-
As the first barrier of the body, the skin is inevitably damaged by radiation during clinical radiotherapy (typically administered at 2–3 Gy per session, for a total of approximately 50–80 Gy to eradicate tumors) or nuclear accidents. Currently, the primary clinical methods for protecting against radiation are limited, and some have serious side effects. 1,2-Propanediol (PPD) is a low-toxicity small-molecule compound that is typically used as a humectant in drug solvents, food additives, pharmaceutical excipients, cosmetics, and electronic cigarettes. Furthermore, PPD can also be used as a co-solvent to enhance drug penetration into the skin. In 1980, both PPD and cysteine were first described to protect against radiation[1]. Correspondingly, in 2019, it was reported that PPD pretreatment effectively promoted hematopoietic stem cell recovery following irradiation[2]. These studies suggest that PPD may be an effective radioprotective agent; however, its protective effect against radiation-induced skin damage has not been reported. In the present study, the protective effects of PPD against X-ray-induced radiation in human epidermal cells were assessed.
Human skin keratinocyte (HaCaT) cells were divided into the following groups: control, irradiation (IR), irradiation + 200 mmol/L PPD (IR + 200 mmol/L), irradiation + 250 mmol/L PPD (IR + 250 mmol/L), and irradiation + 300 mmol/L PPD (IR + 300 mmol/L). The cells were pretreated with varying concentrations of PPD for 2 h and then irradiated with a 10 Gy dose at a rate of 1.375 Gy/min with an X-ray. Thereafter, the cells were immediately cultured in fresh complete medium, and cell proliferation was measured at 0, 24, 48, and 72 h after irradiation. Antioxidative stress products, mRNA expression levels of IL-1β, IL-6, and TNF-α, and the protein expression levels of the associated signaling pathway molecules were measured 24 h after irradiation. The protein levels of IL-1β, IL-6, and TNF-α in the cell culture were determined at 24 and 48 h after irradiation. Apoptosis and cell migration were measured at 48 h after irradiation.
The HaCaT cells were seeded at a density of 5 × 103 cells/well in 96-well plates and cultured overnight. After treatment, cell viability was measured using a cell counting kit-8 (CCK-8) assay (Bimake, China), according to the manufacturer’s instructions. Absorbance was measured at 450 nm using a UV spectrophotometer (Thermo, US), and the viability of the HaCaT cells was calculated using the following formula: Cellular viability = Experimental groupOD450 - Blank groupOD450.
Apoptosis was measured using the phycoerythrin (PE)-Annexin V/7-Aminoactinomycin D (7-AAD) Apoptosis Detection Kit (BD Biosciences). The treated HaCaT cells were collected and incubated with 100 µL of binding buffer containing 5 µL of PE-Annexin-V and 7-AAD for 15 min at room temperature, protected from light. Finally, the apoptosis rate was determined using a FACS Calibur flow cytometer (Becton Dickinson Corporation, USA).
Cell migration was assessed using a wound healing assay. HaCaT cells were grown in 6-well plates for 24 h, and then a scratch was made in the center of the cell monolayer using a sterile 10 µL disposable tip. Thereafter, the culture supernatant was replaced with fresh medium, and cell migration was observed and photographed under a microscope at 0 and 48 h.
Total RNA was extracted using an RNA-Quick Purification Kit (Yishan Biotechnology, China), and the concentration and quality of RNA were determined using a NanoDrop One spectrophotometer (Thermo Fisher Scientific Inc., USA). cDNA synthesis was performed using the Fast All-in-One RT Kit (Yishan Biotechnology, China). Real-time PCR was performed using UNICON® Universal Blue qPCR SYBR Green Master Mix (Yeasen Biotechnology Co., Ltd., China). All data were normalized to GAPDH as an internal control. The primer sequences of GAPDH, IL-1β, IL-6, and TNF-α genes are presented in Table 1.
Genes Sequences (5’-3’) Human GAPDH Forward: 5’-GGTTGTCTCCTGCGACTTCA-3’
Reverse: 5’-TGGTCCAGGGTTTCTTACTCC-3’Human IL-1β Forward: 5’-AGCTACGAATCTCCGACCAC-3’
Reverse: 5’-CGTTATCCCATGTGTCGAAGAA-3’Human IL-6 Forward: 5’-CCTGAACCTTCCAAAGATGGC-3’
Reverse: 5’-TTCACCAGGCAAGTCTCCTCA-3’Human TNF-α Forward: 5’-CCTCTCTCTAATCAGCCCTCTG-3’
Reverse: 5’-GAGGACCTGGGAGTAGATGAG-3’Table 1. mRNA Primer Sequences
The treated cells in 6-well plates were washed twice with PBS, and incubated with a 2′,7′-dichlorofluorescein diacetate (DCFH-DA)-specific fluorescent probe (10 µmol/L) for 20 min according to the manufacturer's instructions (Beyotime Institute of Biotechnology, China). The fluorescence of reactive oxygen species (ROS) was observed using a fluorescence microscope and photographed (Olympus Optical Co., Ltd.).
The cell culture supernatants were collected at 24 h after radiation. Cellular malondialdehyde (MDA) and glutathione (GSH) levels, as well as dismutase (SOD) activity, were determined using a colorimetric method using commercial assay kits (Beyotime Institute of Biotechnology, China).
The treated cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (Beyotime Institute of Biotechnology, China) with phenylmethanesulfonyl fluoride (PMSF; Beijing ComWin Biotech Co. Ltd., China) and protease inhibitors (Beyotime Institute of Biotechnology, China). Proteins were subsequently quantified using a bicinchoninic acid (BCA) Protein Assay Kit (Beyotime Institute of Biotechnology, Shanghai, China). Following this, 20 µg of lysate proteins were separated by 10% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes (Merck Millipore KGaA, Darmstadt, Germany). The membranes were blocked with a rapid blocking buffer (Yangguang Biotech, USA) for 1 h, and incubated overnight with the corresponding primary antibodies. The membranes were then incubated at room temperature for 1 h with horseradish peroxidase (HRP)-conjugated secondary antibodies. Immunoreactive proteins were measured using an electrochemiluminescence-enhanced (ECL) Kit Chemiluminescent Substrate (AB clonal Technology Co. Ltd., China) and ChemiDoc™ MP Imaging System (Bio-Rad Laboratories, USA). The following primary antibodies were used: β-actin (Proteintech Group Inc., USA), nuclear factor-erythroid 2 related factor 2 (NRF2) (Proteintech Group Inc., USA), and NAD(P)H:quinoneoxidoreductase 1 (NQO1) (Abcam, USA).
The experimental data were expressed as x ± s, and statistical analysis was performed using GraphPad Prism 8. One-way ANOVA was used for comparisons between multiple groups, and the unpaired t-test was used for comparisons between two groups. Differences were considered statistically significant at P < 0.05.
Radiation-induced skin injury (RISI) is a common complication of radiotherapy, and effective therapeutic measures other than conservative treatments are currently lacking[3,4]. In this study, we demonstrated for the first time the protective effect of PPD against RISI in a radiation injury model using HaCaT cells.
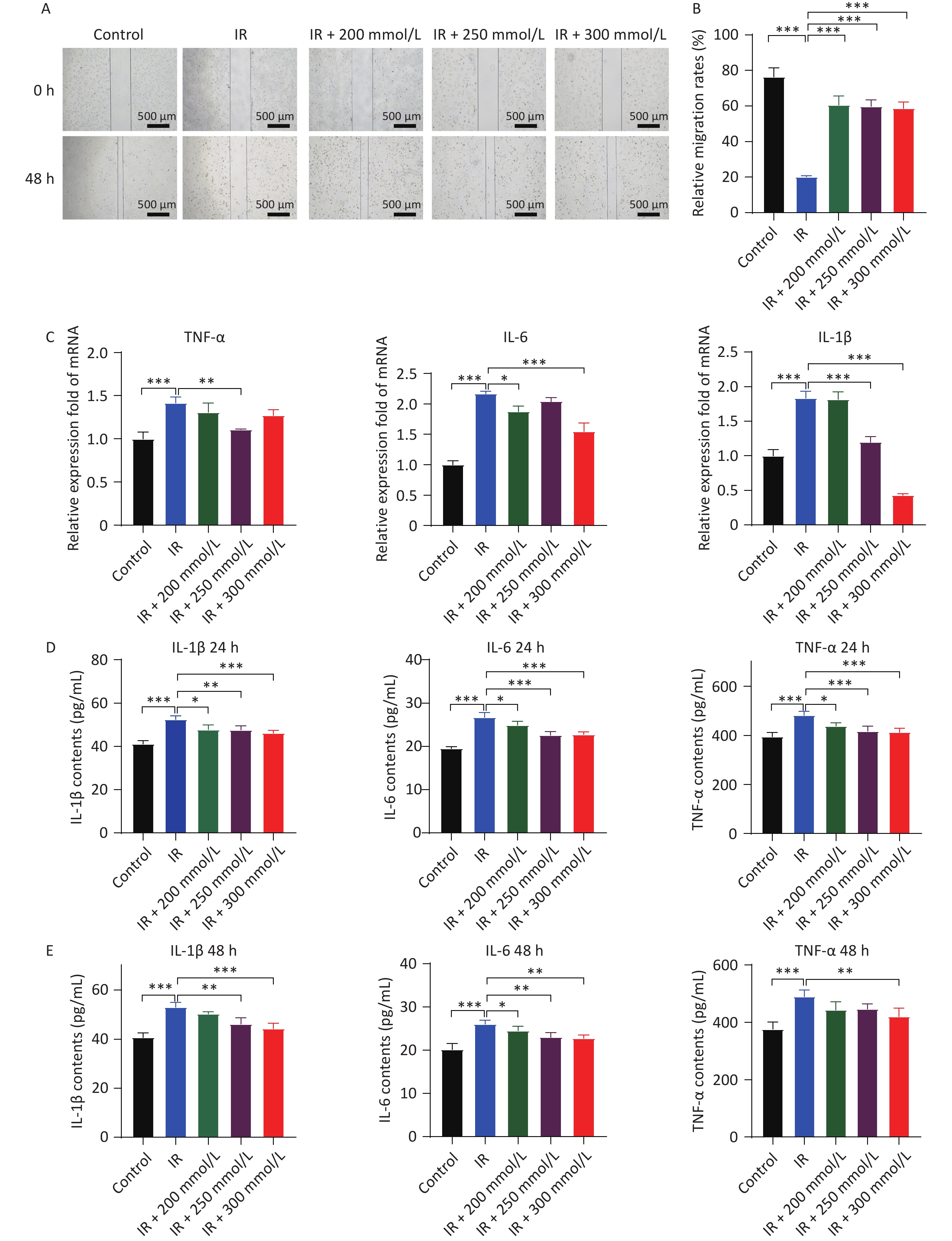
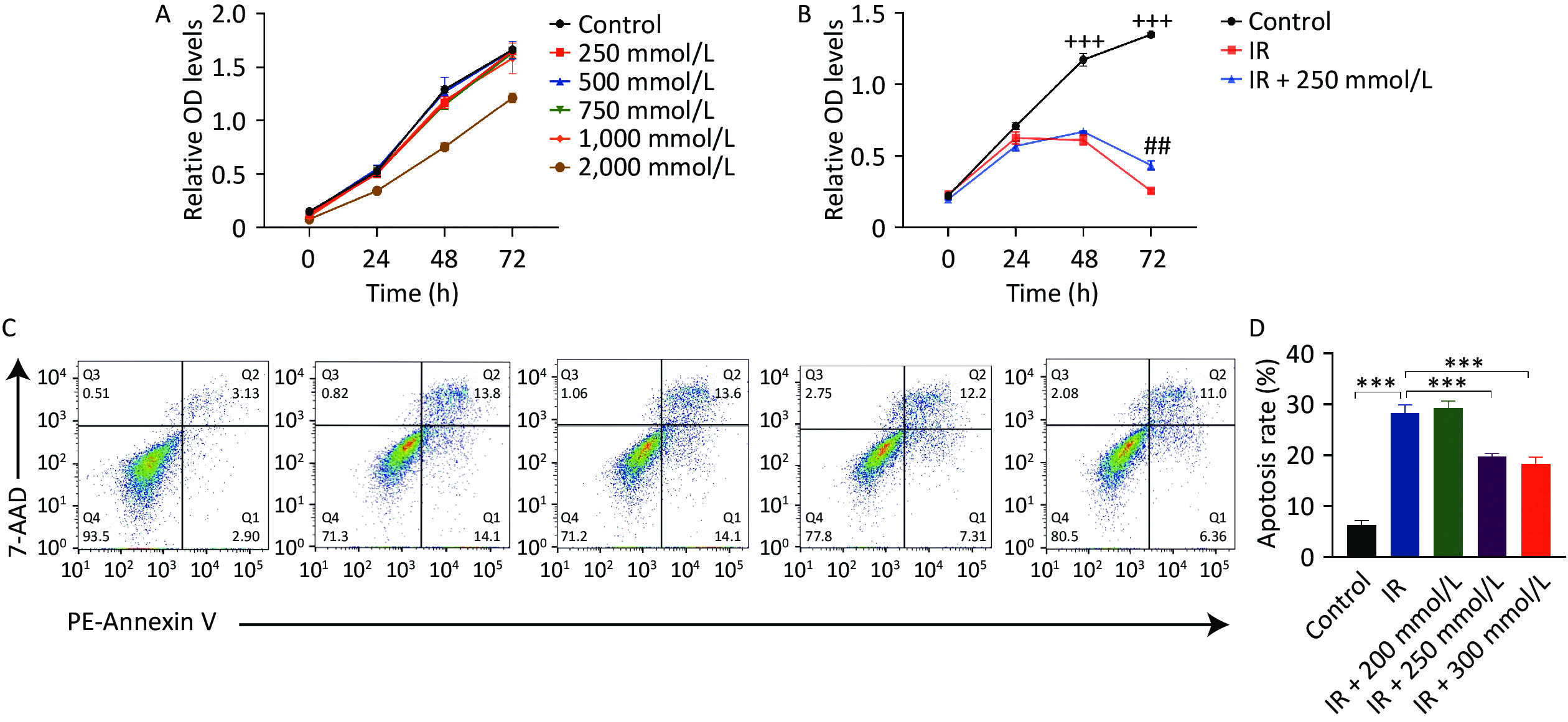
First, the cytotoxic effects of PPD on HaCaT cells were assessed after treating the cells with various concentrations of PPD for 2 h. Treatment with PPD below 1,000 mmol/L did not affect HaCaT cell proliferation (Figure 1A), indicating that PPD had no significant toxic effects on HaCaT cells at concentrations below 1,000 mmol/L. Radiation induces cell cycle arrest in the G2/M phase, impairs cell proliferation and migration, and triggers apoptosis[4]. Our results also demonstrated that the growth of HaCaT cells was significantly inhibited, and apoptosis was induced following irradiation with 10 Gy of X-rays. However, pretreatment of HaCaT cells with PPD at a concentration of 200–300 mmol/L alleviated the suppression of cell proliferation and mitigated apoptosis caused by irradiation (Figure 1B, 1C, and 1D). Moreover, PPD pretreatment significantly improved the cell migration ability of irradiated HaCaT cells (Figure 2A, 2B).
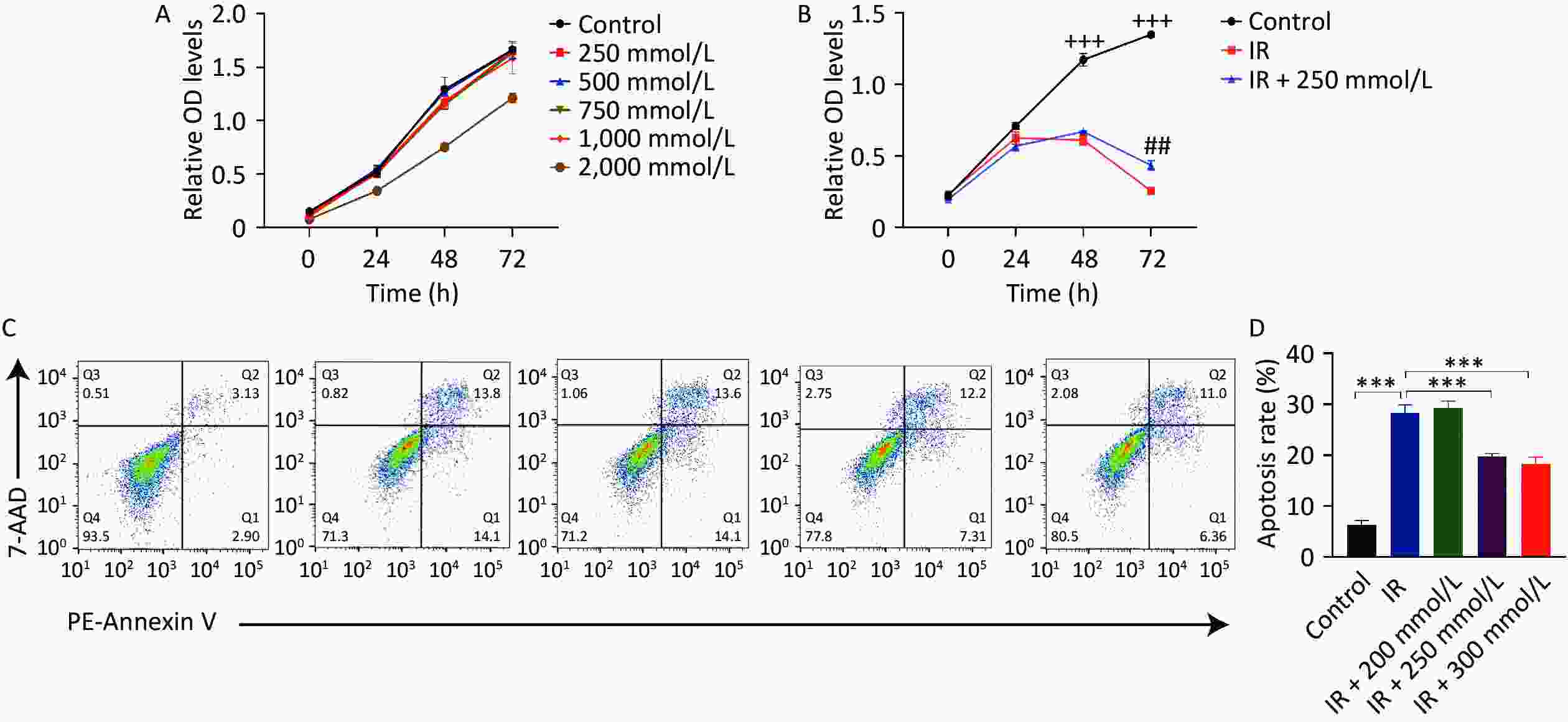
Figure 1. PPD pretreatment promoted the proliferation and inhibited the apoptosis of irradiated HaCaT cells. (A) The proliferation viability of HaCaT cells assayed by CCK-8, after incubation with PPD at 250 mmol/L–2,000 mmol/L for 2 h. (B) HaCaT cells were pretreated with PPD for 2 h and then irradiated with 10 Gy. Cell proliferation was detected by CCK-8 assay at 0 h, 24 h, 48 h, and 72 h. (C) Apoptosis was measured by flow cytometry with PE- Annexin V /7-AAD double staining at 48 h after irradiation. (D) The percentage of total apoptotic cells was calculated. Data were from at least three repeated experiments or the perform in triplicate, and were statistically analyzed by ANOVA (+++P < 0.001, compared with IR group and IR + 250 mmol/L group; ##P < 0.01, compared with IR + 250 mmol/L group; ***P < 0.001. PPD, 1,2-Propanediol; CCK-8, cell counting kit-8; PE, Phycoerythrin; 7-AAD, 7-Aminoactinomycin D).

Figure 2. PPD pretreatment promoted the migration and suppressed the inflammatory response of irradiated HaCaT cells. (A) HaCaT cells were preincubated with PPD and the migration ability of HaCaT cells was analyzed by scratch wound assay at 0 h and 48 h after irradiation (Scale bars: 500 µm). (B) The statistical result of HaCaT cell migration. (C) HaCaT cells were preincubated with PPD and then the mRNA levels of IL-1β, IL-6 and TNF-α were measured with qRT-PCR method at 24 h after irradiation. The cDNA of GAPDH was used as an internal control. (D and E) The protein contents of IL-1β, IL-6 and TNF-α in the cell culture were detected with ELISA kit at 24 h and 48 h after irradiation. All Data from at least three repeated experiments or from the perform in triplicate, and were statistically analyzed by ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001. PPD, 1,2-Propanediol; ELISA, enzyme-linked immunosorbent assay).
Cell damage and excessive ROS generation induced by irradiation stimulate the overproduction of inflammatory factors. Moderate inflammatory factor levels can promote the repair of damaged cells or organisms, whereas excessive inflammatory factor levels may cause further damage. Our results showed that the mRNA expression of the primary cell-secreted inflammatory factors, namely IL-1β, IL-6, and TNF-α, was significantly increased after HaCaT cells were irradiated, and PPD pretreatment significantly inhibited the over-expression of these factors (Figure 2C). Furthermore, these changes were further confirmed by their protein expression results (Figure 2D, 2E). These results suggested that PPD pretreatment dose-dependently attenuated the over-secretion of inflammatory factors following irradiation.
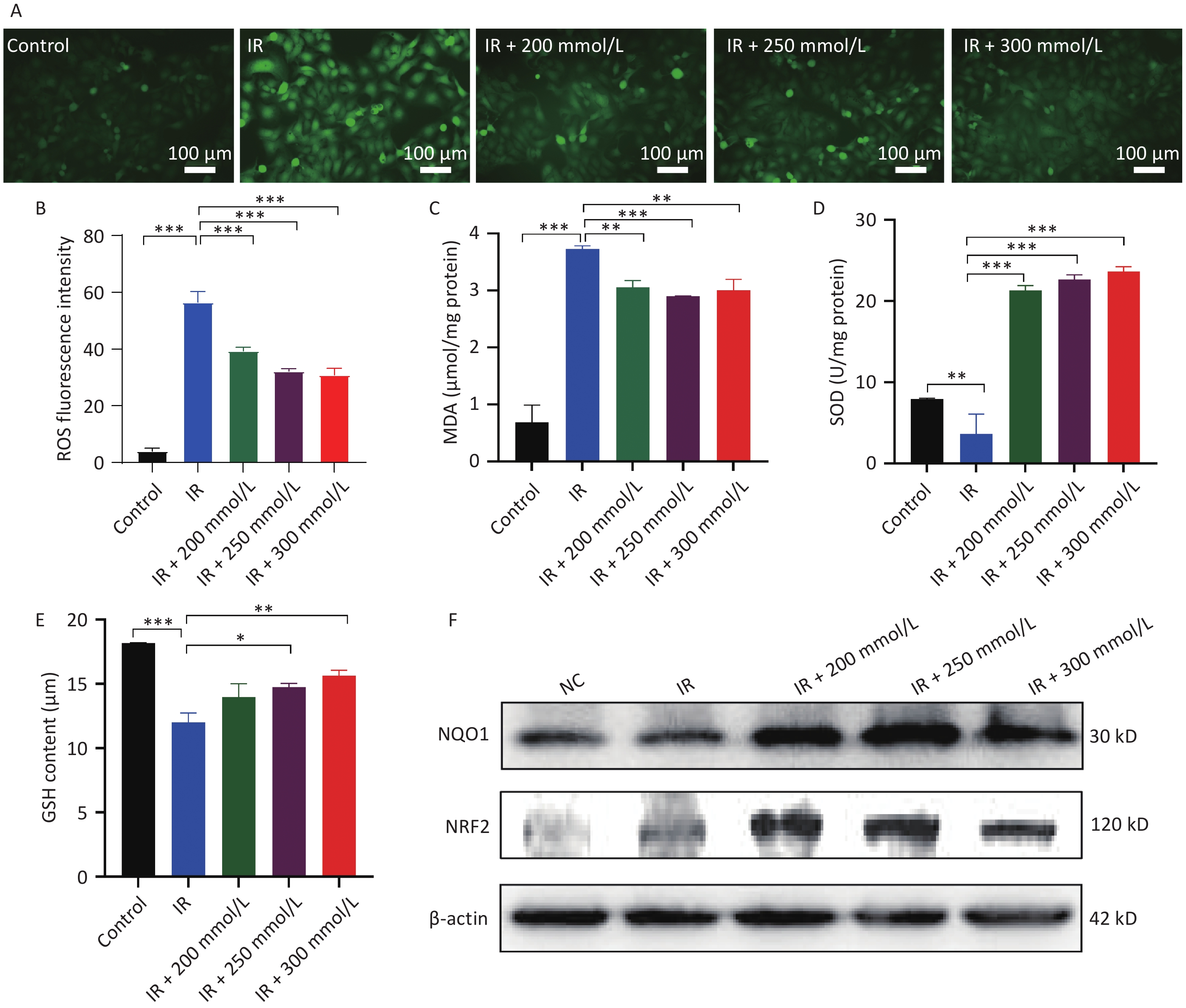
ROS are byproducts of oxygen metabolism and are involved in various physiological functions, including cell signaling, cell division, and the defense of the organism. There is a dynamic balance between ROS and antioxidants[5]. When cells or organisms are exposed to radiation, ROS production is significantly increased, while simultaneously ROS elimination is inhibited, thereby disrupting this balance. Excess ROS irreversibly damages many biological macromolecules, causing lipid peroxidation and the production of MDA, a biomarker of oxidative stress damage, resulting in cell apoptosis[6]. In this study, intracellular ROS and MDA levels were significantly elevated after irradiation, whereas pretreatment with PPD significantly inhibited the increase in intracellular ROS and MDA levels (Figure 3A, 3B, and 3C).
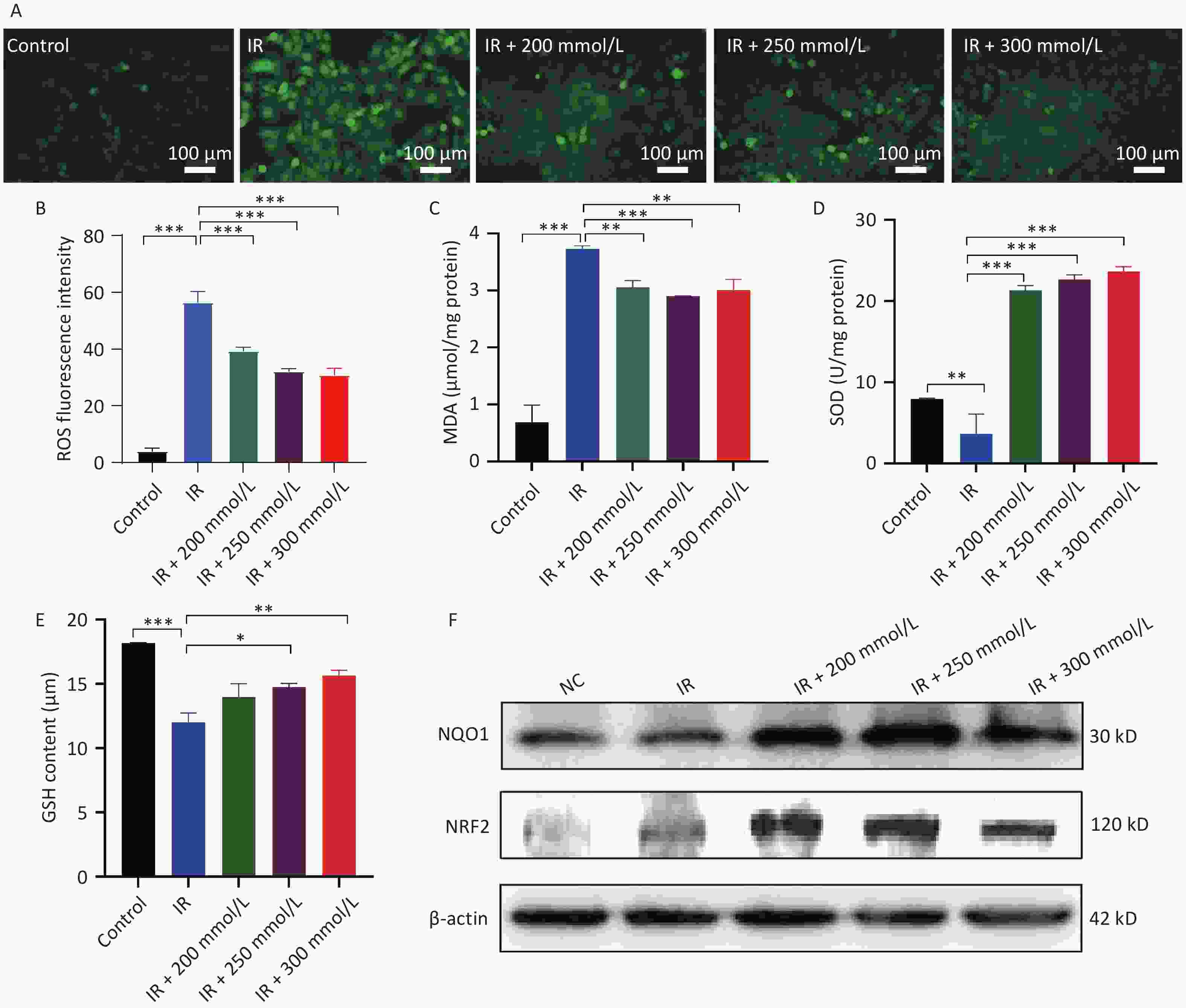
Figure 3. The effect of PPD pretreatment on the oxidative stress reaction in irradiated HaCaT cells. HaCaT cells were preincubated with PPD and then the analysis was performed at 24 h after irradiation. (A) The intracellular ROS was detected by DCFH-DA fluorescent probe (Scale bars: 100 µm). (B) The statistical result of ROS. (C, D and E) The content of MDA, the activity of SOD and the content of GSH in HaCaT cells were detected by colorimetry using corresponding kits. (F) NRF2 and NQO1 protein expression levels in HaCaT cells were analyzed by western blot. Data were from at least three repeated experiments or the perform in triplicate, and were statistically analyzed by ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001. PPD, 1,2-Propanediol; ROS, reactive oxygen species; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione; Nrf2, nuclear factor-erythroid 2 related factor 2; NQO1, NAD(P)H: quinoneoxidoreductase 1).
To maintain cellular redox homeostasis and counteract the harmful effects of ROS, cells possess an intracellular antioxidant system, which consists mainly of enzymes that can decompose ROS as well as substances such as GSH that can neutralize and lower ROS levels. SOD and its mimetics reduce ROS levels, decrease apoptosis, and alleviate radiation-induced skin damage[7]. Some antioxidants, such as ascorbic acid, vitamin E, and coenzyme Q10, also protect against radiation-induced skin damage by scavenging free radicals and stimulating the synthesis of GSH and SOD in cells[8]. Radiation not only generates excessive ROS but also inhibits the intracellular ROS scavenging system, leading to a substantial accumulation of ROS. Our results showed that the antioxidant system in HaCaT cells was disrupted, and the synthesis of SOD and GSH was inhibited. In contrast, PPD pretreatment significantly restored SOD activity and GSH synthesis (Figure 3D, 3E). Collectively, these results suggest that PPD alleviates oxidative stress in HaCaT cells by activating the cellular antioxidant system and scavenging free radicals. NRF2 is a key regulator of anti-oxidative stress that binds to antioxidant response elements, thereby inducing the expression of various antioxidant stress/detoxification enzymes to activate the cellular protective defense system[9]. NQO-1 is an important downstream antioxidant gene in the NRF2 signaling pathway that attenuates oxidative stress and inhibits the progression of radiation damage. Radiation-induced skin damage is inhibited by the activation of NRF2 signaling and its downstream genes[10]. In the present study, we demonstrated for the first time that PPD pretreatment increases the expression of NRF2 and NQO1 after irradiation (Figure 3F). It was previously reported that the activation of the NRF2 pathway inhibited the NF-κB signaling pathway and downregulated pro-inflammatory factor gene expression[10]. Similarly, in the present study, PPD pretreatment suppressed the expression of inflammatory factors (Figure 2C, 2D, and 2E), which may be linked to the activation of the NRF2 signaling pathway.
In summary, these findings demonstrated that PPD pretreatment effectively protects epidermal cells from radiation damage by activating the NRF2 signaling pathway, promoting the synthesis of antioxidant enzymes and antioxidants, eliminating excess free radicals, and reducing the secretion of inflammatory factors. PPD has the potential to be developed as a safe, low-toxicity protection agent against skin radiation. However, these results warrant further validation through animal experiments, and the mechanism of action of PPD needs to be further confirmed through NRF2 knockdown experiments.
1,2-Propanediol Attenuates Radiation-Induced HaCaT Cell Injury by Inhibiting Oxidative Stress
doi: 10.3967/bes2024.189
- Received Date: 2024-04-02
- Accepted Date: 2024-10-15
The authors declare that they have no competing financial interests or personal relationships that may have influenced the work reported in this study.
&These authors contributed equally to this work.
| Citation: | Yun Liu, Boyuan Ren, Yubin Liu, Qiang Li, Jiayan Jin, Chu-tse Wu, Gangqiao Zhou, Jide Jin. 1,2-Propanediol Attenuates Radiation-Induced HaCaT Cell Injury by Inhibiting Oxidative Stress[J]. Biomedical and Environmental Sciences, 2024, 37(12): 1447-1452. doi: 10.3967/bes2024.189 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: