-
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder that primarily affects the central nervous system, with a specific impact on upper and lower motor neurons. The degeneration of these neurons leads to muscle weakness, atrophy, and ultimately the loss of mobility and autonomous respiratory function[1]. The pathogenesis of ALS is complex and has not been fully elucidated, with clinical manifestations likely arising from the interaction of genetic and environmental factors. Established pathogenic mechanisms include oxidative stress, mitochondrial dysfunction, endoplasmic reticulum stress, neuroinflammation, abnormal RNA processing, axonal transport dysfunction, and aggregation of misfolded proteins[2,3]. Despite progress in understanding the disease, the potential risk factors that may initiate these pathogenic processes remain unclear.
The onset of ALS is influenced by a variety of factors, including genetic predisposition, prolonged exposure to metals, viral infections, pollutants, and lifestyle factors, all of which serve as both risk factors and predictors of survival[4,5]. Extensive research has highlighted the involvement of trace elements in the mechanisms underlying motor neuron degeneration, thereby emphasizing their potential critical roles in the pathophysiology of amyotrophic lateral sclerosis (ALS)[6–8]. Elevated environmental concentrations of copper (Cu), zinc (Zn), chromium (Cr), and nickel (Ni) may be associated with the increased incidence of ALS in northern Italy[9]. Furthermore, increased levels of Zn in erythrocytes, and of Cu and iron (Fe) in plasma, have been identified as potential risk factors for ALS[10,11]. Investigations utilizing ALS mouse models have demonstrated that Cu chelators, such as DP-109, DP-460, penicillamine, N-acetylcysteine, and trientine, can effectively delay disease progression and enhance motor function[12–14]. These trace elements function as enzyme cofactors within the central nervous system; however, their presence in excess may contribute to the pathogenesis of disease[10,11]. Research conducted in the United States has further established a positive correlation between the blood Cu level and risk of ALS[15]. Although previous studies highlighted alterations in the levels of these trace elements among patients with ALS, findings have been inconsistent across different ethnic and regional populations. Notably, there has been a lack of correlation studies focusing on patients with sporadic ALS (sALS) in China. This study was performed to evaluate the relations between serum trace element levels and disease progression in patients with sALS in China, explore their influence on survival duration, and offer insights to inform future ALS treatment strategies.
-
The study population consisted of 110 patients diagnosed with sALS who were admitted to our department between October 2018 and October 2021. The inclusion criteria were diagnosis based on the revised El Escorial diagnostic criteria for ALS[16], availability of fasting venous blood sample(s) collected on the morning after admission for serum analysis of calcium (Ca), magnesium (Mg), iron (Fe), copper (Cu), and zinc (Zn), and age > 18 years. The exclusion criteria were a family history of ALS or known genetic mutations; hepatic or renal dysfunction; current use or a history of supplementation with hormones, Ca, Fe, or Zn within 3 months prior to admission; diseases affecting Ca and phosphorus metabolism; and ventilator dependence. The study was approved by the Ethics Committee of the General Hospital of the People’s Liberation Army (S2022-119), and informed consent was obtained from all participants or their legal guardians.
Basic patient data, including sex, age, height, weight, and body mass index (BMI), were collected. Clinical characteristics including age at onset, disease onset time, disease duration, site of onset (medulla oblongata or limb), diagnostic delay (time from onset to diagnosis), date of diagnosis, and date of hospitalization were also recorded. The ALS diagnostic classification was determined by an experienced senior chief physician based on comprehensive neurological examination and categorized as clinically definite, clinically probable, clinically probable-laboratory supported, or clinically possible[16]. ALS functional assessment was conducted by the same physician based on the revised Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R)[17]. The disease progression rate (DPR) was calculated as (48 − ALSFRS-R score at assessment)/time from onset to assessment (in months).
All patients were followed up by telephone or outpatient services, and the last follow-up date was January 2022. Survival time was defined as the time interval (in months) from onset to the date of endpoint event (ALS-related death or tracheotomy). The survival time of surviving patients was defined as the time interval from onset to January 2022.
-
Cubital venous blood samples were collected at 6:00 am after a 6-h period of fasting and water restriction. Serum Ca, Mg, Fe, Cu, and Zn levels in the samples were analyzed within 2 h at the hospital’s laboratory. The concentrations of trace elements were analysed by inductively coupled plasma mass spectrometry (ICP-MS, Thermo).
-
Statistical analyses and figures were generated using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA). The results are presented as the median with minimum and maximum values. The normality of the data distribution was assessed using the Shapiro–Wilk test. Normally distributed data were analyzed using the t test, while non-normally distributed data were evaluated using the Mann–Whitney U test. The relations between serum trace elements and ALSFRS-R score were assessed using Spearman’s correlation. A random forest algorithm was used to rank the importance of serum trace elements in relation to the ALSFRS-R score and disease progression. The impacts of serum Ca and Mg on survival rates in patients with sALS were compared using the log-rank (Mantel–Cox) test. In all analyses, P < 0.05 was taken to indicate statistical significance.
-
The study cohort consisted of 110 patients diagnosed with sALS, including 69 men (62.73%) and 41 women (37.27%), with a male-to-female ratio of 1.68:1. The mean (range) age at hospitalization was 55.32 ± 9.58 (28–74) years. Limb onset occurred in 78.18% of cases, while medulla oblongata onset occurred in 21.82%. The mean (median, range) age at disease onset was 53.95 ± 9.66 (53.88, 25–73) years. The mean (median, range) diagnostic delay was 14.39 ± 14.03 (11, 1–100) months. The mean (median, range) disease duration was 16.44 ± 15.11 (12, 2–108) months. The mean BMI was 23.15 ± 3.03 kg/m2. The mean (median, range) ALSFRS-R score was 38.31 ± 6.02 (39, 7–46). The mean (median, range) DPR was 0.96 ± 0.98 (0.60, 0.12–7) (Table 1).
Clinical variables Items Data Age at hospitalization (years) Mean ± Standard deviation
Age range55.32 ± 9.58
28-74Gender Male
Female
Male: Female ratio69 (62.73% )
41 (37.27% )
1.68:1Site of onset Medulla oblongata
Limb24 (21.82%)
86 (78.18%)Age of onset (years) Mean ± Standard deviation
Median
Age Range53.95 ± 9.66
53.88
25-73Diagnostic delay (months) Mean ± Standard deviation
Median
Month Range14.39 ± 14.03
11
1-100Disease course (months) Mean ± Standard deviation
Median
Month Range16.44 ± 15.11
12
2-108BMI (kg/m2) Mean ± Standard deviation 23.15 ± 3.03 ALSFRS-R Mean ± Standard deviation
Median
Range38.31 ± 6.02
39
7-46DPR Mean ± Standard deviation
Median
Range0.96 ± 0.98
0.60
0.12-7Note. ALS, amyotrophic lateral sclerosis; BMI, body mass index; ALSFRS-R: ALS Functional Rating Scale-revised; DPR: Disease progression rate. Table 1. Clinical characteristics of patients with sALS (n = 110)
-
The mean (median, range) serum concentrations of trace elements in patients with sALS were as follows: Ca, 1.58 ± 0.28 (1.53, 1.15–2.21) mmol/L; Mg, 1.67 ± 0.30 (1.68, 0.82–2.92) mmol/L; Fe, 9.09 ± 2.52 (8.79, 5.94–30.30) mmol/L; Cu, 17.32 ± 5.69 (15.66, 7.30–17.32) μmol/L; and Zn, 95.66 ± 15.99 (93.87, 60.89–161.01) μmol/L (Table 2).
Trace element Items Data Ca (mmol/L) Mean ± Standard deviation
Median
Range1.58 ± 0.28
1.53
1.15-2.21Mg (mmol/L) Mean ± Standard deviation
Median
Range1.67 ± 0.30
1.68
0.82-2.92Fe (mmol/L) Mean ± Standard deviation
Median
Range9.09 ± 2.52
8.79
5.94-30.30Cu (umol/L) Mean ± Standard deviation
Median
Range17.32 ± 5.69
15.66
7.3-17.32Zn (umol/L) Mean ± Standard deviation
Median
Range95.66 ± 15.99
93.87
60.89-161.01Note. ALS, amyotrophic lateral sclerosis; Ca, calcium; Mg, magnesium; Fe, iron; Cu, copper; Zn, zinc. Table 2. Serum trace element levels in patients with sALS
-
Participants were divided into two groups based on the median ALSFRS-R score: ALSFRS-R < 39 and ALSFRS-R ≥ 39. There were significant differences in serum Ca and Mg levels between the two groups, while no significant differences were observed in Fe, Cu, or Zn levels. The ALSFRS-R ≥ 39 group had a serum Ca level of 1.623 ± 0.27 mmol/L, compared to 1.52 ± 0.28 mmol/L in the ALSFRS-R < 39 group (P = 0.0606). Serum Mg levels were 1.620 ± 0.32 mmol/L in the ALSFRS-R ≥ 39 group and 1.73 ± 0.27 mmol/L in the ALSFRS-R < 39 group (P = 0.0567) (Table 3).
Trace element ALSFRS-R < 39 (n = 47) ALSFRS-R ≥ 39 (n = 63) P-value Ca (mmol/L) 1.52 ± 0.28 1.623 ± 0.27 0.0606 Mg (mmol/L) 1.73 ± 0.27 1.620 ± 0.32 0.0567 Fe (mmol/L) 8.89 ± 3.01 9.235 ± 1.64 0.4878 Cu (umol/L) 17.01 ± 4.51 16.81 ± 6.96 0.2760 Zn (umol/L) 97.99 ± 12.79 93.93 ± 19.39 0.1892 Note. Data were represented as mean±SD; ALS, amyotrophic lateral sclerosis; ALSFRS-R: ALS Functional Rating Scale-revised; Ca, calcium; Mg, magnesium; Fe, iron; Cu, copper; Zn, zinc. Table 3. Differences in trace element levels among different ALSFRS-R scores
-
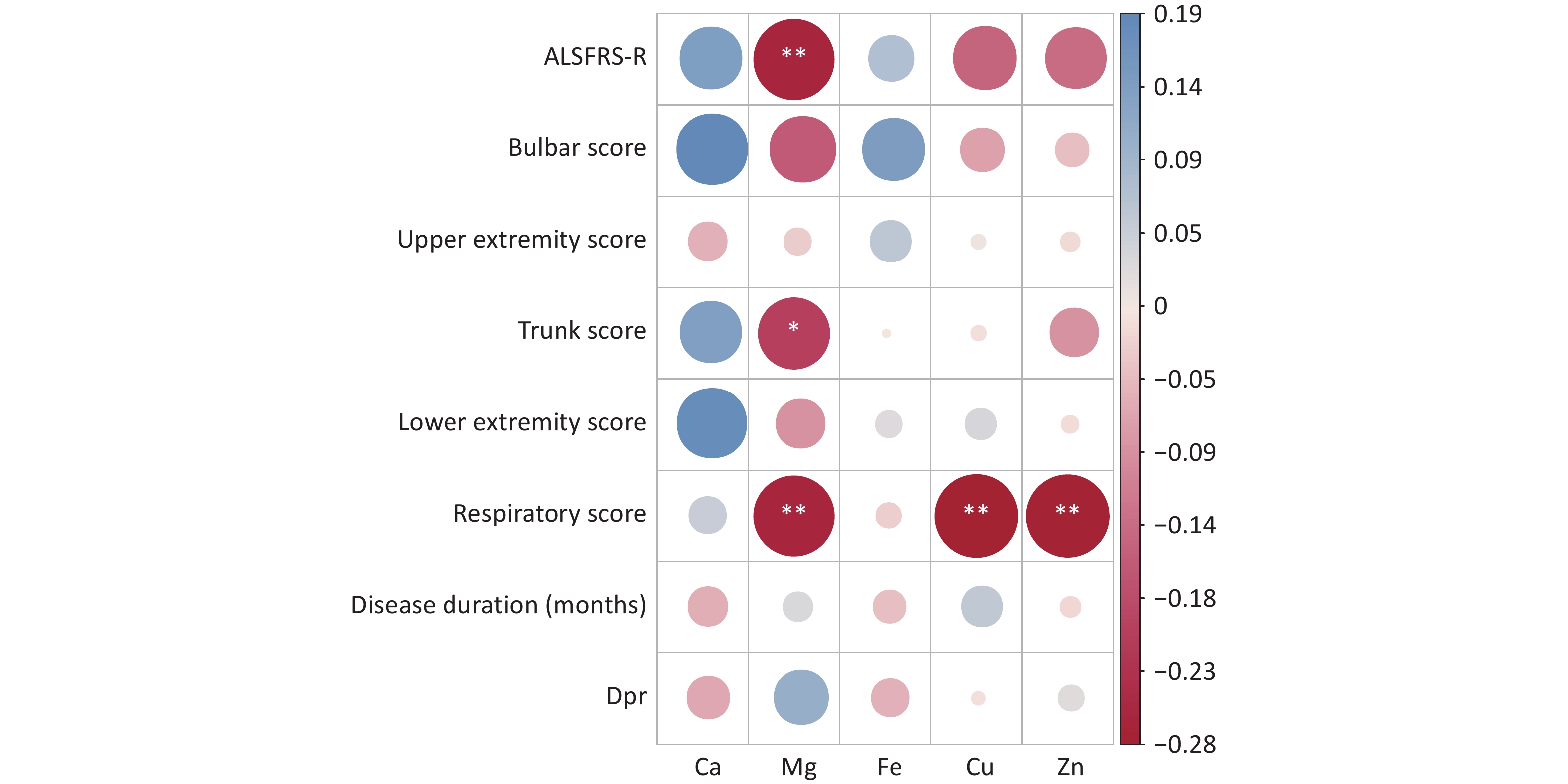
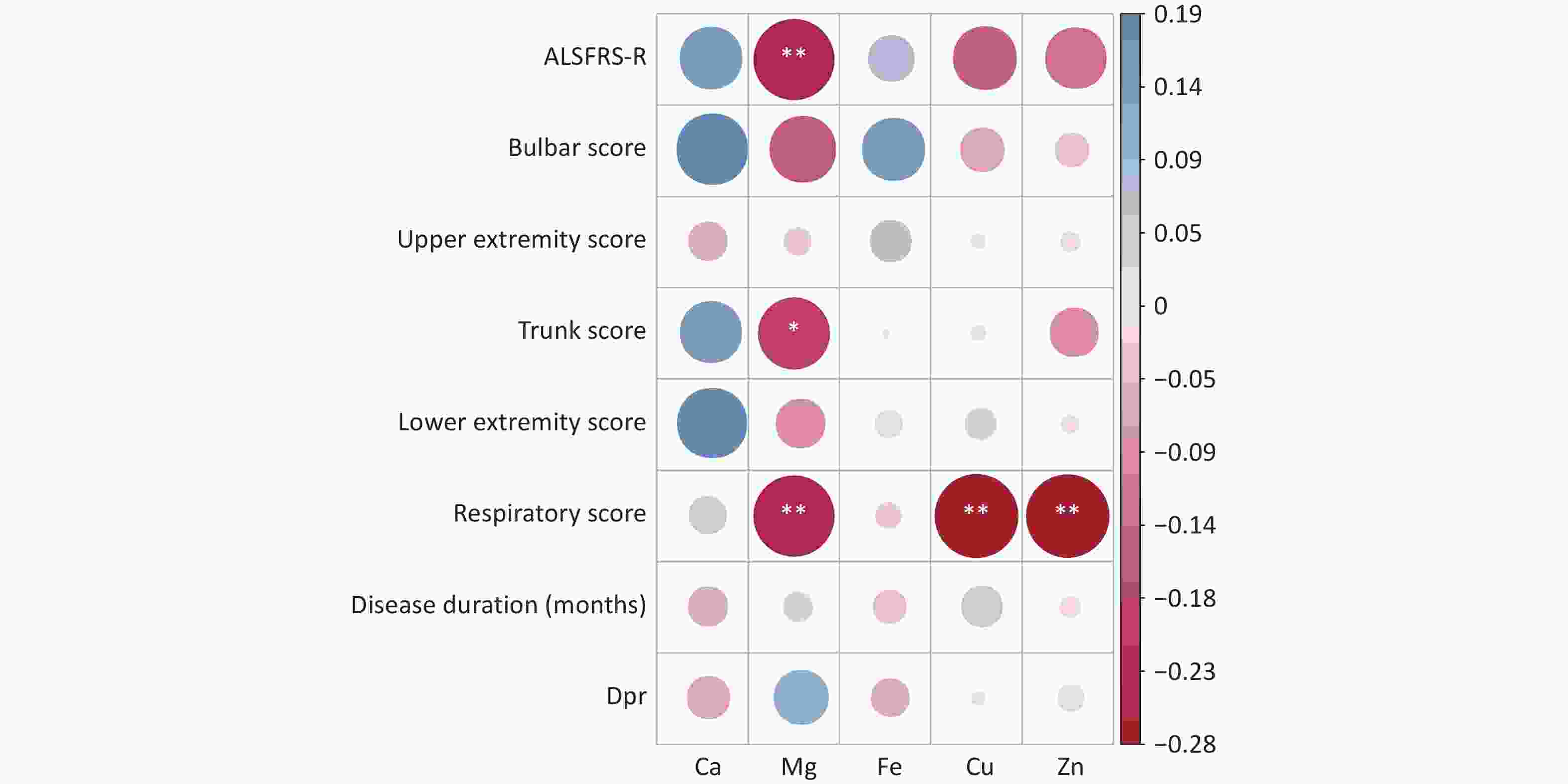
Correlation analyses were performed to assess the relations between serum levels of trace elements (Ca, Mg, Fe, Cu, and Zn) and the total ALSFRS-R score, as well as its subscores including the medulla oblongata, upper limb, trunk, lower limb, and respiratory scores. Significant negative correlations were observed between the serum Mg level and total ALSFRS-R score, trunk score, and respiratory score (P < 0.05), suggesting a potential role of Mg in the pathophysiology of sALS. In addition, serum Cu and Zn levels showed significant negative correlations with the respiratory score (P < 0.05), while Ca and Fe levels were not significantly correlated with any of these scores (Figure 1).
-
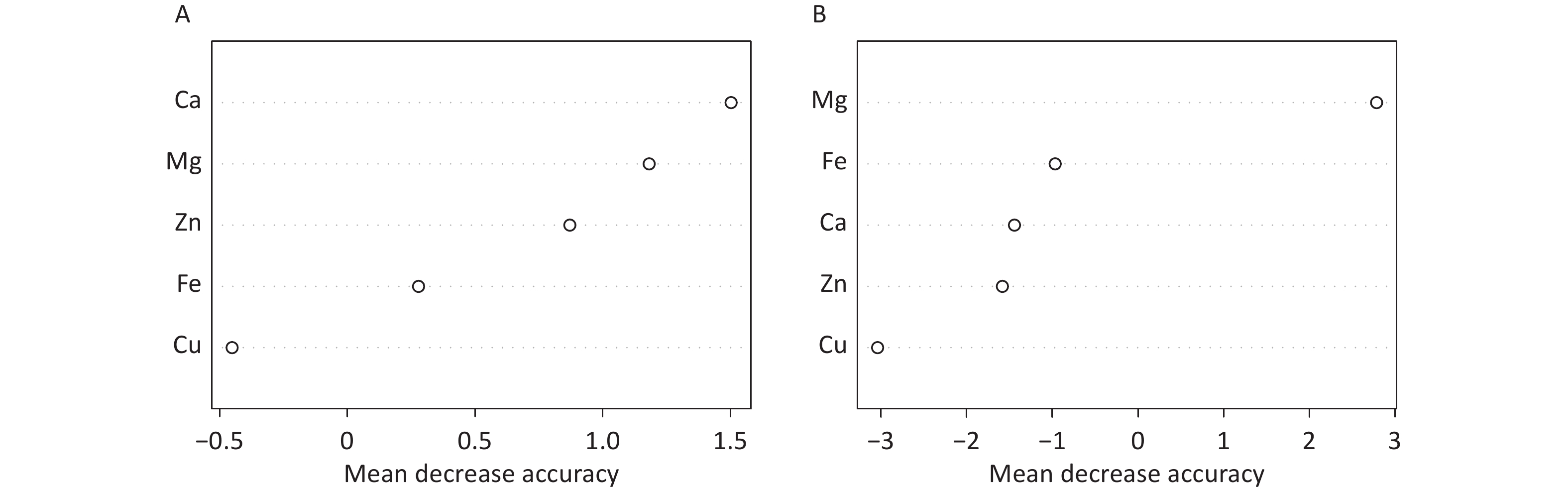
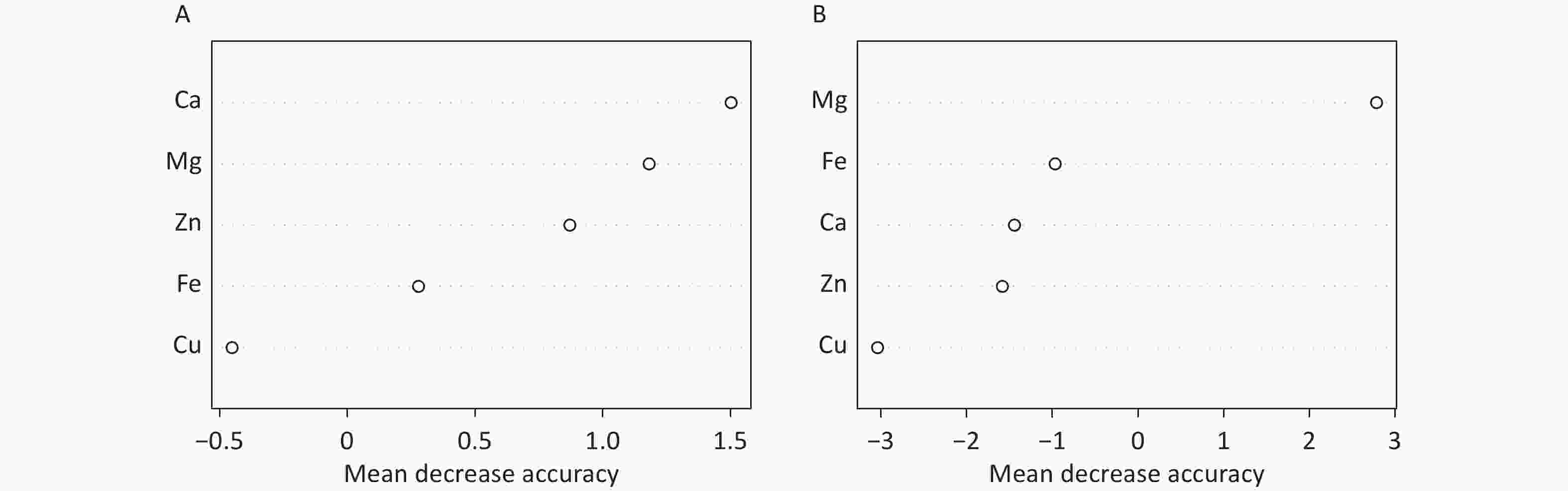
The random forest machine learning prediction model was applied to evaluate the influence of serum trace elements on the total ALSFRS-R score and disease progression. The results indicated that the Ca and Mg levels had a greater influence on the total ALSFRS-R score, with a mean decrease accuracy (MDA) score > 1. In addition, the serum Mg level had a substantial effect on disease progression, with an MDA score > 2 (Figure 2).
-
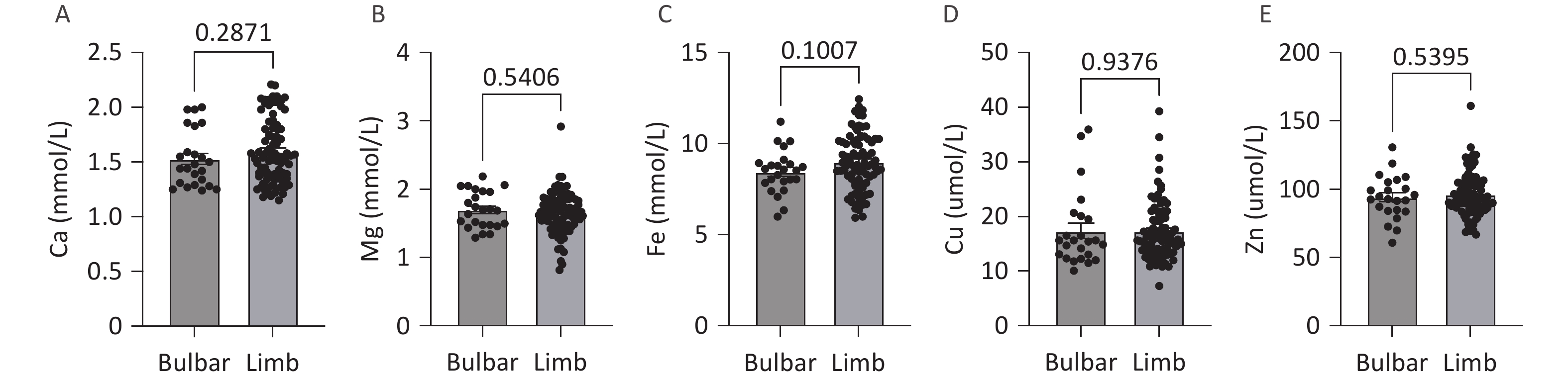
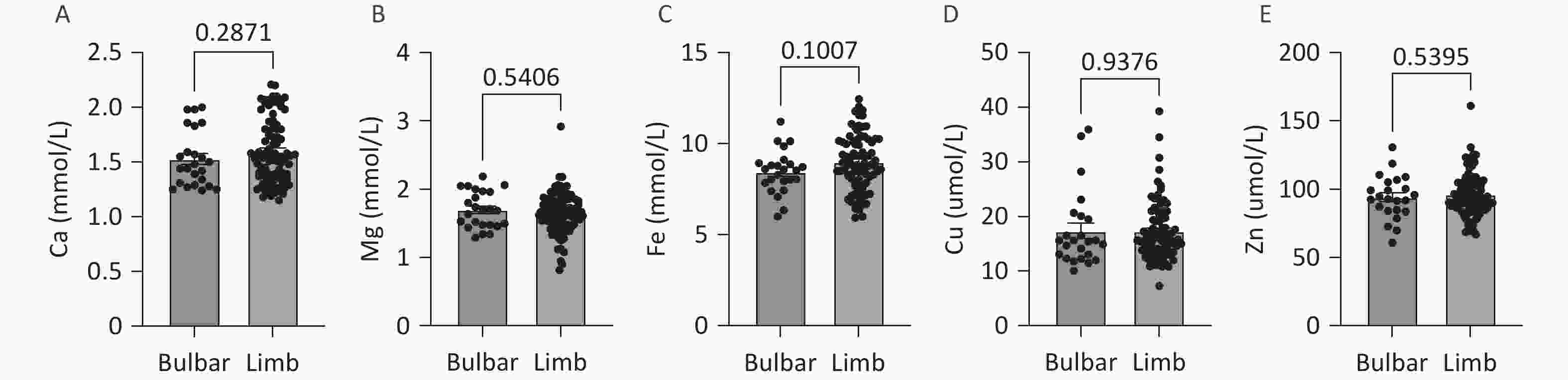
No significant differences in the levels of serum trace elements (Ca, Mg, Fe, Cu, and Zn) were observed between patients with different sites of onset (medulla oblongata onset or limb onset) (Figure 3).
-
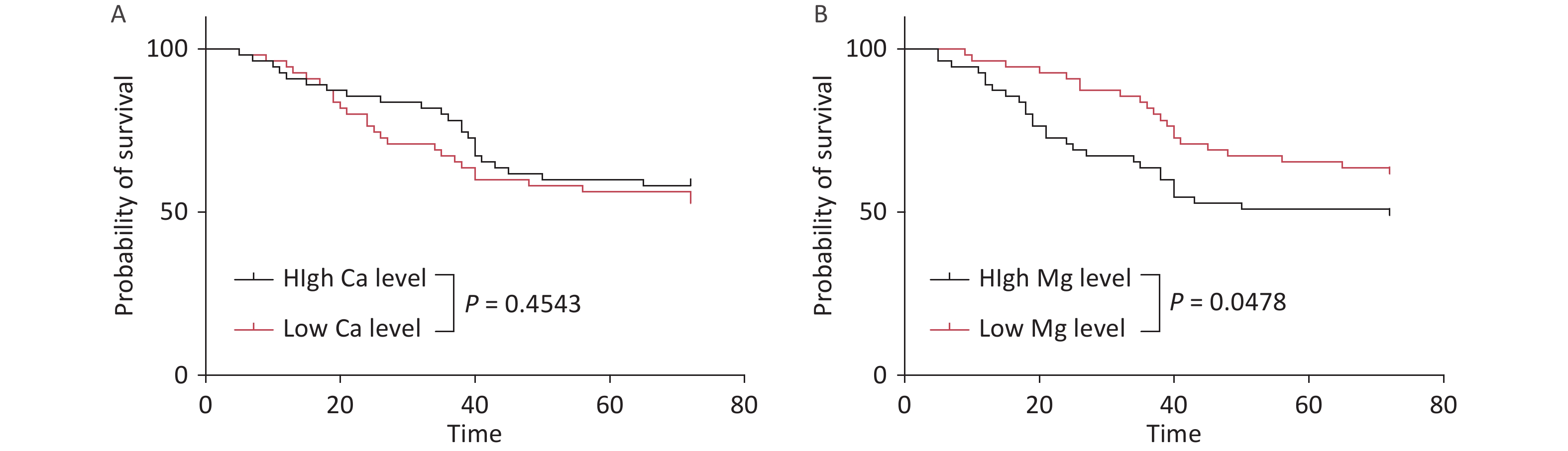
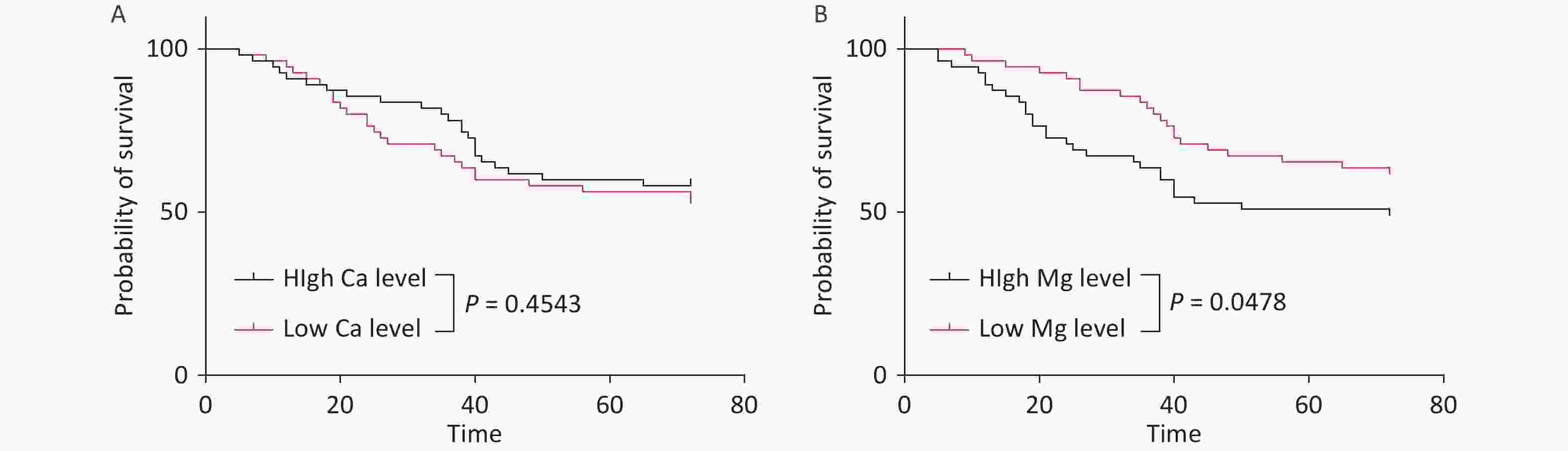
Survival analysis was performed by stratifying the cohort into low- and high-serum-trace-element-concentration groups based on the median values. While the serum Ca level had no significant impact on survival time, patients with low Mg levels showed significantly prolonged survival (P < 0.05) (Figure 4).
-
ALS is a fatal neurodegenerative disorder characterized by progressive muscle weakness, atrophy, spasticity, and fasciculation[18]. This is the first investigation to examine the associations between serum trace element concentrations and the severity and prognosis of the disease in Chinese patients with sALS. The results indicated that serum levels of Ca and Mg significantly influence the progression of sALS, with low serum Mg levels correlating with an increased survival rate. These observations suggest that Mg and Ca may be involved in the pathophysiological mechanisms of ALS and provide a novel perspective for the development of clinical intervention strategies.
In this study, the serum Mg level showed a significant negative correlation with the total ALSFRS-R score, as well as with trunk and respiratory functions, in Chinese patients with sALS (P < 0.05). Interestingly, lower serum Mg levels were associated with enhanced survival outcomes. While this finding may initially appear contradictory, it may be attributable to the dynamic regulatory role of Mg across different pathological stages of ALS. As a natural antagonist of the N-methyl-d-aspartate (NMDA) receptor, Mg can mitigate neuronal damage by inhibiting glutamate excitotoxicity[19]. Furthermore, oxidative stress, characterized by an imbalance between the production of reactive oxygen species (ROS) and the body’s detoxification capabilities, is a critical factor in the pathogenesis of ALS[20]. Mg possesses antioxidant properties that facilitate the neutralization of ROS, thereby safeguarding neurons from oxidative damage[21]. In addition, Mg may modulate inflammatory pathways and decrease the production of proinflammatory cytokines, potentially mitigating inflammatory damage to motor neurons[22]. However, our findings suggest that elevated Mg levels may be associated with increased disease severity in patients with sALS, potentially due to complex interactions involving Mg and factors such as oxidative stress, disruption of metal homeostasis, and abnormal aggregation of pathogenic proteins, including superoxide dismutase 1 (SOD1)[23]. Specifically, mutations in the SOD1 protein in certain ALS patients result in aggregation of the protein, and elevated Mg levels may exacerbate the formation of these abnormal aggregates, thereby accelerating disease progression. These results suggest that electrolyte homeostasis is disrupted at various clinical stages of ALS, with more pronounced disturbances observed in patients with more severe disease. Previous studies did not identify associations between ALS risk and dietary Mg intake alone[24]. Nevertheless, low dietary intakes of Mg and Ca, as well as aluminum (Al), have been linked to the development of ALS–parkinsonism-dementia complex (ALS–PDC) in the Kii Peninsula on Honshu, Japan[25]. Similar dietary patterns have been shown to induce histopathological findings characteristic of human ALS in primates.
In contrast, an elevated serum Mg level was associated with reduced ALSFRS-R functional score in the present study, indicating that the role of Mg in ALS may be more complex and potentially modulated by interactions with other trace elements or disease-related factors. Zn has been implicated in the pathogenesis of ALS[26] and, along with Cu, has been identified as a potential therapeutic target[11]. Previous research suggested that lower Zn levels are associated with an increased risk of ALS, highlighting the possible advantages of dietary Zn supplementation. Moreover, interactions between Mg and other trace elements, particularly Cu and Zn, may impact the progression of ALS. As essential cofactors for SOD1, the balance of Cu and Zn is critical for maintaining its normal function[27,28]. In individuals diagnosed with ALS, disturbances of metal metabolism may result in abnormal accumulation or deficiency of Cu and Zn, thereby affecting the stability and activity of SOD1[29,30]. These findings corroborate the observed negative correlations of serum Cu and Zn levels with respiratory scores.
Random forest modeling indicated significant predictive value of Ca and Mg for ALSFRS-R score and disease duration. Dysregulation of intracellular Ca2+ homeostasis is a hallmark of neuronal degeneration in ALS[31], and Mg can modulate Ca2+ influx by competitively inhibiting voltage-gated Ca channels[32,33]. Although the present study did not establish a direct correlation between the serum Ca level and survival, research using animal models has demonstrated that an elevated Ca/Mg ratio can exacerbate motor neuron apoptosis[34], suggesting that Ca/Mg homeostasis may serve as a more sensitive indicator of disease status. Furthermore, increased Ca levels in the cerebrospinal fluid of ALS patients have been associated with rapid disease progression, reinforcing the potential utility of the Ca/Mg ratio as a disease biomarker[35,36].
In contrast to previous studies[11,26], the results of this investigation identified weak correlations of Fe, Cu, and Zn with disease scores. Cu and Zn levels showed negative correlations with respiratory function. This discrepancy may be attributable to the dietary habits or specific environmental exposure characteristics of the Chinese population. For example, the low Zn content in the soils of northern China may contribute to inadequate Zn intake in the population[37,38]. In addition, abnormal Cu metabolism may be linked to dysfunction of the Cu-binding protein ceruloplasmin[39]. It is important to note that metal-binding protein levels were not examined in this study, which may have affected the assessment of Cu bioavailability.
This study had several limitations. First, confounding factors such as diet and environmental exposure may affect trace element concentrations, so we excluded diseases that affect calcium and phosphorus metabolism in the enrollment criteria, as well as patients currently using or having a history of Ca, Fe, or Zn supplementation in 3 months prior to admission; Second, its single-center design and small sample size restrict the generalizability of the findings; Third, the cross-sectional nature of the study precludes determination of causal relationships between micronutrient levels and disease progression, futher longitudinal studies or experimental studies (e.g., cell/animal models) are required to verify the pathological effects of Mg; Finally, we did not perform comparisons with data from healthy controls or patients with other neurodegenerative disorders. To overcome these limitations, multicenter longitudinal cohort studies incorporating analyses of cerebrospinal fluid or tissue samples are required to thoroughly investigate the dynamic changes in micronutrient levels in sALS.
-
In conclusion, Ca, Mg, Fe, Cu, and Zn, which contribute to normal cellular physiological functions and various pathological processes, including protein folding, oxidative stress, and mitochondrial dysfunction, play crucial roles in the pathogenesis of ALS. Although previous studies have documented alterations in these trace elements among patients with ALS, the findings vary significantly across different regional populations, with limited research focusing on patients with sALS in China. This study analyzed serum levels of these trace elements in patients with sALS in China to explore their potential associations with disease progression and survival time. The results provide new insights into the pathogenesis of ALS and offer a theoretical foundation for the development of therapeutic strategies.
Serum Trace Elements and their Associations with Disease Progression and Survival in Sporadic Amyotrophic Lateral Sclerosis: Insights from a Chinese Cohort
doi: 10.3967/bes2025.094
- Received Date: 2025-04-20
- Accepted Date: 2025-08-01
-
Key words:
- Amyotrophic lateral sclerosis /
- Metal/metalloid /
- Microelement /
- Risk factor
Abstract:
The authors declare no conflicts of interest.
The study was approved by the Ethics Committee of the First Medical Center of Chinese PLA General Hospital(No.: S2022-119), and written informed consent was obtained from all participants.
&These authors contributed equally to this work.
| Citation: | Hongfen Wang, Qionghua Sun, Rongrong Du, Shiya Wang, Yan Wang, Jiongming Bai, Mao Li, Xusheng Huang. Serum Trace Elements and their Associations with Disease Progression and Survival in Sporadic Amyotrophic Lateral Sclerosis: Insights from a Chinese Cohort[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2025.094 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: