-
Silicosis, a major persistent occupational disease in China, is a progressive and irreversible pulmonary fibrosis disease with unclear pathogenesis. Cellular senescence, a state of stable cell cycle arrest that is recognized as a key underlying factor in age-related fibroproliferative disorders, plays an important role in chronic lung diseases, particularly pulmonary fibrosis. We previously reported that SiO2-stimulated mice and alveolar type II epithelial cells develop cellular senescence, which is involved in silicosis formation in alveolar type II epithelial cells[1]. Cellular senescence may play an important role in silicosis development; however, the exact underlying mechanisms are not fully understood.
MicroRNA (miRNA)-411 is a tumor suppressor that inhibits the progression of multiple cancers, and miR-411-3p attenuates silica-induced pulmonary fibrosis by regulating myofibroblast transformation[2]. As a predictive target gene for miR-411-3p, Ras homolog gene family member B (RhoB) is a member of the Ras superfamily of small GTPases. Rho-associated coiled-coil containing protein kinase (ROCK) is a downstream effector of RhoA and RhoC[3]. RhoB inhibits proliferation, invasion, epithelial–mesenchymal transition, and phosphatase and tensin homolog/AKT serine/threonine kinase pathway signaling in breast tumor cells[4]. Additionally, RhoB/ROCK signaling is involved in fibrosis regulation[5]. More importantly, RhoB regulates cellular aging, and the mechanisms regulating senescence-associated cellular, oncogene-induced, and stress-induced (e.g., irradiation) senescence need to be explored[6]. Myocardin-related transcription factor A (MRTF-A) is a coactivator of serum response factor (SRF). MRTF-A is a key regulator of fibroblast differentiation and induces COL1 accumulation[7]. The RhoA-SRF-MRTFA pathway promotes invasion and metastasis of nasopharyngeal carcinoma[8]. RhoA/ROCK signaling regulates TGF-β1-induced fibroblast transformation via MRTF-A[9].
To explore the anti-silicotic mechanisms of miR-411-3p by inhibiting cell senescence, a silicotic model was induced by intratracheal instillation of 2.5 mg silica turbid liquids (0.05 mL) into each mouse, which were euthanized on day 28 after SiO2 treatment. The mice were randomly divided into four groups (n = 6 per group): control, SiO2, SiO2 plus agomir-NC, and SiO2 plus agomir miR411-3p. In the in vitro experiment, MLE-12 cells were cultured and treated with SiO2, miRNA, or siRNA, as previously reported[10].
Silicotic lesions were identified using H&E staining, Masson’s trichrome staining, micro-CT (NEMO-II NMC-200 micro-CT device, PINGSENG Healthcare Inc., Kunshan, China), and lung function measurements (FinePointe whole-body plethysmography system). Cell cycle and apoptosis were detected by flow cytometry. A double-luciferase reporter assay was performed to verify whether RhoB was the target gene of miR-411-3p. The levels of miR-411-3p, fibrotic markers (COL1, α-SMA, MRTF-A, and SRF), cell proliferation indicators (cyclin D, cleaved caspase 3, and cleaved caspase 9), senescence markers (P21, P16, and p-P53), and Rho signaling pathway markers (RhoB, ROCK1, and p-phosphorylated-myosin phosphatase target) were detected or measured by immunofluorescence and immunohistochemistry staining, real-time reverse transcription, and western blot. Quantitative results were normalized to the corresponding controls and housekeeping genes.
All data are presented as median ± standard deviation, and multiple comparisons were used for the analysis of one-way variance. In addition, constant variance was analyzed using the least significant difference test, and heterogeneity of variance was determined using the Tamhane’s test. An independent-samples t-test was used to compare the means of the two samples, and Levene's test was used for equality of variances. All statistical analyses were performed using Statistical Package for the Social Sciences version 23.0. Statistical significance was set at P < 0.05, with a 95% confidence interval.
In this study, we found that treatment with the miR-411-3p mimic reduced the percentage of apoptotic cells induced by silica, along with decreasing the levels of cleaved caspase 3 and 9 in MLE 12 cells (Supplementary Figure S1A–D), which was reversed by the miR-411-3p inhibitor. Furthermore, the cell counts in the S and G2 phases increased in the miR-411-3p mimic treatment group, as measured by flow cytometry (Supplementary Figure S2A) with an increase in cyclin D1 levels in SiO2-treated MLE 12 cells (Supplementary Figure S2B–E). In contrast, the miR-411-3p inhibitor exerted opposite effects, suggesting that miR-411-3p inhibits cellular apoptosis and promotes cellular proliferation in SiO2-induced MLE 12 cells.
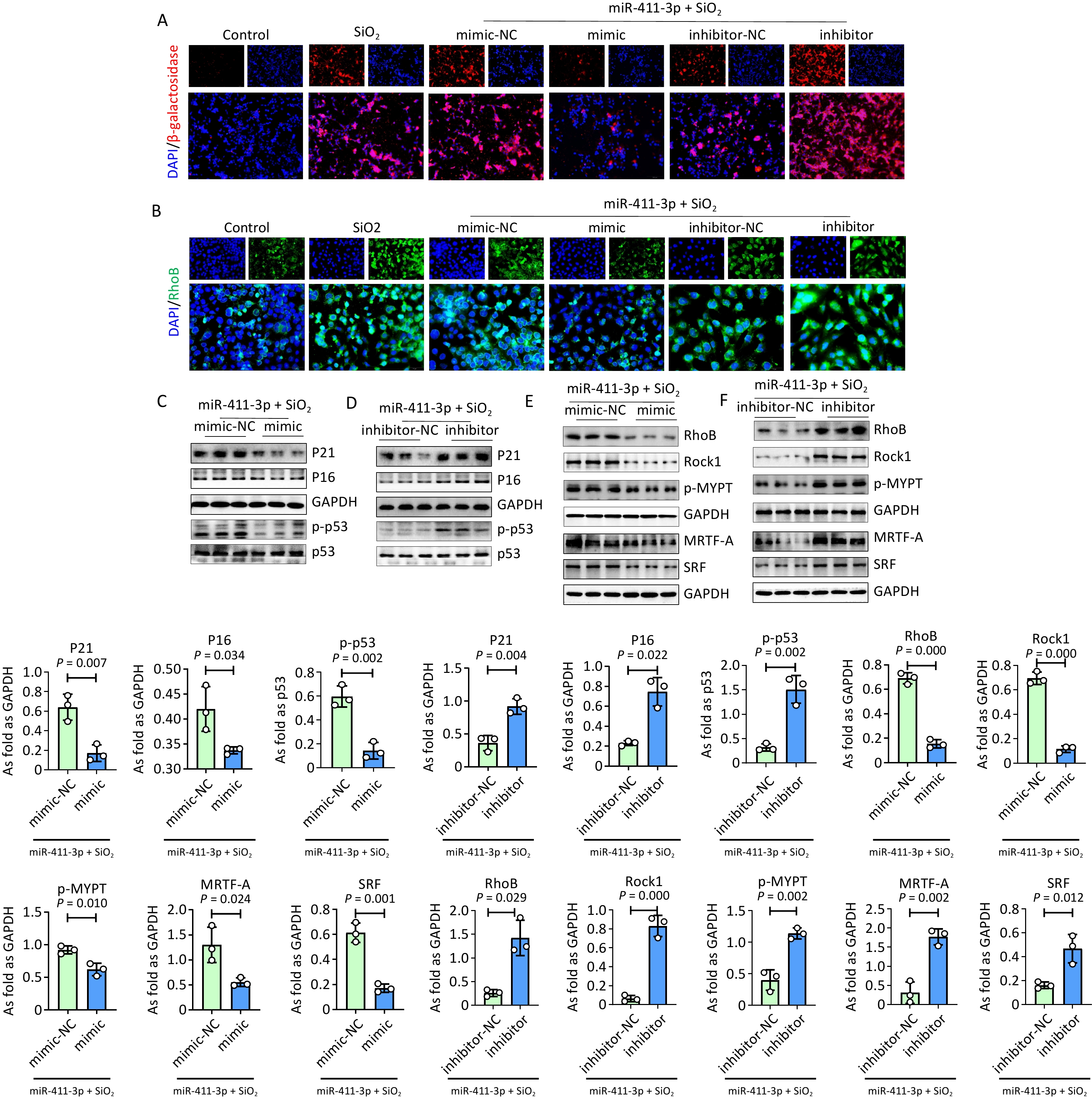
Furthermore, treatment with silica resulted in cellular senescence, with increased levels of RhoB, Rock1, and p-MYPT in MLE-12 cells (Supplementary Figure S3). A double-luciferase reporter assay revealed that RhoB was one of the target genes regulated by miR-411-3p (Supplementary Figure S3C). Silica-induced cellular senescence and upregulation of RhoB signaling were reduced by miR-411-3p mimic treatment in MLE-12 cells (Figure 1). Consistent with previous studies[2,10], MRTF-A and SRF levels were inhibited by miR-411-3p in SiO2 treated MLE-12 cells.
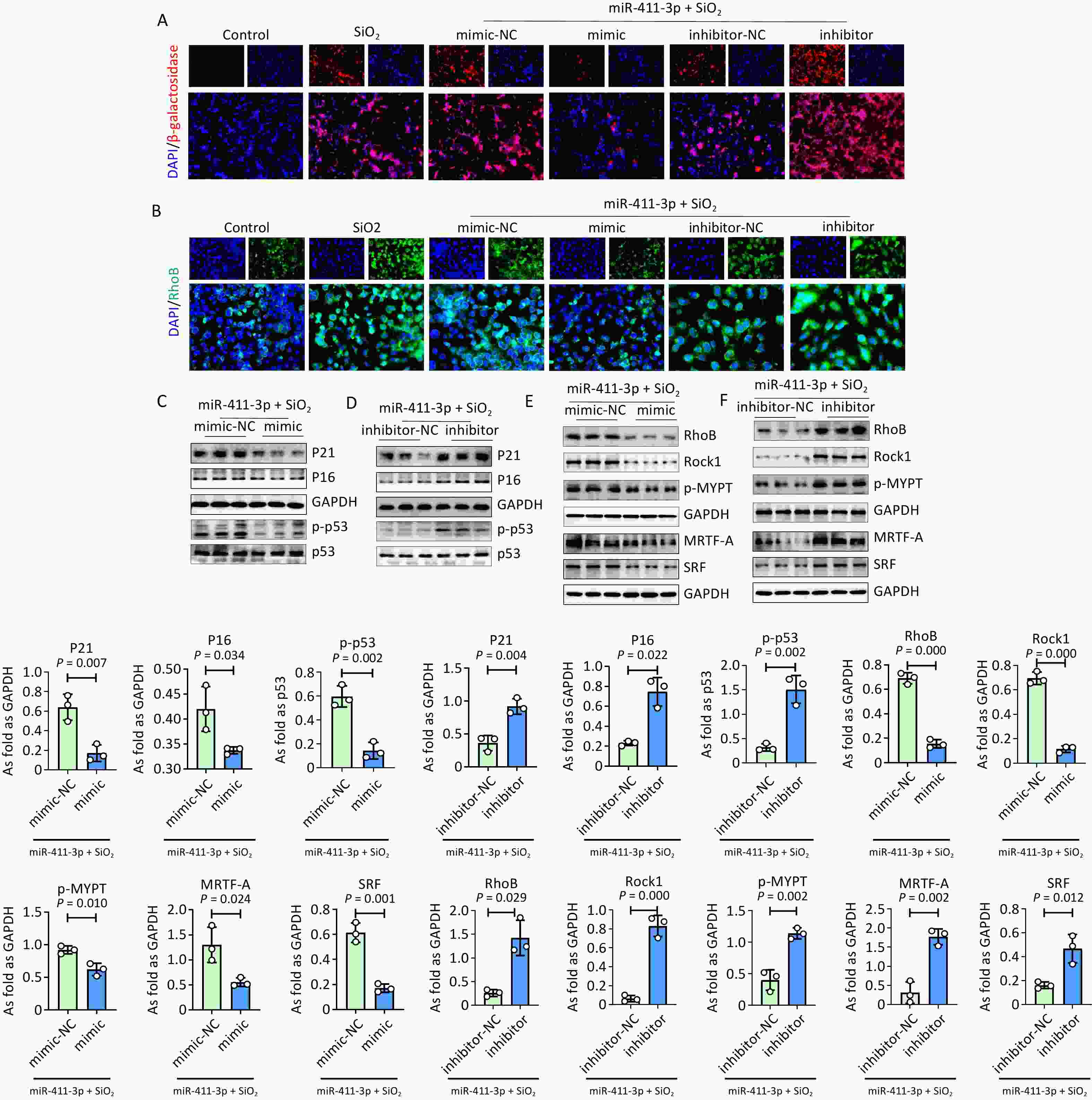
Figure 1. MiR-411-3p inhibited senescence and suppressed MRTF-A/SRF signaling in MLE12 cells induced by SiO2. (A) The expression of β-galactosidase was detected by immunofluorescence staining in SiO2 treated MLE12 cells transfected with miR-411-3p mimic/inhibitor (scale bars: 50 μm); (B) Immunofluorescence staining for RhoB in SiO2 treated MLE12 cells transfected with
To further explore the role of RhoB in silica-induced cellular senescence, RhoB was silenced in MLE 12 cells (Supplementary Figure S4A). Immunofluorescence staining and western blot results showed that the expression levels of MRTF-A, β-galactosidase, and senescence markers were significantly reduced in SiO2-induced MLE 12 cells transfected with Rhob siRNA (Figure 2A and Supplementary Figure S4B). Overexpression of Rhob promoted cellular senescence and MRTF-A expression in silica-treated MLE-12 cells (Supplementary Figure S4C). Treatment with Mrtfa siRNA also reduced the levels of β-galactosidase, MRTF-A, P21, P16, and p-p53 in SiO2-treated MLE 12 cells (Figure 2B). These results showed that the downregulation of RhoB and MRTF-A inhibited silica-induced AT2 cell senescence, which may be regulated by miR-411-3p.
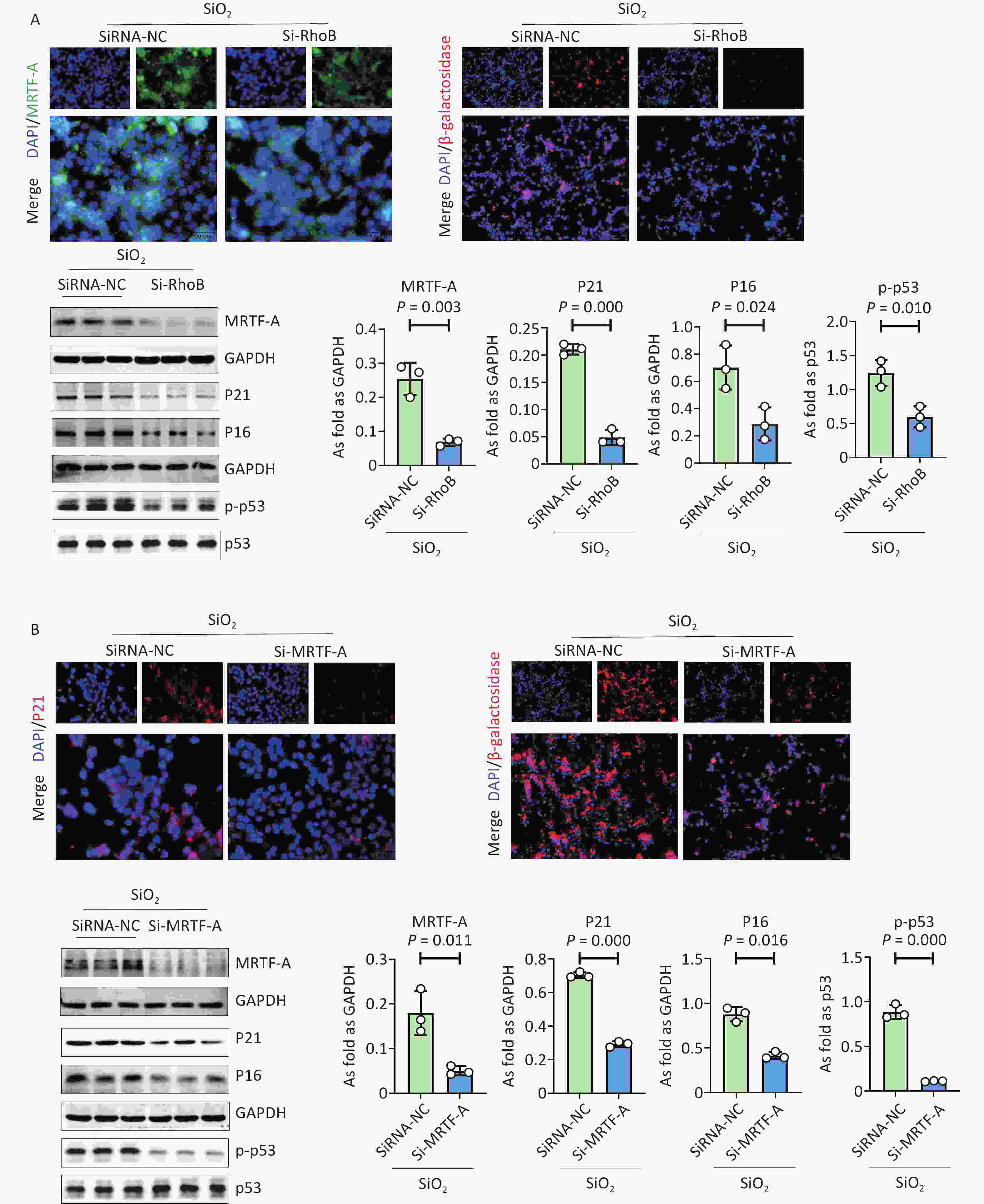
Figure 2. The effect of silencing RhoB or silencing MRTF-A in inhibited MLE12 cell senescence induced by SiO2. (A) Immunofluorescence staining for MRTF-A and β-galactosidase (scale bars: 50μm) and the protein expression of MRTF-A、P21、P16 and p-p53 in SiO2 induced MLE12 cells treated with RhoB gene silencing; (B) Immunofluorescence staining for P21 and β-galactosidase (scale bars: 50μm) and western blot for the protein expression of MRTF-A, P21, P16 and p-p53 in SiO2 induced MLE12 cells treated with MRTF-A gene Silencing.
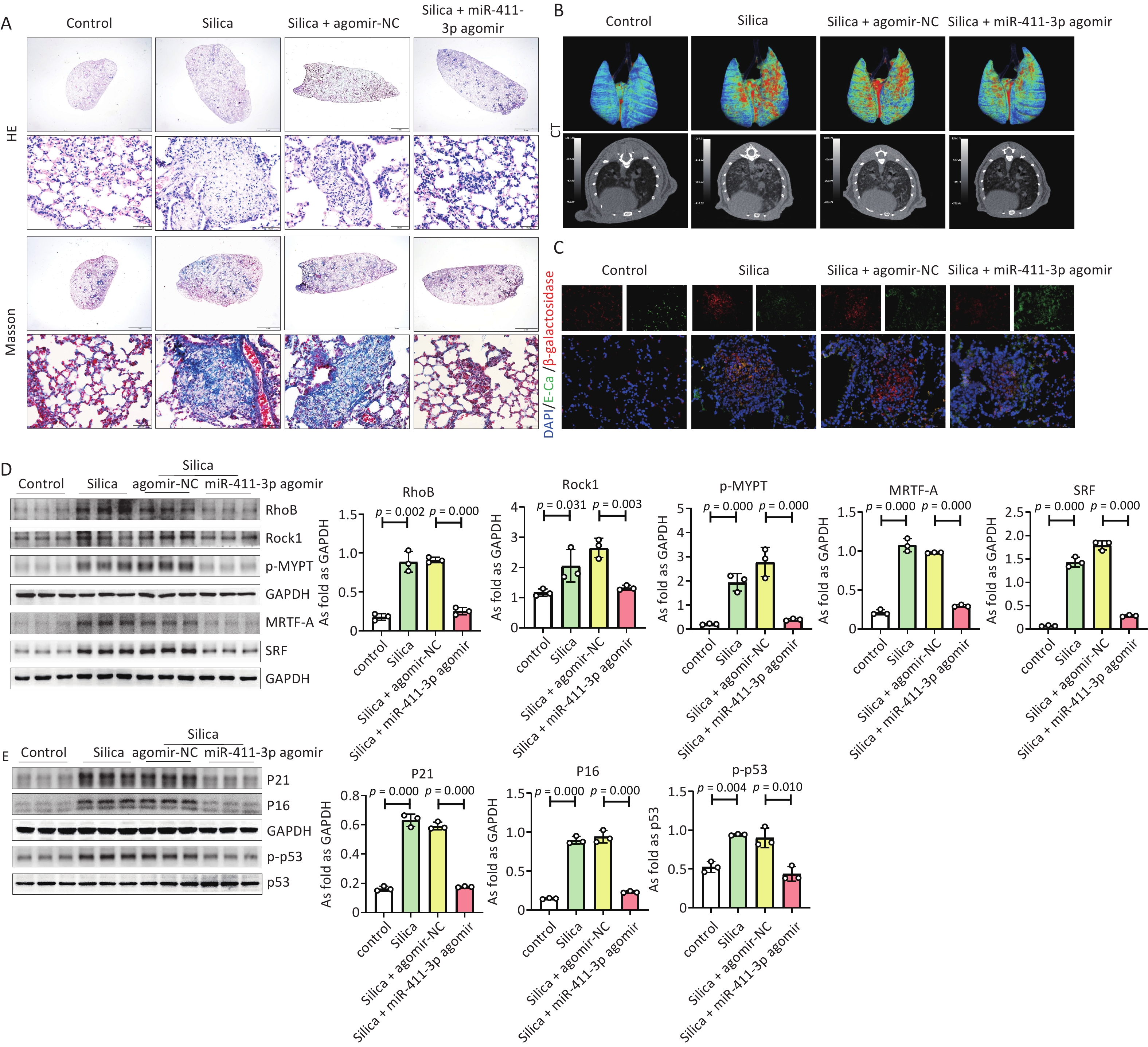
In addition, we observed a potential anti-fibrotic effect of miR-411-3p on silicosis by regulating cell senescence via the RhoB/MRTF-A/SRF signaling pathway. The results showed that treatment with miR-411-3p agomir reduced the silicotic nodules (Figure 3A, Supplementary Figure S5A), collagen and α-SMA expression (Supplementary Figure S5C, D), and percentage of CT high density volume (Figure 3B, Supplementary FigureS5A) and improved lung function (Supplementary Figure S5B), suggesting that miR-411-3p agomir alleviated SiO2-induced pulmonary fibrosis in mice. Immunohistochemistry staining and western blotting results indicated that miR-411-3p agomir reduced high levels of RhoB/ROCK1 and MRTF-A/SRF signaling in silicotic mice. MiR-411-3p agomir also decreased the expression of β-galactosidase, P21, P16, and p-p53 in SiO2-induced mouse lungs (Figure 3C, D, F, Supplementary FigureS5F). These results show that miR-411-3p agomir attenuates silicosis by inhibiting alveolar epithelial cell senescence through the RhoB/MRTF-A/SRF signaling pathway and that it is a potential therapeutic target for silicosis. This study had some limitations, such as the lack of verification of human silicosis samples, which will require further in-depth experiments.
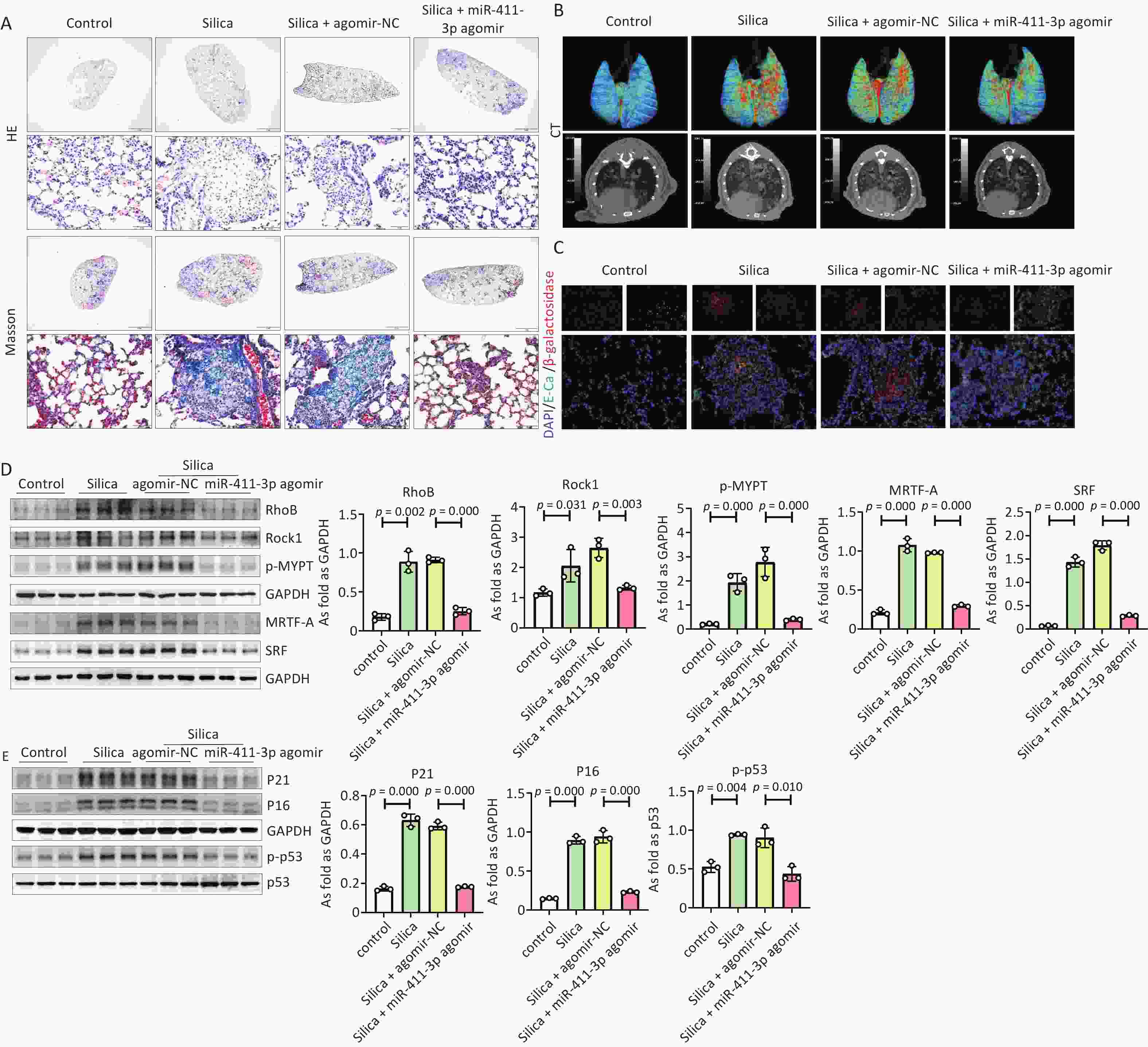
Figure 3. MiR-411-3p agomir alleviated silica-induced pulmonary fibrosis by RhoB/Rock1 and MRTF-A/SRF signaling and inhibited cell senescence in mice. (A) The H.E. staining, Masson staining of lung tissue of mice treated with silica (scale bars: 2 mm/50 μm); (B) The CT of lung tissue of mice treated with silica (scale bars: 2 mm/50 μm); (C) The co-expression of β-galactosidase and E-Ca was detected by immunofluorescence staining (scale bars: 50μm); (D) The protein expression of RhoB, Rock1, p-MYPT, MRTF-A and SRF in mice lung tissue; (Data are means ± SD; n = 6 independent experiments); (E) The protein levels of P21, P16 and p-p53 in lung tissue independent experiments). of mice treated with silica measured by Western blot. (Data are means ± SD; n = 6 independent experiments).
Overall, this study showed that miR-411-3p attenuates cell senescence by inhibiting RhoB/MRTF-A/SRF signaling to inhibit silicosis in vivo and in vitro.
MicroRNA-411-3p Attenuates Cell Senescence in SiO2-induced Pulmonary Fibrosis
doi: 10.3967/bes2025.097
- Received Date: 2025-02-09
- Accepted Date: 2025-06-04
The authors declare that no competing interests exist.
This study was approved by the Ethics Committee for Animal Experiments of North China University of Science and Technology (LX2021147) and conformed to the guidelines set by the National Institutes of Health.
&These authors contributed equally to this work.
| Citation: | Zelin Xu, Siqi Liu, Xiao Yu, Siyi Wang, Bingbing Li, XinYu Wang, Siyuan Shan, Hong Xu, Bonan Zhang, Yiwei Shi, Xuemin Gao. MicroRNA-411-3p Attenuates Cell Senescence in SiO2-induced Pulmonary Fibrosis[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2025.097 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: