. Data were presented as means ± SD (n = 3 / group for intestinal injury analysis, n = 5 / group for the gut microbiota analysis). *P < 0.05, **P < 0.01, no marker indicates no significant difference (P > 0.05).
HTML
Conflict of Interest The authors declare that they have no conflicts of interest.
&These authors contributed equally to this work.
Reference







 Quick Links
Quick Links
 DownLoad:
DownLoad: