-
Iodine is a trace element critical for the biosynthesis of thyroid hormones, including triiodothyronine (T3) and thyroxine (T4), which strongly affect an extended range of biochemical functions, such as cellular metabolism, normal growth, and brain development[1,2]. Historically, iodine deficiency has puzzled vulnerable groups of the population and its adverse consequences have been well documented[3]. However, the current concern regarding iodine intake is gradually changing from deficiency to excess with the enforcement of the universal salt iodization (USI) policy because excess iodine intake may precipitate hyperthyroidism, hypothyroidism, goiter, and thyroid autoimmunity[4]. Both iodine deficiency and excess can potentially threaten human health, and the relationship between iodine intake and thyroid disease appears as a U-shaped curve. From the public health perspective, suitable iodine intake is vital for maintaining good health.
As previously reported by the World Health Organization (WHO)[5], approximately 2 billion people worldwide had been deeply suffering from the troubles of iodine deficiency. Thus, a dietary iodine reference intake (DRIs) is recommended to eliminate iodine deficiency disorder (IDDs). Adequate iodine intake was suggested as 90 μg/day for children aged 0–5 years, 120 μg/day for children aged 6–12 years, 150 μg/day for aged over 12 years and adults, and 250 μg/day for pregnancy and lactation[6,7]. The Chinese government also began implementing the USI policy in 1995, and 97.8% of the counties eliminated IDDs nationwide in 2019[8,9]. Currently, a growing number of iodine-rich foods are emerging in the market, such as marine products, fish, dairy, and eggs, and are becoming excellent iodine sources in addition to iodized salt. Although most individuals tolerate excessive iodine intake, cases of iodine-associated thyroid disorders have been increasingly reported[10-12]. Thyroid disorders are susceptible to increased iodine intake in individuals previously exposed to iodine deficiency, even slightly above the physiological needs[13]. Moreover, the tendency seems to be exacerbated in those living in Asian countries or regions[12,14,15]. This is the main reason why iodine DRIs are currently mentioned when facing an increasing number of reported thyroid disorders.
Balance studies are the most widely used methods for assessing iodine requirements and formulating iodine DRIs in the population[16,17]. However, it should be noted that the zero iodine balance in the body can be affected by the baseline iodine status[18]. Three modified balance studies have been conducted to explore iodine requirements in Chinese adults[19-22]. However, the modified balance study still has a high time cost and logistical challenges. Meanwhile, we further observed that 24 h urinary iodine excretion (24 h UIE) presented a progressive decline as low-iodine experimental diets were offered. In essence, the 24 h UIE change can reflect the integrated functioning of the body’s iodine metabolism; thus, the simulation of the 24 h UIE change has important implications.
Therefore, the current study aimed to re-explore the iodine requirement using the obligatory iodine loss hypothesis based on three previous modified balance studies in Shenzhen, Yinchuan, and Changzhi, and to further support the amendment of iodine DRIs in Chinese adults, including the Estimated Average Requirement (EAR) and Recommended Nutrient Intake (RNI).
-
The current study aimed to reanalyze the available data derived from three modified balance studies in Chinese adults conducted in the southern metropolis of Shenzhen and the northern cities of Yinchuan and Changzhi[19-22]. Some obvious dietary differences include a high intake of wheat, tubers, eggs, and liquor in the northern regions and a high intake of rice, vegetables, meat, poultry, and fish in the southern regions. All participants were recruited according to inclusion and exclusion criteria. They were 18–24 years old, with a body mass index of 18–25 kg/m2, normal thyroid, liver, and kidney function, without overt constipation, no use of iodine-containing drugs and supplements or algae products, or exposure to iodinated contrast media within the last 6 months. A smart questionnaire was used to collect information on disease history and dietary and living habits, including consumption of iodized salt and regular fish and seafood intake. Physical examinations were performed by professional medical workers at baseline and at the end of each experiment. All participants were required to comply with the study requirements if they consented to participate. Signed informed consent was obtained from each participant after the nature and protocol of the study were explained. The study protocol was established according to the guidelines of the Declaration of Helsinki of the World Medical Association[23].
-
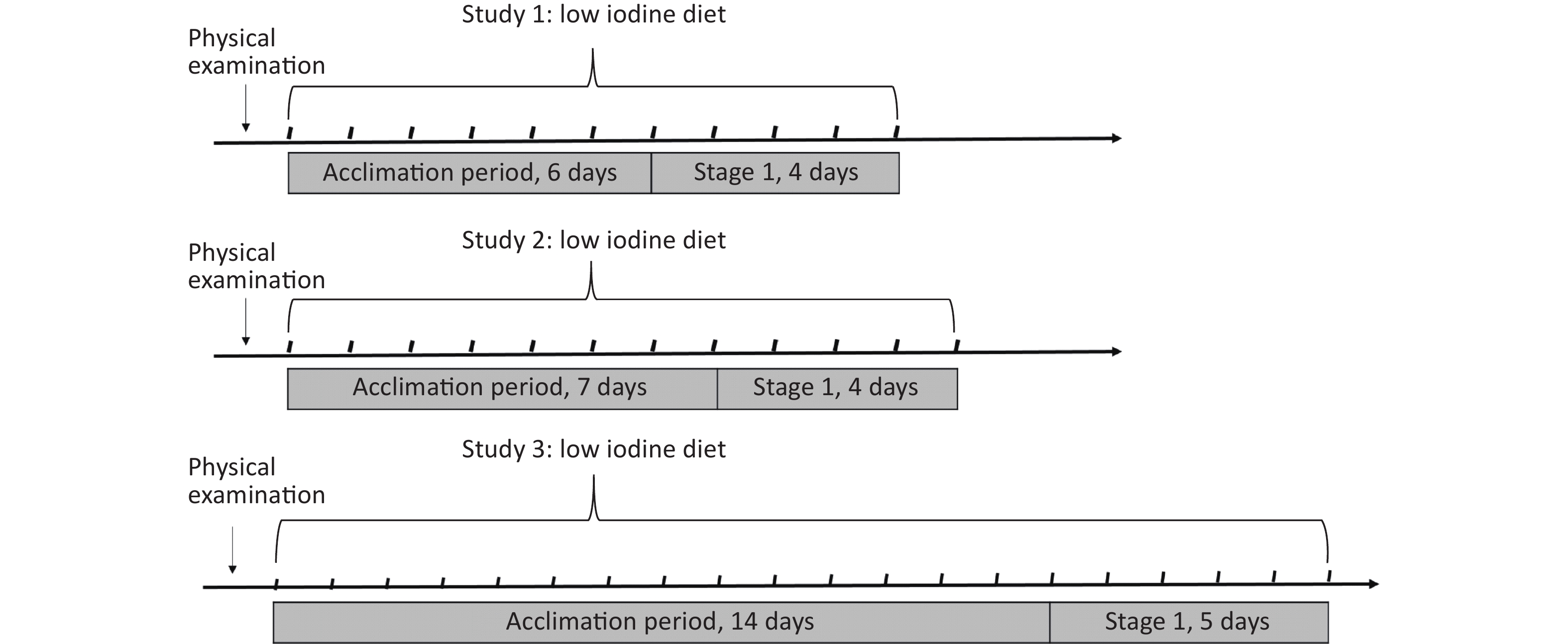
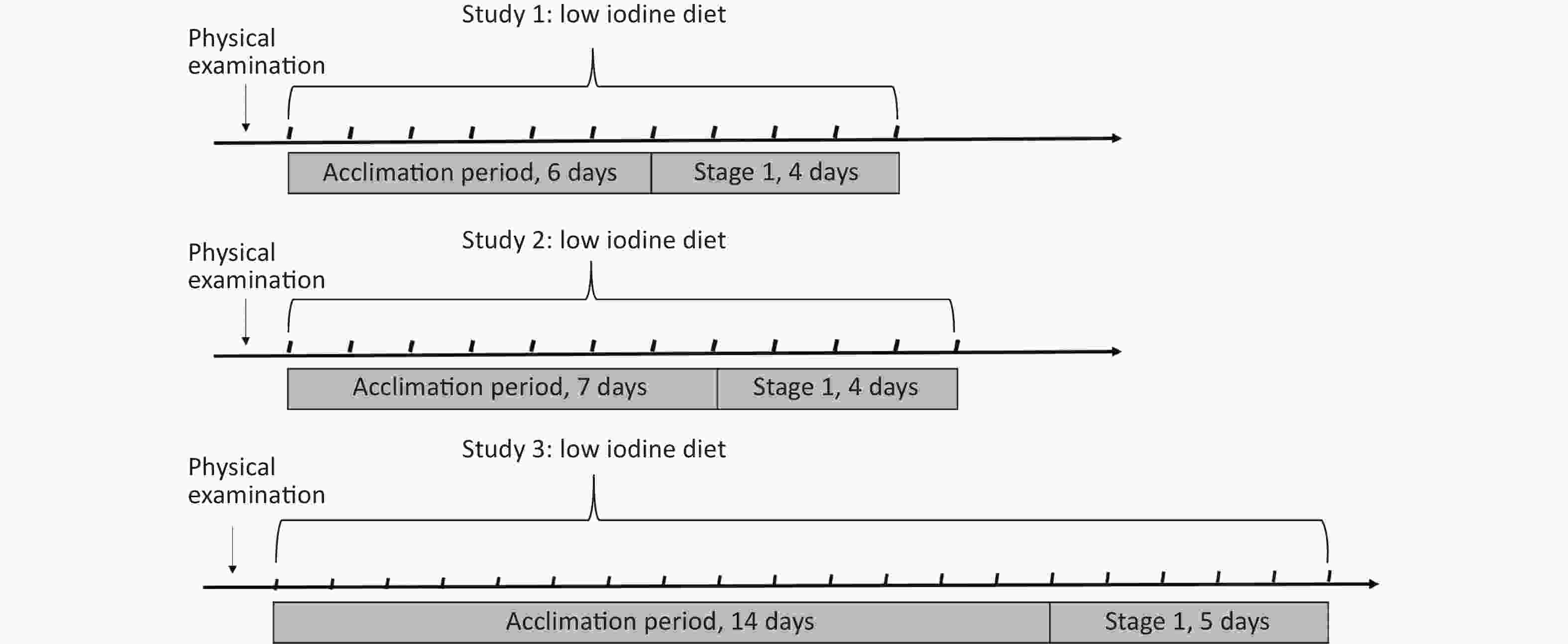
The schedules of the three balance studies were similar and divided into two sections: accumulation and supplementation. The former aims to acclimatize to a low-iodine experimental diet to reduce the overstored iodine in the thyroid, whereas the latter aims to elevate iodine provision by adding milk or eggs. In this study, the available data are derived primarily from the previous section. As previously stated[19-22], the experimental diets were designed in advance according to balanced meals to avoid the use of condiments and foods with high iodine contents, including iodized salt, kelp, seaweed, and marine products. Throughout the study, food servings were uniformly prepared using non-iodized salt and purified drinking water.
There was a significant difference in the accumulation duration between 6 and 14 days. In Study 1, 37 healthy adults (14 males, and 23 females) aged 20.2 years were recruited from Shenzhen in 2018, and the acclimation period was set to six days. In Study 2, 60 subjects (30 males, and 30 females) aged 20.3 years were selected from Yinchuan in June 2017 and 7 days were assigned for adaptation to a low-iodine diet. Study 3, 74 subjects (38 males, and 36 females) aged 19.9 years were included in Changzhi in 2018−2019, which selected the 14-days as the initial acclimation period, as shown in Figure 1.
-
On physical examination, blood specimens were drawn by venous puncture after fasting for over 8 h. Daily food samples were collected using the duplicate portion method and daily iodine intake was calculated by multiplying the iodine content by the amount of food consumed. The 24 h urine specimens were collected and weighed upon delivery, and the estimated amount of missed urine was recorded. The 24 h urinary iodine excretion (24 h UIE) was calculated based on the iodine concentration and 24 h urine specimen amount. All food and urine specimens were stored in a cryogenic refrigerator at −20 °C and then sent to the laboratory of National Institute for Nutrition and Health (NINH) in Chinese Center for Disease Control and Prevention (China CDC) for analysis.
Serum alanine transaminase (ALT), aspartate aminotransferase (AST), urea, and uric acid were measured by an automatic biochemical analyzer (COBAS INTEGRA® 400 Plus, Roche Diagnostic, Switzerland). Thyroid-stimulating hormone (TSH), free thyroxine (FT4), and free triiodothyronine (FT3) levels were determined using an automated electrochemiluminescence immunoassay (ADVIA Centaur Immunoassay System, Bayer Healthcare, Germany; COBAS E601 analyzer, Roche Diagnostic, Switzerland) according to standard operation procedures. Urinary creatinine (Cr) concentration was measured based on a modified Jaffé procedure using a clinical analyzer (Beckman Instruments, Brea, CA, USA). Measurements were required to satisfy analytical requirements. Urinary iodine concentration (UIC) was determined using quantitative rapid test kits (Conson Biochemicals, China) in daily monitoring and inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7700, Agilent Technologies, USA) in the laboratory[24]. Urine-certified reference materials (CRMs) (GBW 09108 and GBW 09110) were used for quality control during urinary iodine determination. The recoveries ranged between 92.3% and 106.7%, whereas the intra- and inter-day coefficients of variance (CV) ranged from 1.6% to 5.7%.
-
Iodine intake and excretion jointly affect the iodine balance, which involves iodinated substance synthesis and breakdown in vivo. Insufficient iodine intake causes iodine stored in the thyroid to be utilized and reduces iodine excretion, which could be interpreted as an adaptation to low iodine intake. Iodine excretion rapidly decreases during the initial period of low iodine intake and then begins to decline slowly during the iodine accumulation period. From this, the obligatory iodine loss hypothesis holds that the trajectory of iodine excretion is a progressive descent curve, and the inflection point of the curve is considered the iodine balance status. In this case, the iodine excretion at the inflection point is equivalent to the minimum iodine excretion. Thus, if iodine intake could be provided at the inflection point, it would satisfy the physiological requirement for iodine.
-
Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA) and Origin 9.0 (OriginLab, USA) for data analyses and visualization. The Shapiro-Wilk test was used to assess the normal distribution of the data, which are expressed as mean ± SD, while the non-normally distributed data are expressed as the median and range interquartile (IQR). Differences in height, weight, urine volume, 24 h UIC, 24 h UIE, ALT, AST, urea, Cr, uric acid, serum TSH, FT3, and FT4 levels were compared using nonparametric analysis of variance with the Kruskal-Wallis test for multiple comparisons. A P value < 0.05 was considered significant.
In this study, a single exponential equation was used to simulate the trajectory of 24 h UIE change as low-iodine experimental diets were offered, which are listed as follows:
$$ \overline{y}={p}_{1}{e}^{-{p}_{2}*{t}_{s}}+{p}_{3} $$ (1) In this equation, $ \overline{y} $ is the 24 h UIE change with the time of iodine intake from adequate to low level, p1 is the difference in the 24 h UIE from the initial to asymptotic stability, p2 is the fractional rate constant, p3 is the asymptotic value for $ \overline{y} $, $ {t}_{s} $ is the time to the stability of the 24 h UIE.
Although the 24 h UIE only partly reflects the net effect of iodine metabolism, it has been prominently used to assess obligatory iodine loss. Iodine excretion was assumed to be the sum of urinary and fecal iodine losses. In addition, there are two parts to the factorial approach for setting DRIs recommendations: calculation of basic requirements and application of a bioavailability factor. Iodine bioavailability is the combination of iodine absorption, thyroid uptake, and utilization. A correction factor is generally assumed to be 1.4, and is used for the estimation of the iodine RNI from the iodine EAR, considering the bioavailability and variation of iodine requirements in the population.
-
In this study, data on 171 subjects with a mean age of 19−21 years were obtained derived from the three balance studies and included to analyze, and they were severally recruited in Shenzhen, Yinchuan, and Changzhi. As shown in Table 1, the sex ratio was nearly 1:1, which is similar to that in Shenzhen. The median iodine concentrations and excretion of 24 h urine specimens for the subjects were 126 μg/L in Study 1, 318 μg/L in Study 2, and 230 μg/L in Study 3, respectively. The TSH, FT4, and FT3 concentrations were within the normal clinical reference ranges (TSH, 0.35−5.5 μIU/mL; FT3, 3.5−6.5 pmol/L; FT4, 11.5−22.7 pmol/L), though obvious differences were observed in TSH (P < 0.05), FT3 (P < 0.01), and FT4 (P < 0.01). Significant fluctuations were observed in 24 h urine volume (P < 0.01), 24 h UIC (P < 0.01), 24 UIE (P < 0.01), and creatinine (P < 0.01), which might be due to regional and habitational differences. In addition, there were no significant differences in health indicators, including age, height, weight, ALT, AST, urea, and uric acid levels (P > 0.05).
Variables Study 1 Study 2 Study 3 N 37 60 74 Age (year) 20.1 ± 1.0 20.3 ± 0.9 19.9 ± 1.6 Sex (male/female) 14/23 30/30 38/36 Height (cm) 165 ± 9 165 ± 7 167 ± 10 Weight (kg) 56.6 ± 13.2 57.7 ± 9.4 59.1 ± 11.1 Urine Volume (L) 2.0 (1.5–2.4)a 1.4 (1.0–1.8)b 1.0 (0.8–1.3)c 24 h UIC (μg/L) 126 (76–165)a 318 (278–420)b 230 (175–287)c 24 h UIE (μg/day) 214 (113–347)a 448 (279–657)b 220 (169–264)c ALT (U/L) 13.7 ± 8.3 13.1 ± 3.9 12.3 ± 5.7 AST (U/L) 18.7 ± 4.0 21.3 ± 3.8 16.3 ± 3.6 Urea (mmol/L) 3.8 ± 0.9 3.4 ± 0.6 4.2 ± 1.2 Creatinine (μmol/L) 63.8 ± 12.7a 63.6 ± 10.1a 71.8 ± 18.4b Uric acid (mmol/L) 0.35 ± 0.08 0.30 ± 0.07 0.36 ± 0.10 TSH (μIU/mL) 1.9 ± 0.9a 2.4 ± 1.2b 2.4 ± 0.8b FT3 (pmol/L) 5.3 ± 0.9a 2.9 ± 0.4b 5.6 ± 0.4a FT4 (pmol/L) 18.6 ± 2.7a 13.2 ± 1.8b 14.9 ± 3.7b Note. The data were represented in the form of n or mean ± standard deviation. 24 h UIC, 24-hour urinary iodine concentration; 24 h UIE, 24-hour urinary iodine excretion; ALT, alanine transaminase; AST, aspartate aminotransferase; TSH, thyroid-stimulating hormone; FT3, free triiodothyronine; FT4, free thyroxine. Values not sharing the same superscript letter (a–c) denote a significant difference between the same variable of three balance studies. Table 1. The characteristics of subjects at baseline among the three balance studies
-
As shown in Table 2, there was a lack of data on iodine intake, whereas there was a higher level of 24 h UIE at baseline in three balance studies. Overall, the highest 24 h UIE of subjects was observed in Yinchuan (448 μg/day) as compared to that of Shenzhen (214 μg/day) and of Changzhi (220 μg/day), while there was a slight difference in 24 h UIE between male and female, indicating a great difference in their dietary preferences and iodine intakes. During the last 5 days for the experimental diets with an extremely low iodine level, iodine intakes of the subjects were severally kept at 17−26 μg/day in Study 1, 14−23 μg/day in Study 2, and 11−18 μg/day in Study 3, whilst the 24 h UIE were correspondingly dropped to a relatively low stable plateau at 44−65 μg/day in Study 1, 50−59 μg/day in Study 2, and 45−56 μg/day in Study 3. In contrast, there was a visible difference in iodine intake and excretion among the three balance studies. Still, a slight alteration was observed in the last five days in each study.
Variables Day 0 Day 1 Day 2 Day 3 Day 4 Day 5 Study 1 Iodine intake − 26 (22−28) 20 (18−23) 17 (16−20) 17 (16−20) 20 (17−25) 24 h UIE 214 (113−347) 56 (42−82) 54 (46−65) 44 (36−55) 65 (54−91) 46 (41−58) 24 h UIE male 192 (111−329) 70 (65−100) 65 (52−79) 55 (49−62) 67 (57−93) 57 (49−65) 24 h UIE female 216 (128−333) 51 (40−58) 49 (44−55) 39 (34−45) 61 (50−91) 42 (38−48) Study 2 Iodine intake − 18 (16−22) 17 (15−22) 15 (12−18) 23 (19−27) 14 (12−18) 24 h UIE 448 (279−656) 59 (50−72) 53 (42−65) 50 (41−59) 52 (36−66) 51 (38−69) 24 h UIE male 557 (424−818) 58 (50−71) 52 (41−64) 50 (40−59) 51 (36−64) 63 (52−81) 24 h UIE female 341 (207−465) 56 (44−66) 44 (39−58) 43 (35−54) 38 (31−55) 40 (33−48) Study 3 Iodine intake − 11 (9−15) 11 (8−13) 18 (14−21) 18 (9−24) 16 (12−18) 24 h UIE 220 (169−264) 49 (36−58) 45 (36−61) 48 (35−56) 52 (40−66) 56 (43−72) 24 h UIE male 208 (158−256) 43 (34−54) 39 (35−50) 44 (33−58) 44 (34−58) 43 (32−54) 24 h UIE female 230 (184−310) 55 (43−61) 57 (44−69) 49 (39−54) 61 (47−70) 44 (39−51) Note. The data were expressed as the median and range interquartile (IQR). S1, means the study in Shenzhen; S2, means the study in Yinchuan; S3, means the study in Changzhi; Day 0, means the data at baseline; Day 1−Day 5, means the data at last 5 days in the period with low iodine provision, 24 h UIE, 24-hour urinary iodine excretion. Table 2. Iodine intake and excretion at baseline and the last 5 days for the experimental diets from the three balance studies (μg/day)
-
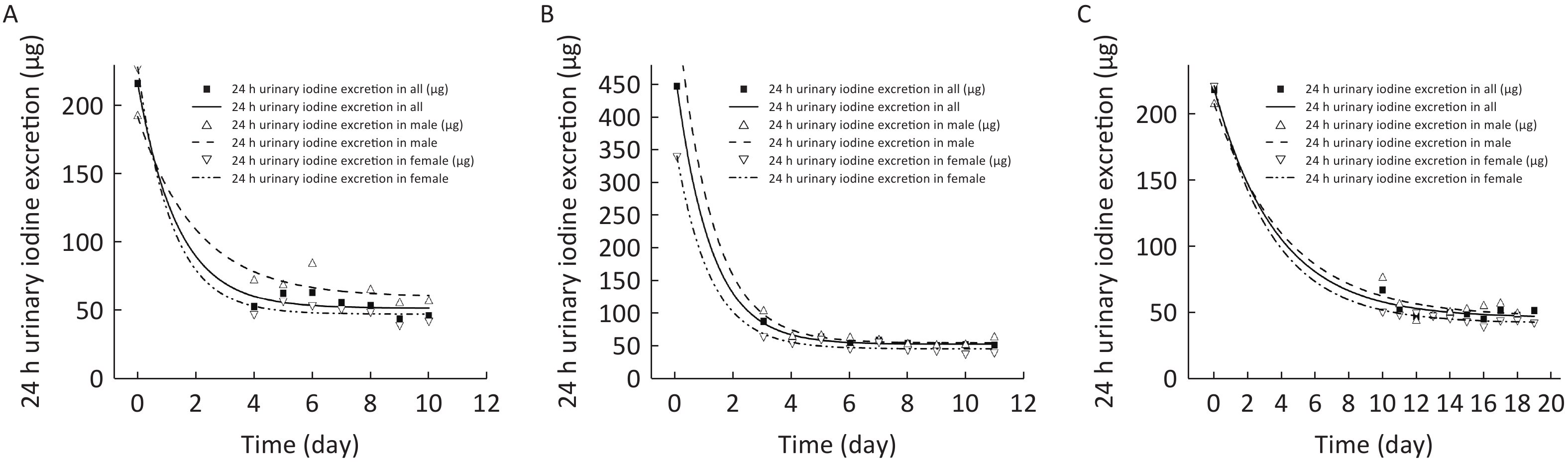
In order to simulate the dynamic trajectory of the 24 h UIE change as the low-iodine experimental diets were offered, a single exponential model was employed to estimate the minimum iodine requirement according to the obligatory iodine loss hypothesis, which was proposed based on the derivation of the obligatory nitrogen loss. As illustrated in Figure 2 (A, B, C), the trajectory of the 24 h UIE change progressively approached the asymptote of the curve. As shown in Table 3, although there was an obvious difference in the iodine status at baseline among the three balance studies, the minimum iodine excretion (p3) were severally sustained at 51.5 μg/day for Study 1, 52.3 μg/day for Study 2, and 45.6 μg/day for Study 3. Here, a trivial difference might be due to the similar minimum iodine requirements in young adults. In addition, there was also a particular difference in the estimation of obligatory iodine loss between males and females among the three balance studies (60.0 vs. 47.1 μg/day in Study 1, 54.3 vs. 45.3 μg/day in Study 2, and 46.1 vs. 41.8 μg/day in Study 3), which might be due to the physiological differences.
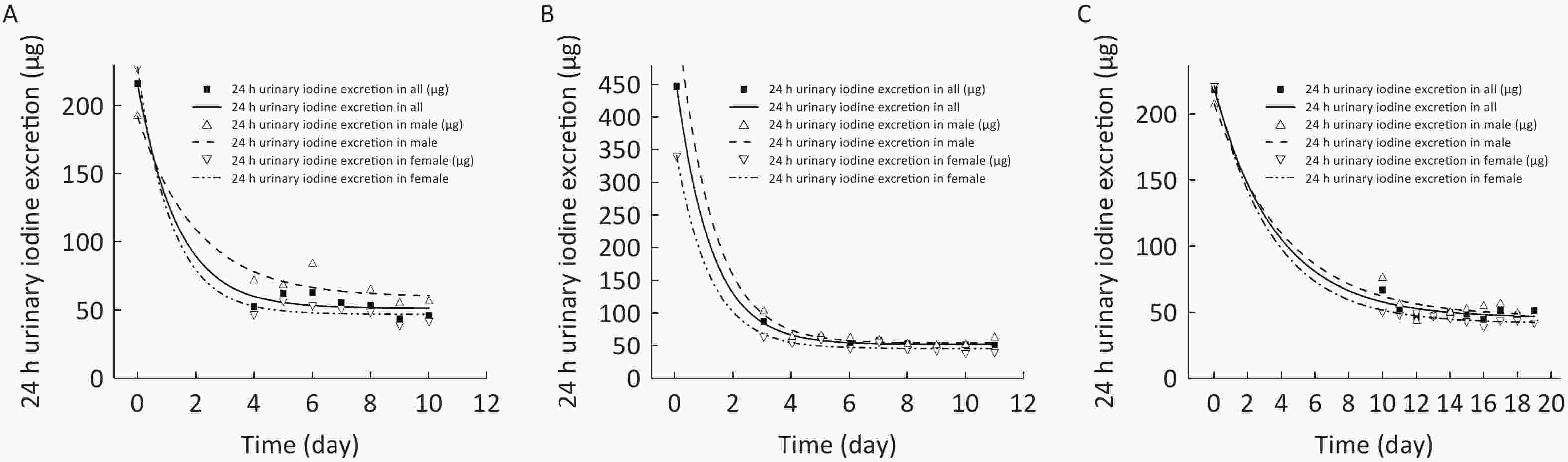
Figure 2. Estimation for obligatory iodine loss for Chinese adults in in Shenzhen (A), Yinchuan (B), and Changzhi (C).
Parameters Study 1 Study 2 Study 3 Male Female All Male Female All Male Female All p1 131.7 180.9 164.6 502.6 295.0 395.3 162.0 180.1 173.6 p2 64.8 155.0 119.9 409.5 251.2 330.0 34.3 48.0 42.2 p3 60.0 47.1 51.5 54.3 45.3 52.3 46.1 41.8 45.6 Note. p1, the difference between iodine excretion at baseline and the asymptote of the curve (μg/day); p2, the rate of change of iodine excretion; p3, iodine excretion at the asymptote of curve (μg/day). Table 3. Statistical and estimated parameters for subjects by sex among the three balance studies
In this study, obligatory iodine loss was estimated using a mathematical model to fit dynamic iodine excretion. In theory, nearly 90% of the daily consumed iodine is excreted in urine[5], and 10% of the consumed iodine is excreted in feces; thus, obligatory iodine loss can be calculated based on the 24 h UIE and fecal iodine excretion. As shown in Table 4, it was severally estimated as 57, 58, and 51 μg/day, which was equal to the specific iodine EAR. Furthermore, the 20% variable coefficient (CV) of EAR was used to estimate the iodine RNI[25]. The iodine RNI was thus calculated as 80, 81, and 71 μg/day, or expressed as 1.42, 1.41, and 1.20 μg/(day·kg) of body weight.
Variables Sex Obligatory iodine loss (μg) Iodine EAR (μg) Iodine RNI (μg) Each day Per body weight (Kg) Study 1 All 57 57 80 1.42 Male 67 67 93 1.40 Female 52 52 73 1.45 Study 2 All 58 58 81 1.41 Male 60 60 84 1.36 Female 50 50 70 1.32 Study 3 All 51 51 71 1.20 Male 51 51 72 1.11 Female 46 46 65 1.22 Note. Study 1 , means the study in Shenzhen; Study 2, means the study in Yinchuan; Study 3, means the study in Changzhi; EAR, estimated average requirement; RNI, recommended nutrient intake. Table 4. Estimated iodine EAR and RNI from obligatory iodine loss by sex among the three balance studies
-
Many expert organizations have insisted that the derivation of iodine DRIs should be based on balance studies or repletion and depletion studies[26]. Nevertheless, interference by the baseline iodine status, its high time cost, and logistic challenges should be noted. In this study, we reanalyzed the available data of 171 Chinese adults aged 19–21 years derived from three balance studies in Shenzhen, Yinchuan, and Changzhi, according to the obligatory iodine loss hypothesis. The single exponential equations were fitted to delineate the non-linear change of 24 h UIE as the low iodine experimental diets offered (iodine intake 11–26 μg/day) and to further estimate the iodine requirements in Chinese adults. Accordingly, it was severally estimated as 57, 58, and 51 μg/day for the physiological requirement or EAR for iodine, whilst it was further calculated as 80, 81, and 71 μg/day for iodine RNI, or expressed as 1.42, 1.41, and 1.20 μg/(day·kg) of body weight when considering the correction factors.
In this study, the median UIC of subjects ranged from 126 μg/L to 318 μg/L at baseline, indicating that most subjects were at adequate and more than adequate iodine status, and the results were consistent with the findings in the studies[9,27]. However, concerns regarding excessive iodine intake have been increasingly exacerbated[12,28,29]. In this context, three modified iodine balance studies have been conducted to explore the iodine requirements of Chinese adults[19-22]. The physiological requirements of iodine in Chinese adults were finally estimated to be 47.0, 48.0, 52.2, and 63.4 μg/day, respectively. By comparison, these values were relatively close to the corresponding derived results in this study using the single exponential equation according to the obligatory iodine loss hypothesis, such as 57, 58, and 51 μg/day. Furthermore, these derived results were in line with that reported in a previous balance study[16], whereas the derived iodine RNI, such as 80, 81, and 71 μg/day, were significantly lower than the current recommended iodine intakes by the Chinese Nutrition Society (120 μg/day)[30], the European Food Society Authority (EFSA, 150 μg/day), World Health Organization (WHO, 150 μg/day), Food and Agriculture Organization of the United Nations (FAO, 150 μg/day)[31,32], and far below that of healthy Chinese women[33] (154.7 μg/day). This discrepancy may be related to the baseline iodine status of participants. In addition, the study design, based on different assumptions, is also a crucial factor in assessing iodine requirements.
In addition, there was a remarkable difference in the duration of iodine accumulation between the three balance studies. As compared to the former two studies, the latter one in Changzhi had a much longer duration of iodine depletion (6–7 days vs. 14 days) and a lower estimation of iodine requirement (from 45.6 µg/day to 51.5 µg/day), which might indicate that obligatory iodine loss could be further decreased to a more stable plateau if low iodine accumulation is moderately prolonged. In this study, according to the obligatory iodine loss hypothesis, dynamic iodine metabolism was precisely simulated based on the available 24 h UIE data using a single exponential equation. The inflection point of the asymptote curve of 24 h UIE can be regarded as the obligatory iodine loss excreted in the urine when iodine metabolism reaches an equilibrium state. The derived iodine values were equal to the sum of all excreted iodine, including urine and fecal iodine. Hence, fecal iodine should be considered when iodine EAR and RNI are further deduced based on the obligatory iodine loss premise.
This study has several advantages and limitations. First, this study analyzed the available data derived from three previous balance studies according to the obligatory iodine loss hypothesis. Moreover, the estimation of the iodine requirement can be obtained using a single exponential equation with a short iodine accumulation and time cost, and the logistical challenge can be greatly controlled. Second, although three balance studies were conducted in southern and northern regions with different dietary habits, the derived iodine requirements were fairly similar in Chinese adults. The mathematical estimation approach exhibits good robustness. Third, there were noticeable differences in baseline status and iodine accumulation among the three balance studies. Fecal iodine excretion was estimated empirically. Thus, an increase in low iodine accumulation and fecal iodine determination might be a good choice for reducing interference and improving precision in the estimation of iodine requirement in Chinese adults.
In conclusion, throughout the statistical analysis of the available data derived from the previous three balance studies, we obtained the minimum iodine excretion in Chinese adults using the single exponential equation according to the obligatory iodine loss hypothesis, which was severally estimated as 57, 58, and 51 μg/day. Accordingly, it was further calculated as 57, 58, and 51 μg/day for iodine EAR, as well as 80, 81, and 71 μg/day for iodine RNI, or expressed as 1.42, 1.41, and 1.20 μg/(day·kg) of body weight to satisfy the iodine requirement. The obligatory iodine loss hypothesis is an effective tool for exploring iodine requirements. These values provide scientific evidence for the formulation of new iodine DRIs in China and may be warranted to provide a new perspective in nutrient requirement research.
HTML
Subjects
Study Design
Sample Collection and Laboratory Analysis
‘Obligatory Iodine Loss’ Hypothesis
Statistical Analysis
Characteristics of Subjects
Iodine Intake, Excretion, and Balance
Obligatory Iodine Loss and Mathematical Model Estimation
Competing Interests All authors declare no conflict of interest.
Ethics The study was approved by the Ethics Committee of the National Institute for Nutrition and Health (NINH), and registered in Chictr. org. cn (ChiCTR1800014877, ChiCTR1800016184).
&These authors contributed equally to this work.







 Quick Links
Quick Links
 DownLoad:
DownLoad: