-
Life expectancy, a fundamental demographic measure of mortality, represents the average number of remaining years an individual is expected to live under current age-specific mortality conditions[1,2]. As a central indicator of population health and aging, life expectancy holds advantages over traditional metrics such as mortality and morbidity rates, being less affected by population size and age structure, while offering more direct insights into the interplay between aging-related factors and senescence[3,4]. Considerable individual variation in life expectancy arises from intricate interactions between genetic and environmental influences[5–8]. Though earlier work has focused primarily on population-level life expectancy, recent methodological developments have made it possible to estimate individualized expected years of life lost (YLL), enabling finer-grained analyses of aging-related factors at the individual level[9,10]. Despite this progress, large-scale studies evaluating individualized YLL remain scarce.
Telomere length (TL), a core marker of biological aging, consists of protective DNA sequences at chromosome termini that preserve genomic stability. TL is closely tied to life expectancy, as shorter telomeres have been associated with increased cellular senescence and reduced regenerative capacity[11]. Population-based studies have shown that shorter telomeres at age 40 are associated with a decrease in life expectancy by about 2.5 years[12]. Recent findings suggest significant sex differences in telomere biology, with females generally exhibiting longer telomeres than males. Hormonal regulation, particularly the effect of estrogen in increasing telomerase activity and limiting oxidative stress-induced telomere loss, may contribute to this difference[13,14]. This sex gap is reflected in an estimated 7.4-year divergence in telomere attrition rates between males and females[15]. Despite these insights, the sex-specific impact of TL on individualized life expectancy remains poorly characterized. Additionally, the association between TL and aging-related diseases remains a topic of debate[16,17]. While some research has linked longer telomeres with lower risks of specific cancers, cardiovascular disease, and Alzheimer’s disease, with varying effects by sex[18–23], other findings challenge the assumption that preserving telomere length consistently improves health outcomes. In particular, longer telomeres have been associated with increased vulnerability to certain malignancies, including B-cell and T-cell lymphomas and myeloid cancers[24]. Given these multifaceted relationships, further research is necessary to clarify how sex-specific differences in telomere dynamics affect disease risk and life expectancy[15,25].
This study aims to examine sex-specific associations between leukocyte telomere length (LTL) and individualized YLL, and to explore potential differences in how TL relates to aging-related diseases between males and females[26–28]. A prospective cohort design was applied, drawing on data from 445,399 participants (203,731 males and 241,668 females) in the UK Biobank (UKB).
-
The UKB is a large-scale prospective cohort study that enrolled approximately 500,000 individuals aged 40–70 years between 2006 and 2010. At baseline, participants completed a wide array of assessments including self-administered questionnaires, physical measurements, interviews, and blood sampling. The study received ethical approval from the North West Multi-Center Research Ethics Committee. Detailed information is available at https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics.
A total of 445,399 participants (203,731 males and 241,668 females) were included based on the following exclusion criteria (Supplementary Figure S1): (1) invalid TL measurements; (2) missing baseline mortality data on cause of death, date of death, or age at death (UKB categories 40001, 40000, 40007); (3) non-white ethnicity.
-
TL was assessed using leukocyte DNA from peripheral blood leukocytes (Supplementary Figure S2), a method known to yield consistent values that align with TL in other tissues within the same individual[15,29]. LTL was quantified through a multiplex qPCR approach, calculating the ratio of telomere repeat copy number (T) to a single-copy reference gene (S, HBB, encoding the human hemoglobin subunit β)[30]. Quality control was conducted in three stages: (1) determination of technical parameters based on Bayesian criteria; (2) assessment of two-way interactions among significant effects; (3) inspection of DNA purity and storage conditions. To normalize the distribution and allow for cross-study comparisons, LTL data were subjected to loge transformation and subsequently Z-standardized (UKB category 22192)[15]
-
Individualized YLL served as the primary outcome measure, calculated using actuarial life table, a demographic methodology that estimates remaining life expectancy through probabilistic analysis of mortality patterns. The life table was constructed from the UK Office for National Statistics (2015–2017), incorporating three consecutive years of population estimates, birth cohorts, and all-cause mortality data[10,31]. Unlike traditional population-level methods to calculate life expectancy , which face limitations in capturing the complex influence of aging-related diseases on life expectancy[8], this approach permits direct, individual-level evaluation of study variables, offering distinct advantages for assessing personalized outcomes[9,31]. YLL reflects the expected years lost between the time of death, loss to follow-up, or study end, based on actuarial predictions. For instance, if a man dies at age 60 and the life expectancy at that age is 22.57 years, then his YLL is 22.57. To allow for cross-study comparison, the non-normally distributed YLL data were converted to Z-standardized values.
-
Multiple linear regression was used to evaluate sex-specific associations between LTL and YLL, adjusting for age, menopause status (females only), body mass index (BMI), smoking status, white blood cell count (WBC), townsend deprivation index, college education, alcohol status, physical activity, and the interaction term between LTL and age[12,15]. To aid interpretation, LTL values were divided into four groups based on standard deviation (S.D.) from the mean: the shortest group (LTL < -1 S.D.); the shorter group (-1 S.D. ≤ LTL < 0 S.D.); the longer group (0 S.D. ≤ LTL < 1 S.D.); and the longest group (LTL ≥ 1 S.D.)[12].
Stratified analyses were performed to examine sex-specific variation across covariates. Subgroup analyses were conducted to assess the relationship between LTL and individualized YLL within major aging-related diseases: cancers (ICD-10: C00–C97; 45,473 males and 46,960 females), cardiovascular diseases (ICD-10: I00–I99; 101,937 males and 96,622 females), and chronic respiratory conditions including COPD (ICD-10: J41–J44) and asthma (ICD-10: J45–J46; 24,582 males and 30,327 females)[8]. Sensitivity analyses were carried out using linear models with various covariate combinations to assess the robustness of results.
All statistical analyses were conducted using R software version 4.3.1. Statistical significance was defined as P ≤ 0.05 (two-sided).
-
A total of 445,399 participants (203,731 males and 241,668 females) were included to examine associations between LTL and individualized YLL. Baseline characteristics stratified by LTL groups are shown in Supplementary Table S1. In both sexes, participants with longer LTL tended to have lower BMI, lower townsend deprivation index, and were generally younger. These individuals were also more likely to be non-smokers and engage in moderate physical activity. Initial analyses revealed clear differences between premenopausal and postmenopausal females (Supplementary Table S2), prompting additional stratified analyses based on menopausal status.
Sex-specific patterns emerged in the relationship between LTL and individualized YLL (Table 1). In males, longer LTL was associated with lower YLL. Each S.D. increase in LTL corresponded to a 0.965-year decrease in YLL (95% CI: −1.025, −0.900; P < 0.001). This association intensified across LTL categories, with the longest LTL group experiencing a 3.264-year reduction in YLL (95% CI: −3.497, −3.026; P < 0.001). In contrast, females showed an opposite trend. Each S.D. increase in LTL was linked to a 0.102-year increase in YLL (95% CI: 0.057, 0.146; P < 0.001), and the longest LTL group exhibited a 0.287-year increase in YLL (95% CI: 0.121, 0.452; P = 0.001).
Characteristics Males Females Individualized YLL (95% CI) P Individualized YLL (95% CI) P LTL Increased Per S.D. −0.965 (−1.025, −0.900) < 0.001 0.102 (0.057, 0.146) < 0.001 LTL Groups LTL<−1 S.D. Ref. − Ref. − −1 S.D. ≤LTL<0 −1.340 (−1.537, −1.138) < 0.001 −0.013 (−0.166, 0.134) 0.850 0 ≤ LTL<1 S.D. −2.371 (−2.573, −2.174) < 0.001 0.019 (−0.127, 0.172) 0.780 LTL≥1 s.d −3.264 (−3.497, −3.026) < 0.001 0.287 (0.121, 0.452) 0.001 Age −0.715 (−0.715, −0.709) < 0.001 −0.751 (−0.758, −0.751) < 0.001 WBC 0.048 (0.042, 0.048) < 0.001 0.032 (0.032, 0.038) < 0.001 Smoking Status Never Ref. − Ref. − Former 0.316 (0.292, 0.346) < 0.001 0.166 (0.140, 0.185) < 0.001 Current 0.101 (0.083, 0.119) < 0.001 0.102 (0.089, 0.121) < 0.001 BMI 0.012 (0.006, 0.012) < 0.001 0.000 (−0.001, 0.127) 0.500 Townsend Deprivation Index Quintile 1 Ref. − Ref. − Quintile 2−4 0.107 (0.083, 0.125) < 0.001 0.070 (0.057, 0.089) < 0.001 Quintile 5 0.262 (0.232, 0.292) < 0.001 0.134 (0.108, 0.153) < 0.001 Education Other Ref. − Ref. − College/University 0.030 (0.006, 0.048) 0.007 0.001 (−0.013, 0.013) 0.904 Alcohol Status Never Ref. − Ref. − Former 0.423 (0.346, 0.506) < 0.001 0.108 (0.064, 0.153) < 0.001 Current 0.083 (0.018, 0.155) 0.010 −0.070 (−0.102, −0.045) < 0.001 Physical Activity Low Ref. − Ref. − Moderate −0.161 (−0.191, −0.137) < 0.001 −0.019 (−0.038, −0.003) 0.023 High −0.232 (−0.256, −0.203) < 0.001 −0.019 (−0.038, 0.002) 0.074 Menopausal Status Pre − − Ref. − Post − − 0.439 (0.420, 0.458) < 0.001 Note. LTL, leukocyte telomere length; Individualized YLL, individualized expected years of life lost; 95% CI, 95% confidence interval; S.D., standard deviation; Ref., reference group; WBC, white blood cell count; BMI, body mass index. Table 1. Sex-specific associations between leukocyte telomere length and individualized expected years of life lost
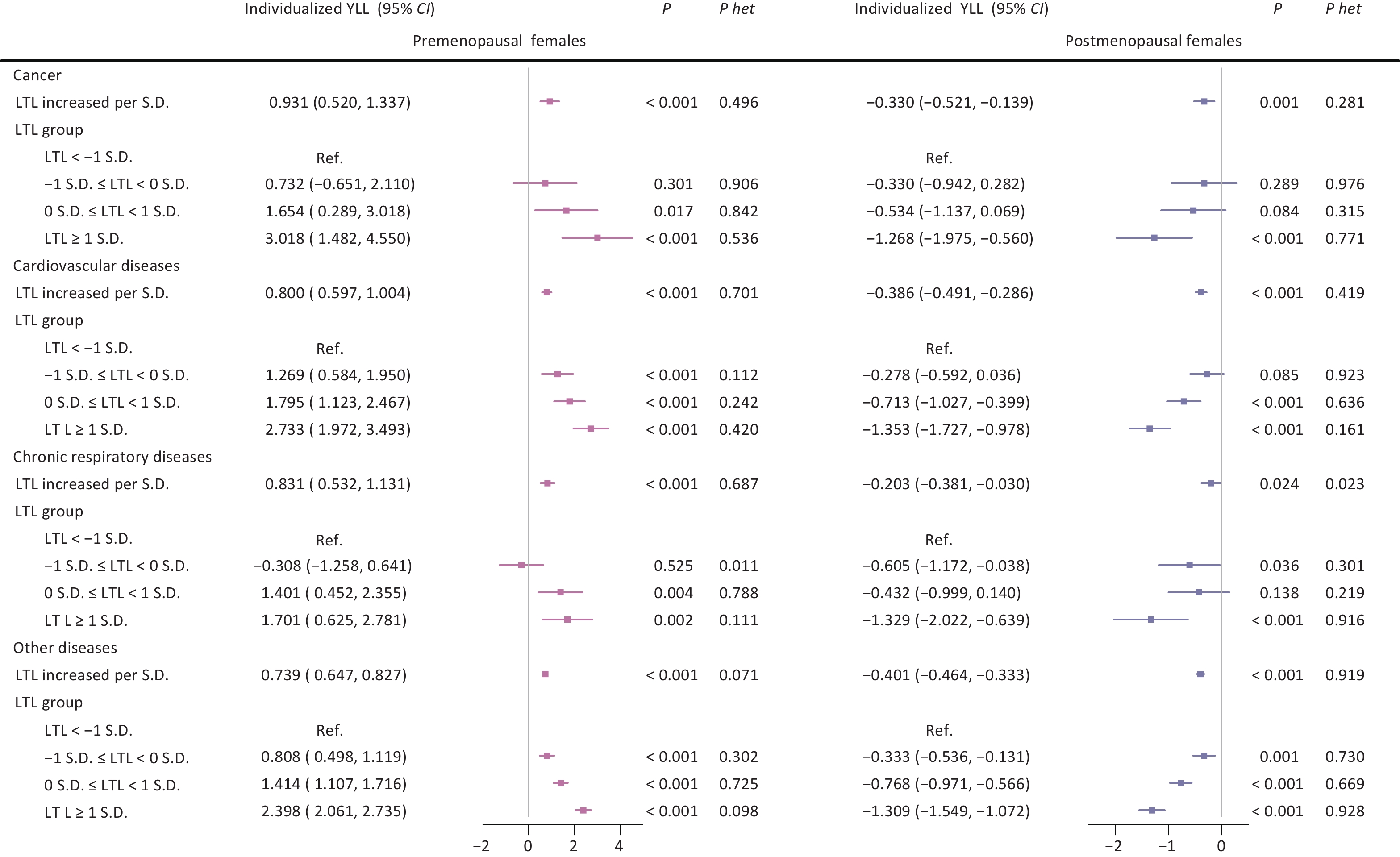
Stratification by menopausal status revealed further divergence in LTL–YLL associations (Table 2). Among premenopausal females, longer LTL was related to higher individualized YLL. Each S.D. increase in LTL was associated with a 0.705-year increase in YLL (95% CI: 0.625, 0.785; P < 0.001), with the longest LTL group showing a 2.285-year increase (95% CI: 1.986, 2.589; P < 0.001). By contrast, postmenopausal females exhibited a pattern resembling that of males, with longer LTL linked to lower YLL. Each S.D. increase was associated with a 0.387-year reduction in YLL (95% CI: −0.446, −0.328; P < 0.001), and the longest LTL group experienced a 1.276-year reduction (95% CI: −1.490, −1.065; P < 0.001).
Characteristics Premenopausal Females Postmenopausal Females Individualized YLL (95% CI) P Individualized YLL (95% CI) P LTL Increased Per S.D. 0.705 (0.625, 0.785) < 0.001 −0.387 (−0.446, −0.328) < 0.001 LTL Groups LTL<−1 S.D. Ref. − Ref. − −1 S.D.≤LTL< 0 S.D. 0.750 (0.474, 1.031) < 0.001 −0.320 (−0.501, −0.135) 0.001 0 S.D.≤LTL<1 S.D. 1.387 (1.114, 1.660) < 0.001 −0.741 (−0.922, −0.560) < 0.001 LTL≥1 S.D. 2.285 (1.986, 2.589) < 0.001 −1.276 (−1.490, −1.065) < 0.001 Age −0.515 (−0.515, −0.512) < 0.001 −0.493 (−0.493, −0.488) < 0.001 WBC 0.023 (0.015, 0.027) < 0.001 0.021 (0.017, 0.025) < 0.001 Smoking Status Never Ref. − Ref. − Former 0.011 (−0.011, 0.038) 0.313 0.143 (0.122, 0.160) < 0.001 Current 0.045 (0.030, 0.064) < 0.001 0.072 (0.059, 0.080) < 0.001 BMI 0.000 (−0.002, 0.002) 0.895 0.001 (0.000, 0.002) 0.211 Townsend Deprivation Index Quintile 1 Ref. − Ref. − Quintile 2−4 0.053 (0.034, 0.072) < 0.001 0.038 (0.025, 0.051) < 0.001 Quintile 5 0.099 (0.072, 0.121) < 0.001 0.076 (0.059, 0.088) <0.001 Education Other Ref. − Ref. − College/University 0.011 (−0.004, 0.027) 0.110 0.000 (−0.013, 0.013) 0.921 Alcohol Status Never Ref. − Ref. − Former −0.019 (−0.080, 0.042) 0.562 0.118 (0.084, 0.152) < 0.001 Current −0.064 (−0.110, −0.023) 0.003 −0.025 (−0.051, −0.004) 0.030 Physical Activity Low Ref. − Ref. − Moderate 0.027 (0.004, 0.049) 0.015 −0.034 (−0.051, −0.017) < 0.001 High 0.042 (0.019, 0.064) < 0.001 −0.042 (−0.059, −0.025) < 0.001 Note. LTL, leukocyte telomere length; Individualized YLL, individualized expected years of life lost; 95% CI, 95% confidence interval; S.D., standard deviation; Ref., reference group; WBC, white blood cell count; BMI, body mass index. Table 2. Associations between leukocyte telomere length and individualized expected years of life lost, stratified by menopausal status
Analyses of major aging-related diseases consistently showed that longer LTL was associated with protective effects in males. For cancer, cardiovascular diseases, and chronic respiratory diseases, each S.D. increase in LTL corresponded to reductions in individualized YLL by 1.127 years (95% CI: −1.326, −0.932), 1.026 years (95% CI: −1.131, −0.922), and 0.557 year (95% CI: −0.761, −0.359), respectively (all P < 0.001). These associations were strongest in the longest LTL group. In females, the relationships varied by disease type. For chronic respiratory diseases, longer LTL was linked to an increase in YLL (0.155 year per S.D.; 95% CI: 0.012, 0.298; P = 0.030). Cardiovascular diseases showed a modest protective association (−0.115 year per S.D.; 95% CI: −0.201, −0.034; P = 0.006), while no significant association was observed for cancer (Figure 1).
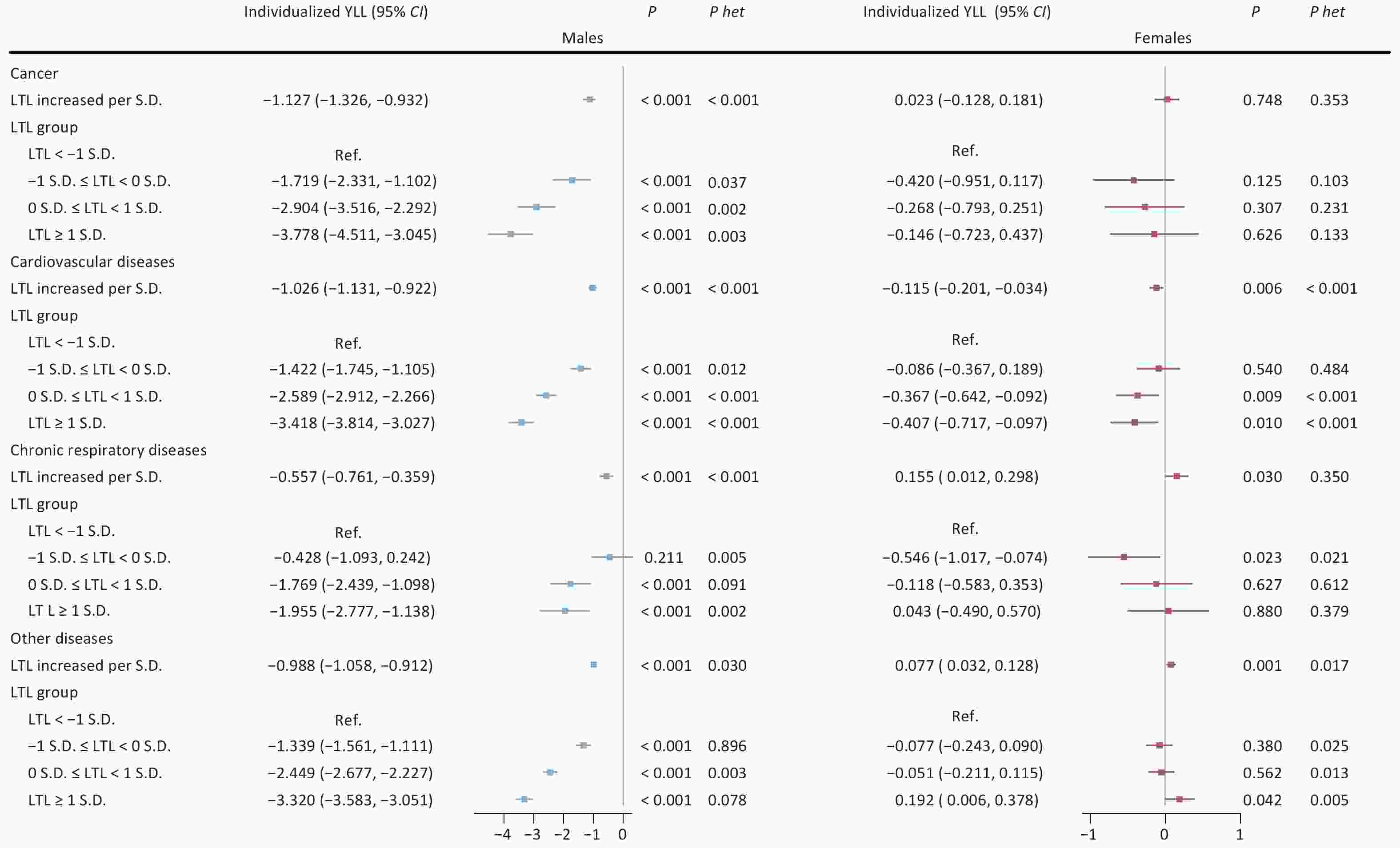
Figure 1. Sex-specific associations between leukocyte telomere length and individualized years of life lost across three major aging-related diseases. Note: LTL, leukocyte telomere length; Individualized YLL, individualized expected years of life lost; 95% CI, 95% confidence interval; S.D., standard deviation; Ref., reference group; P het, p-value for heterogeneity between groups with and without major aging-related diseases (cancer, cardiovascular diseases, chronic respiratory diseases).
Further analysis stratified by menopausal status revealed divergent patterns across disease categories. Among premenopausal females, longer LTL was associated with increased individualized YLL across all three conditions, with the strongest effects found in cancer (3.018 years; 95% CI: 1.482, 4.550; P < 0.001) and cardiovascular diseases (2.733 years; 95% CI: 1.972, 3.493; P < 0.001) in the longest LTL group. In contrast, postmenopausal females exhibited protective effects across all diseases, with the most pronounced association seen in cardiovascular diseases (−1.353 years; 95% CI: −1.727, −0.978; P < 0.001) in the longest LTL group (Figure 2).
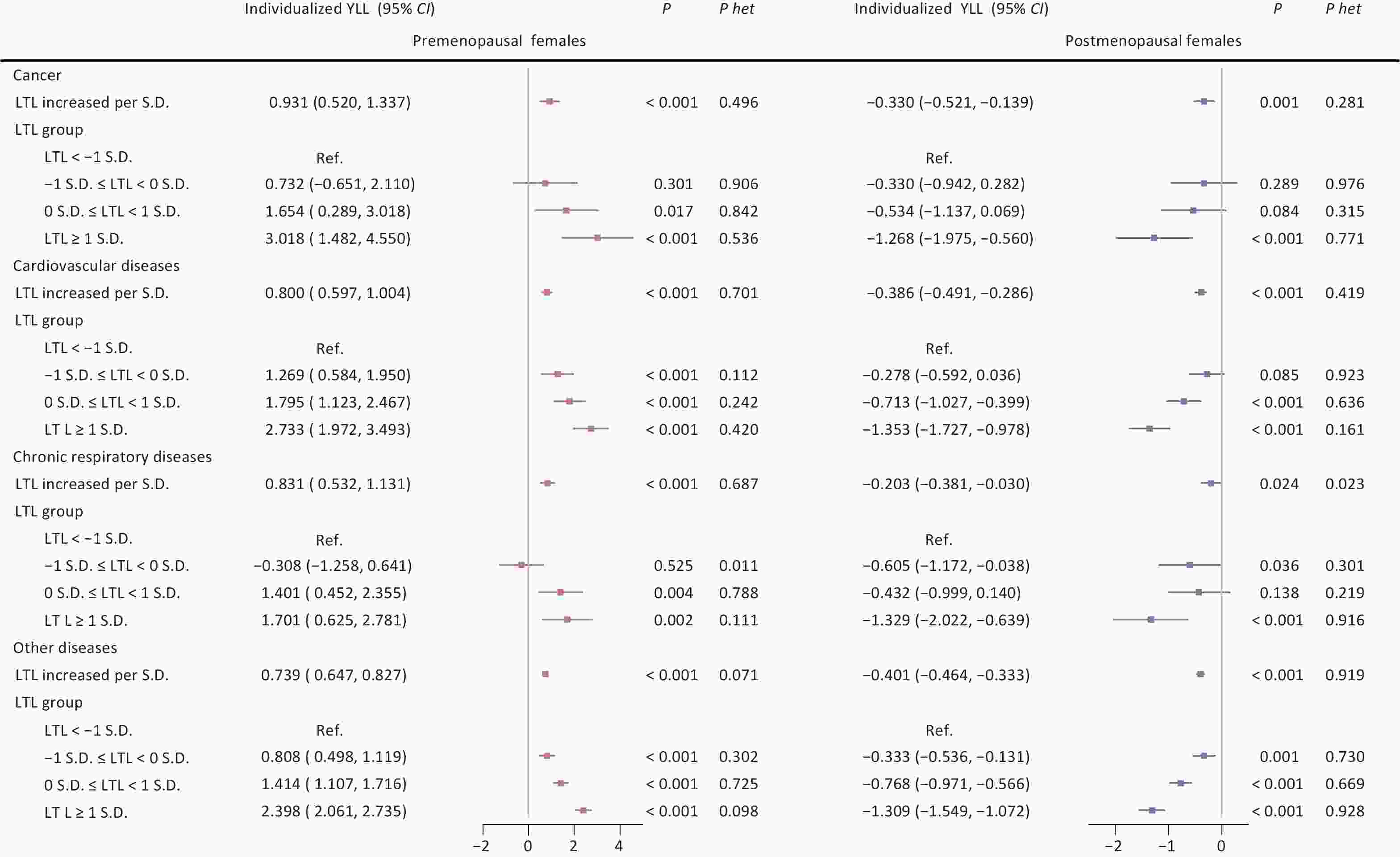
Figure 2. Associations between leukocyte telomere length and individualized years of life lost across three major aging-related diseases according to menopausal status. Note: LTL, leukocyte telomere length; Individualized YLL, individualized expected years of life lost; 95% CI, 95% confidence interval; S.D., standard deviation; Ref., reference group; P het, p-value for heterogeneity between groups with and without major aging-related diseases (cancer, cardiovascular diseases, chronic respiratory diseases).
Sensitivity analyses that incorporated various covariate adjustments confirmed the consistency of these results (Supplementary Tables S3–S4).
-
This study revealed distinct sex-specific associations between LTL and individualized YLL. Longer LTL was linked to reduced YLL in males, while in females, it was associated with increased YLL. Among females, this pattern became more complex when stratified by menopausal status: premenopausal females showed increased YLL with longer LTL, whereas postmenopausal females showed reduced YLL.
Epidemiological studies increasingly support sex-specific links between TL and aging-related diseases, which may help explain the divergent impacts on YLL observed here. For example, females with shorter TL face higher cancer risks than males[20], particularly in cancers like pancreatic ductal adenocarcinoma[19]. TL has also been associated with atherosclerosis in a sex-specific manner[21], and in Alzheimer’s disease, females show distinct cognitive aging trajectories based on TL[22,23]. While earlier research has described sex-specific effects of TL on aging-related diseases, our study adds to this by quantifying the overall influence of LTL on aging using individualized YLL. The findings suggest stronger protective effects of longer telomeres in males, indicating potential biological differences in how TL shapes aging between the sexes.
The complex relationship between LTL and individualized YLL in females appears to involve hormonal factors, especially in relation to menopause. Stratified analyses showed that longer LTL correlated with increased YLL before menopause, but with decreased YLL after menopause. In contrast, males displayed a consistent protective association across the lifespan. These patterns are supported by prior findings on the role of sex hormones. Elevated testosterone has been linked to reduced all-cause mortality in males[32,33], but to harmful effects in females[34]. Hormones such as testosterone and estradiol have been associated with increased risks for breast and endometrial cancer in females[35–37]. In addition, higher testosterone levels have shown inverse associations with cardiovascular risk in females[38,39]. Other studies have linked higher estradiol and progesterone levels with increased mortality[40]. Estrogen may affect TL by acting on immune and antioxidant pathways[41–44]. These hormonal dynamics likely contribute to the observed increase in YLL among premenopausal females. Although our findings show clear contrasts between premenopausal and postmenopausal females, the mechanisms by which sex hormones influence the relationship between LTL and individualized YLL remain to be clarified. Further research is needed to explore these pathways in detail.
Furthermore, this study introduces individualized YLL as a comprehensive metric to assess the overall impact of TL on aging across multiple diseases, addressing an unresolved question regarding its influence on life expectancy[26–28]. Previous work has shown that longer telomeres are associated with increased life expectancy at the population level[12]. A separate Mendelian randomization (MR) study also identified a significant link between longer telomeres and increased life expectancy[45]. However, traditional population-level approaches such as Arriaga’s decomposition are limited in capturing the complex effects of aging-related diseases on life expectancy[8,12,46]. In addition, TL shows varied associations across aging-related diseases[12,16]. Longer telomeres have been linked to lower risks of cardiovascular disease[47], diabetes[48], and Alzheimer’s disease[49], yet paradoxically, they are also associated with higher risks of certain cancers, including gliomas, and ovarian, lung, and bladder cancers[16]. Unlike population-level metrics, individualized YLL permits direct comparison of the net effects of TL across disease types. Our findings indicate that increased LTL is associated with reduced YLL from cancer, cardiovascular disease, and chronic respiratory disease in males, whereas in females, YLL reduction was observed only in cardiovascular disease. These results suggest that individualized YLL may serve as a useful metric for evaluating the complex relationships between TL and aging.
Several limitations should be acknowledged when interpreting these findings. First, incomplete data on sex hormones, especially estradiol levels in both pre- and postmenopausal females, limit our ability to fully explore hormonal mechanisms. Second, TL was measured at a single time point at baseline, which may not capture long-term exposure dynamics, although stringent quality control procedures improved measurement reliability[15]. Third, the UKB cohort’s narrow age range (40–70 years) and predominantly White ethnic background reduce the applicability of these results to more diverse populations. Additionally, the UK Biobank is affected by a ‘healthy volunteer’ bias, as participants tend to be healthier and more health-conscious than the general population. This selection bias may result in underestimation of disease incidence and restrict the broader generalizability of the findings.
In conclusion, this prospective cohort study identified sex-specific associations between LTL and individualized YLL. LTL was inversely related to individualized YLL in males but positively associated with individualized YLL in females. Among females, longer LTL appeared to increase YLL in the premenopausal group, while showing a protective association in the postmenopausal group. These results suggest that individualized YLL may serve as an effective unified index for evaluating the influence of TL on aging across a range of diseases. Further mechanistic and longitudinal studies are needed to examine causal pathways, with particular attention to hormonal factors and disease-specific patterns.
Sex-specific Association of Telomere Length with Individualized Expected Years of Life Lost among 203,731 Males and 241,668 Females
doi: 10.3967/bes2025.053
- Received Date: 2025-02-15
- Accepted Date: 2025-04-18
Abstract:
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The UKB received approval from the Northwest Multi-Center Research Ethics Committee (MREC). Full details can be found at https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics.The generation and use of the data presented in this paper was approved by the UKB access committee under UKB application number 79151.
&These authors contributed equally to this work.
| Citation: | Feifei Xu, Chenjie Li, Yifan Wang, Xiao Wang, Yumnah Babar, Shuang Liang, Fan Yang, Zhazheng He, Honggang Yi, Juncheng Dai. Sex-specific Association of Telomere Length with Individualized Expected Years of Life Lost among 203,731 Males and 241,668 Females[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2025.053 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: