-
Sarcopenia, a geriatric syndrome characterized by the progressive loss of muscle strength, muscle mass, and physical performance, begins to affect individuals at approximately of 40 years, with a marked decrease observed after 70 years[1,2]. It has emerged as a major public health challenge with a global prevalence ranging from 10% to 16% among older adults[3]. This condition notably affects physical function and increases the risk of several chronic diseases, including falls, diabetes, cardiovascular disease (CVD), cognitive impairment, and depression[4-8].
Social isolation, defined as an objective lack of social contact and limited social engagement, is another pervasive global concern, affecting approximately 25% of community-dwelling older adults[9,10]. Previous studies have linked social isolation to various adverse health outcomes, including hypertension, diabetes, and CVD[11-13].
Emerging evidence suggests that sarcopenia and social isolation often coexist[14,15] and may exacerbate each other’s effect. Social isolation can exacerbate sarcopenia by reducing physical activity levels[16], impairing nutritional intake[17], and inducing chronic inflammation[18]. Conversely, older adults with sarcopenia may experience loss of physical independence, frequent falls, and poor quality of life, resulting in restricted social networks and intensified social isolation[19]. This bidirectional relationship underscores the need for comprehensive research to understand how these conditions interact and contribute to mortality among older adults. Although previous studies have found that sarcopenia and social isolation are independently associated with mortality[20,21], limited research has explored their joint associations. The interaction between these conditions may result in synergistic effects, potentially leading to worse health outcomes than with either condition alone. Investigating these joint associations is crucial for identifying vulnerable populations and developing targeted interventions to mitigate mortality risk.
To address this gap, we analyzed data from two large-scale prospective cohorts, the Chinese Longitudinal Healthy Longevity Survey (CLHLS) and the UK Biobank, to investigate the joint associations of sarcopenia and social isolation with mortality risk.
-
The CLHLS is a nationally representative cohort that focuses on the determinants of longevity among adults aged 65 years and above in China, and it has been followed up every 2–3 years since the baseline in 1998. Owing to the lack of exposure information in previous visits, the 2008–2009 follow-up visit was selected as the baseline for the current analysis. The UK Biobank is a prospective cohort of over 500,000 participants aged 37–73 years from 2006 to 2010. The CLHLS provides unique insights into aging patterns among Chinese older adults, whereas the UK Biobank offers extensive data on Western middle-aged and older populations. Their comprehensive assessments of age-related health determinants and outcomes provide sufficient statistical power for longitudinal analyses and enable valuable cross-cultural comparisons. The CLHLS study maintained ethical standards with approval from the Research Ethics Committee of Peking University (IRB00001052-13074), and the UK Biobank study was approved by the North West Multicenter Research Ethics Committee. All the participants or their legal representatives provided written informed consent.
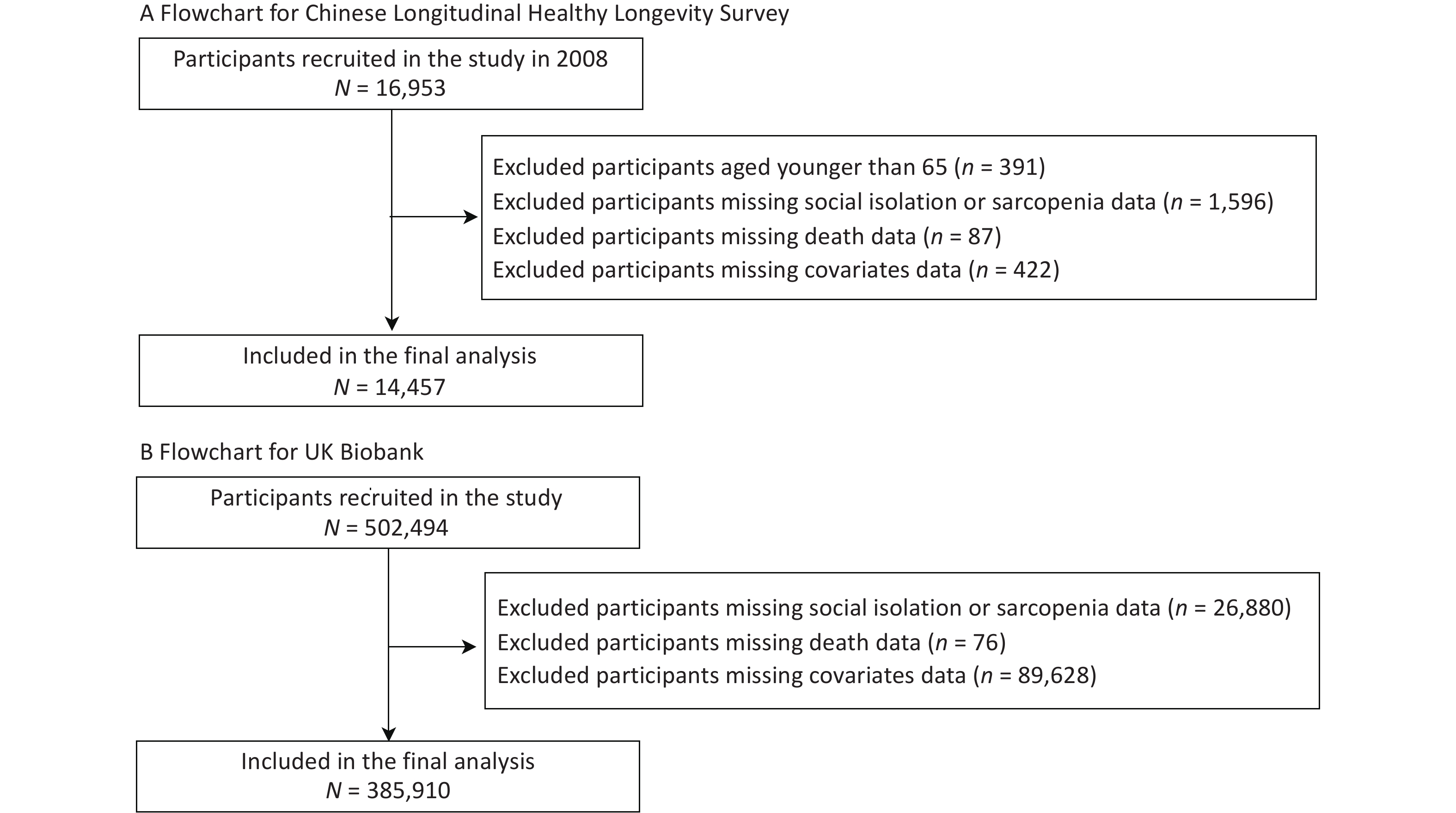
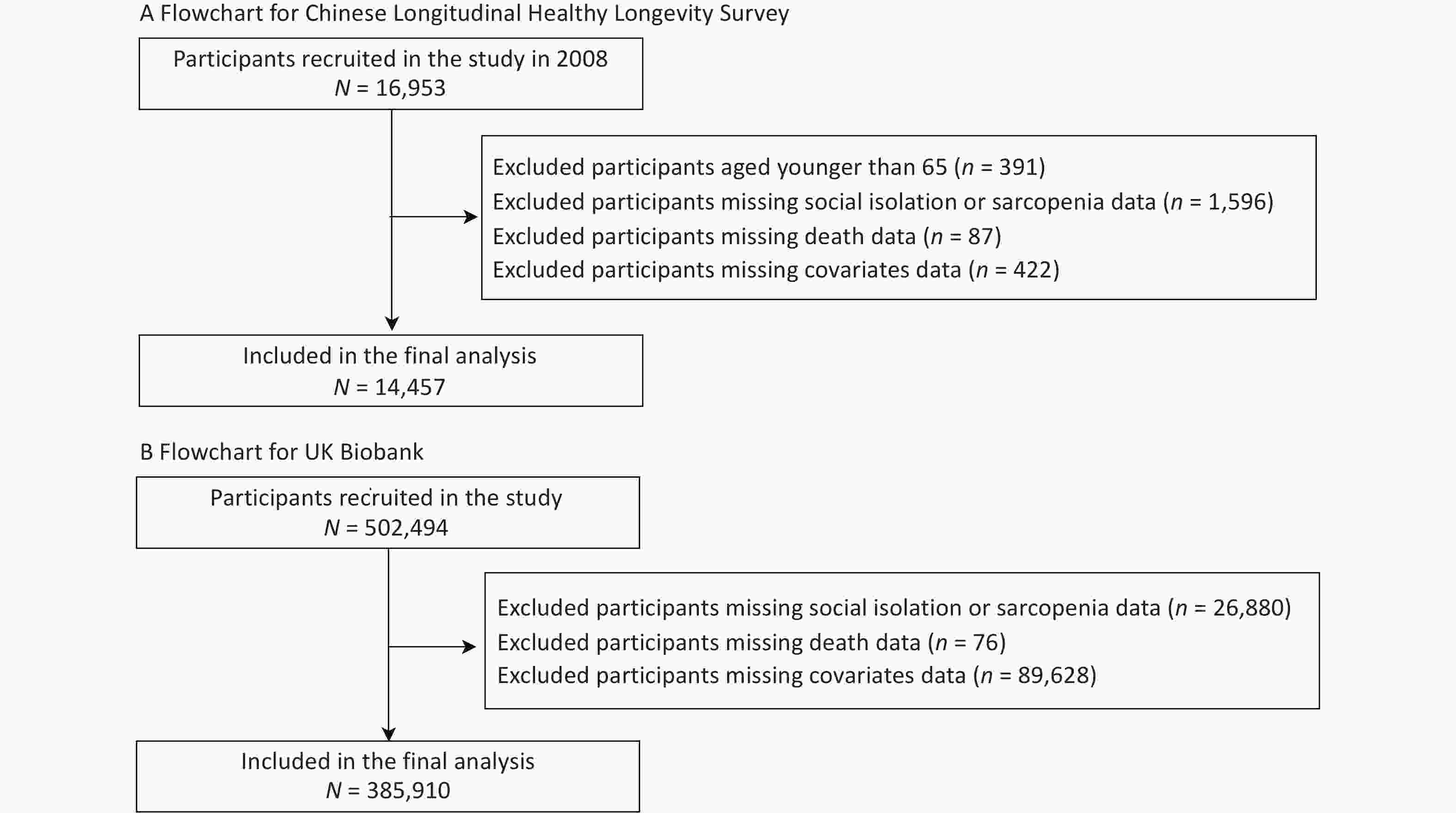
After excluding participants in the CLHLS who were younger than 65 years (n = 391) and those with missing data on sarcopenia or social isolation (n = 1,596), death (n = 87), and the covariates of interest (n = 422), 14,363 Chinese adults were included in the analyses. Similarly, we included 385,910 European adults from the UK Biobank after excluding participants with missing data on sarcopenia or social isolation (n = 26, 880), death (n = 76), or the covariates (n = 89,628). To fully utilize the comprehensive age coverage and statistical power of the UK Biobank, we included the full age range in our main analysis. Figure 1 shows the selection process for the study population.
-
In the CLHLS, sarcopenia was defined according to the Asian Working Group on Sarcopenia in Older People 2019 (AWGSOP2) criteria, which includes three dimensions: low muscle strength, low muscle mass, and low physical performance[22]. Low muscle strength was identified by self-reported difficulty in lifting 5 kg, and low physical performance by self-reported difficulty walking 1 km or crouching and standing three times[23]. Muscle mass was assessed using the appendicular skeletal muscle mass (ASM) index, with thresholds of < 3.98 kg/m2 for women and < 6.37 kg/m2 for men, calculated based on a specific equation for the Chinese population[24,25]. In the UK Biobank, sarcopenia was defined according to the European Working Group on Sarcopenia in Older People 2019 (EWGSOP2) criteria, covering three dimensions[26]. Low muscle strength was defined by grip strength (< 27 kg for men and < 16 kg for women), and low muscle mass was represented by appendicular lean mass (ALM/height2 < 7.0 kg/m2 for men and < 5.5 kg/m2 for women). Due to the absence of direct physical performance measurements, a slow walking pace (< 3 mph) was used as a proxy[27,28] (Supplementary method).
In the CLHLS, participants with either low muscle strength or low physical performance but without low muscle mass were classified as having probable sarcopenia. Those who additionally exhibited low muscle mass were classified as having confirmed sarcopenia. In the UK Biobank, possible sarcopenia was identified based on low muscle strength alone, whereas confirmed sarcopenia required the presence of both low muscle strength and mass. In addition, those who had low muscle strength, low muscle mass, and low physical performance were classified as having severe sarcopenia in both cohorts[22,26]. However, as only 204 (0.05%) participants in the UK Biobank had severe sarcopenia at baseline, and the corresponding number in the CLHLS was 1,799 (12.4%), they were grouped with confirmed sarcopenia in the analyses[27].
-
To maintain consistency with previous studies, distinct methods were employed to assess social isolation in the two cohorts[29,30]. Social isolation was evaluated according to the sum of the following items: living alone, frequency of leisure/social activities, frequency of contact with friends or family, and marital status (for the CLHLS only). Each item was assigned a value of 0 or 1, with 1 representing living alone, fewer social activities, less contact with others, and lack of a spouse. The social isolation index ranged from 0 to 3 in the UK Biobank and from 0 to 4 in the CLHLS. Participants were then divided into three groups based on the social isolation index: least social isolation (score = 0), moderate social isolation (score = 1), and most social isolation (scored ≥ 2)[12] (Supplementary method).
-
The primary outcome was all-cause mortality in both cohorts, and the secondary outcome was cause-specific mortality, including CVD and cancer mortality in the UK Biobank. In the CLHLS, the survival status and date of death were ascertained through follow-up interviews with close family members or community doctors, with data available until 2018. Unfortunately, the causes of death were not available in the CLHLS. In the UK Biobank, the date and cause of death were retrieved from the mortality databases maintained by the National Health Service (NHS) Information Centre (England and Wales) and NHS Central Register Scotland (Scotland). ICD-10 codes were used to define CVD mortality (I00-I99) and cancer-related mortality (C00-C97). Mortality data were available until April 1, 2022 in England; March 1, 2022, in Scotland; and October 7, 2021, in Wales. Person-years were calculated from the date of entry to the date of death or the last date of follow-up, whichever occurred first.
-
Covariates for adjustment were selected a priori based on their availability and general knowledge. In the CLHLS, the covariates included age (years, continuous), sex (women, men), residence (city, rural), economic level (rich, fair/poor), education level (high, low), smoking status (never, former, and current), alcohol intake (never, former, and current), healthy diet score (continuous), physically active (yes, no), body mass index (BMI, underweight: < 18.5, normal: 18.5–24.0, and overweight/obesity: ≥ 24.0 kg/m2), and the number of baseline diseases (0, 1, and ≥ 2). Baseline diseases were assessed based on self-reported hypertension, diabetes, coronary heart disease, stroke, and cancer.
Correspondingly, in the UK Biobank, we chose age (years, continuous), sex (women, men), ethnicity (white, others), Townsend Deprivation Index (TDI, continuous), education level (high, low), smoking status (never, former, and current), alcohol intake (never, former, and current), healthy diet score (continuous), physically active (yes, no), BMI (underweight: < 18.5, normal: 18.5–25.0, and overweight/obesity: ≥ 25.0 kg/m2), and the number of baseline diseases (0, 1, and ≥ 2) as covariates (Supplementary method).
-
Baseline characteristics are presented as mean (standard deviation, SD) or median (interquartile range, IQR) for continuous variables and number (percentages) for categorical variables. Cox proportional hazards regression models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CI) of outcomes associated with sarcopenia and social isolation. The proportional hazards assumption was assessed using the Schoenfeld residual method, and the results showed no significant deviation from the assumption (Supplementary Figure S1). In both cohorts, two models were generated for the analysis: Model 1 was adjusted for age and sex; Model 2 was further adjusted for ethnicity and TDI (UK Biobank only), residence and economic level (CLHLS only), education level, smoking status, alcohol intake, healthy diet score, physically active, BMI, the number of baseline diseases, and sarcopenia (in the social isolation analysis) or social isolation (in the sarcopenia analysis).
We conducted a stratified analysis by sarcopenia class to investigate the association between social isolation and health outcomes in patients with different sarcopenia statuses. As only 955 (0.2%) European adults and 2,229 (15.4%) Chinese adults had confirmed sarcopenia, we merged possible and confirmed sarcopenia into one group (sarcopenia) to avoid small-sample issues and increase statistical power. Additive and multiplicative interactions were assessed by incorporating a cross-product term for social isolation (least, moderate, and most) and sarcopenia (non-sarcopenia and sarcopenia) into multivariable Cox models.
To understand the joint effects of social isolation and sarcopenia on mortality, we classified participants into six groups according to sarcopenia (non-sarcopenia and sarcopenia) and social isolation (least, moderate, and most), using individuals without sarcopenia and least social isolation as the reference group. Kaplan–Meier curves were plotted to visualize the differences in survival probability between the groups. We also tested the association between the components of social isolation and all-cause mortality.
Several sensitivity analyses were conducted to test the robustness of the results. (1) We repeated the analyses after excluding those who died within the first 2 years of enrollment to minimize the potential impact of reverse causality. (2) We refined our findings by excluding participants with cancer or CVD at baseline to mitigate the potential bias of short-term survival associated with these conditions. (3) We repeated the main analyses using multiple imputations for missing covariates. (4) We incorporated loneliness and psychological scores into the model to reduce confounding effects. (5) We further advanced the censoring date to December 31, 2019, in the UK Biobank to reduce the potential bias introduced by the COVID-19 pandemic. (6) We included only those aged 65 years and older in the UK biobank to better align with the CLHLS cohort. (7) We selected participants in the same age range from the two cohorts and adjusted for the same covariates in the model. (8) We restricted the follow-up duration to within 5 years for both cohorts.
All statistical analyses and visualizations were conducted using SAS (version 9.4; SAS Institute) and R software (version 4.1.0; R Foundation for Statistical Computing, Vienna, Austria). A two-sided P value of < 0.05 was used to indicate statistical significance for all analyses.
-
Table 1 shows the baseline characteristics of participants from the CLHLS and UK Biobank. Among the 14,457 participants with CLHLS (mean age, 86.4 years; 56.1% women), 8,877 (61.4%) were identified as having sarcopenia. In the UK Biobank, among the 385,910 participants (mean age, 56.3 years; 52.5% women), 24,372 (6.3%) had sarcopenia. The patients in the CLHLS cohort were older and had a higher prevalence of sarcopenia than those in the UK Biobank cohort. Those with sarcopenia tended to be women, non-white, urban dwellers, less educated, never-smokers, never-drinkers, and lower economic status. They also exhibited poorer health and a higher likelihood of experiencing social isolation.
Characteristics CLHLS UK Biobank Total Population Non-sarcopenia Probable and Confirmed Sarcopenia Total Population Non-sarcopenia Probable and Confirmed Sarcopenia (n = 14,457) (n = 5,580) (n = 8,877) (n = 385,910) (n = 361,538) (n = 24,372) Age (years), mean±SD 86.4 (11.3) 78.3 (9.5) 91.5 (9.1) 56.3 (8.1) 56.0 (8.1) 59.7 (7.3) Sex, n (%) Women 8,103 2,348 (29.0) 5,755 (71.0) 202,475 187,243 (92.5) 15,232 (7.5) Men 6,354 3,232 (50.9) 3,122 (49.1) 183,435 174,295 (95.0) 9,140 (5.0) Ethnicity, n (%) White NA NA NA 366,234 343,830 (93.9) 22,404 (6.1) Others NA NA NA 19,676 17,708 (90.0) 1,968 (10.0) Residence, n (%) City 2,871 1,055 (36.8) 1,816 (63.2) NA NA NA Rural 11,586 4,525 (39.1) 7,061 (60.9) NA NA NA TDI, mean±SD NA NA NA −1.4 (3.0) −1.4 (3.0) −1.0 (3.1) Economic level, n (%) Rich 1,961 866 (44.2) 1,095 (55.8) NA NA NA Fair/Poor 12,496 4,714 (37.7) 7,782 (62.3) NA NA NA Education level, n (%) High 5,617 3,078 (54.8) 2,539 (45.2) 135,915 128,974 (94.9) 6,941 (5.1) Low 8,840 2,502 (28.3) 6,338 (71.7) 249,995 232,564 (93.0) 17,431 (7.0) Smoking status, n (%) Never 9,565 3,140 (32.8) 6,425 (67.2) 211,712 197,788 (93.4) 13,924 (6.6) Former 2,318 950 (41.0) 1,368 (59.0) 135,117 126,816 (93.9) 8,301 (6.1) Current 2,574 1,490 (57.9) 1,084 (42.1) 39,081 36,934 (94.5) 2,147 (5.5) Alcohol intake, n (%) Never 9,921 3,439 (34.7) 6,482 (65.3) 14,921 13,265 (88.9) 1,656 (11.1) Former 2,001 785 (39.2) 1,216 (60.7) 12,880 11,723 (91.0) 1,157 (9.0) Current 2,535 1,356 (53.5) 1,179 (46.5) 358,109 336,550 (94.0) 21,559 (6.0) Healthy diet score, median (IQR) 10.0
(8.0, 11.0)10.0
(9.0, 12.0)9.0
(8.0, 11.0)3.0
(2.0, 4.0)3.0
(2.0, 4.0)3.0
(2.0, 4.0)Physically active, n (%) No 10,291 3,269 (31.8) 7,022 (68.2) 69,980 65,770 (94.0) 4,210 (6.0) Yes 4,166 2,311 (55.5) 1,855 (44.5) 315,930 295,768 (93.6) 20,162 (6.4) BMI, n (%) Underweight 4,641 1,239 (26.7) 3,402 (73.3) 1,881 1,628 (86.6) 253 (13.4) Normal 7,793 3,320 (42.6) 4,473 (57.4) 128,460 120,142 (93.5) 8,318 (6.5) Overweight/Obesity 2,023 1,021 (50.5) 1,002 (49.5) 255,569 239,768 (93.8) 15,801 (6.2) No. of diseases at baseline, n (%) 0 9,818 3,916 (39.9) 5,902 (60.1) 150,959 142,619 (94.5) 8,340 (5.5) 1 3,088 1,155 (37.4) 1,933 (62.6) 186,764 174,876 (93.6) 11,888 (6.4) ≥ 2 1,551 509 (32.8) 1,042 (67.2) 48,187 44,043 (91.4) 4,144 (8.6) Social isolation, n (%) Least 1,204 962 (79.9) 242 (20.1) 176,952 166,340 (94.0) 10,612 (6.0) Moderate 4,287 2,426 (56.6) 1,861 (43.4) 155,092 145,042 (93.5) 10,050 (6.5) Most 8,966 2,192 (24.5) 6,774 (75.6) 53,866 50,156 (93.1) 3,710 (6.9) Living alone, n (%) 2,273 955 (42.0) 1,318 (58.0) 70,287 64,583 (91.9) 5,704 (8.1) Less contact with friends/family, n (%) 73 12 (16.4) 61 (83.6) 84,387 79,305 (94.0) 5,082 (6.0) Fewer leisure/social activities, n (%) 12,068 3,970 (32.9) 8,098 (67.1) 112,978 105,899 (93.7) 7,079 (6.3) Lack of a spouse, n (%) 9,741 2,587 (26.6) 7,154 (73.4) NA NA NA Note. BMI, body mass index; CLHLS, Chinese Longitudinal Healthy Longevity Survey; IQR, interquartile range; SD, standard deviation; TDI, Townsend deprivation index. Table 1. Baseline characteristics of the study participants by categories of sarcopenia
-
During the median follow-up of 3.0 years (up to 10 years) in the CLHLS group, 8,249 deaths were recorded. In the UK Biobank, during the median follow-up of 13.1 years (up to 15 years), 26,670 deaths occurred, including 5,285 from CVD and 13,805 from cancer. In the CLHLS, sarcopenia was associated with an increased risk of all-cause mortality, with an HR of 1.71 (95% CI: 1.59–1.86) for those with confirmed sarcopenia compared to those without sarcopenia. Similarly, in the UK Biobank, confirmed sarcopenia was linked to higher risks of all-cause mortality (HR: 1.95, 95% CI: 1.70–2.23), CVD mortality (HR: 2.17, 95% CI: 1.62–2.91), but not cancer mortality (HR: 1.07, 95% CI: 0.82–1.39) (Table 2). Social isolation showed varying associations with mortality across cohorts. In the CLHLS, only the most social isolation group displayed a significant association with all-cause mortality, with an HR of 1.30 (95% CI: 1.16–1.45). In the UK Biobank, both moderate and most social isolation status were significantly associated with an increased risk of all-cause mortality, exhibiting HRs of 1.16 (95% CI: 1.13–1.19) and 1.38 (95% CI: 1.33–1.43), respectively. Similar results were observed for CVD- and cancer-related mortality (Table 2).
CLHLS UK Biobank All-cause Mortality All-cause Mortality CVD Mortality Cancer Mortality Cases/
Person-yearsHR
(95% CI)Cases/
Person-yearsHR
(95% CI)Cases/
Person-yearsHR
(95% CI)Cases/
Person-yearsHR
(95% CI)Sarcopenia Non-sarcopenia 2,187/32,251 1 24,298/4,616,786 1 4,793/4,616,786 1 12,731/4,616,786 1 Probable sarcopenia 4,300/22,839 1.63
(1.53–1.73)2,147/292,228 1.12
(1.07–1.17)444/292,228 1.21
(1.10–1.34)1,017/292,228 1.01
(0.94–1.07)Confirmed sarcopenia 1,762/5,803 1.71
(1.59–1.86)225/10,941 1.95
(1.70–2.23)48/10,941 2.17
(1.62–2.91)57/10,941 1.07
(0.82–1.39)Social isolation Least 396/6,827 1 10,683/2,267,399 1 2,010/2,267,399 1 5,830/2,267,399 1 Moderate 1,939/22,564 1.07
(0.95–1.19)11,091/1,974,721 1.16
(1.13–1.19)2,193/1,974,721 1.21
(1.13–1.28)5,677/1,974,721 1.10
(1.07–1.15)Most 5,914/31,502 1.30
(1.16–1.45))4,896/677,835 1.38
(1.33–1.43)1,082/677,835 1.55
(1.44–1.68)2,298/677,835 1.25
(1.19–1.31)Note. Model adjusted for age, sex, ethnicity (UK Biobank only), residence (CLHLS only), Townsend deprivation index (UK Biobank only), economic level (CLHLS only), education level, smoking status, alcohol intake, healthy diet score, physically active, body mass index, the number of baseline diseases, and sarcopenia (in the social isolation analysis) or social isolation (in the sarcopenia analysis). Abbreviations: CLHLS, Chinese Longitudinal Healthy Longevity Survey; CVD, cardiovascular disease; CI, confidence interval; HR, hazard ratio. Table 2. Independent associations of sarcopenia and social isolation with mortality
-
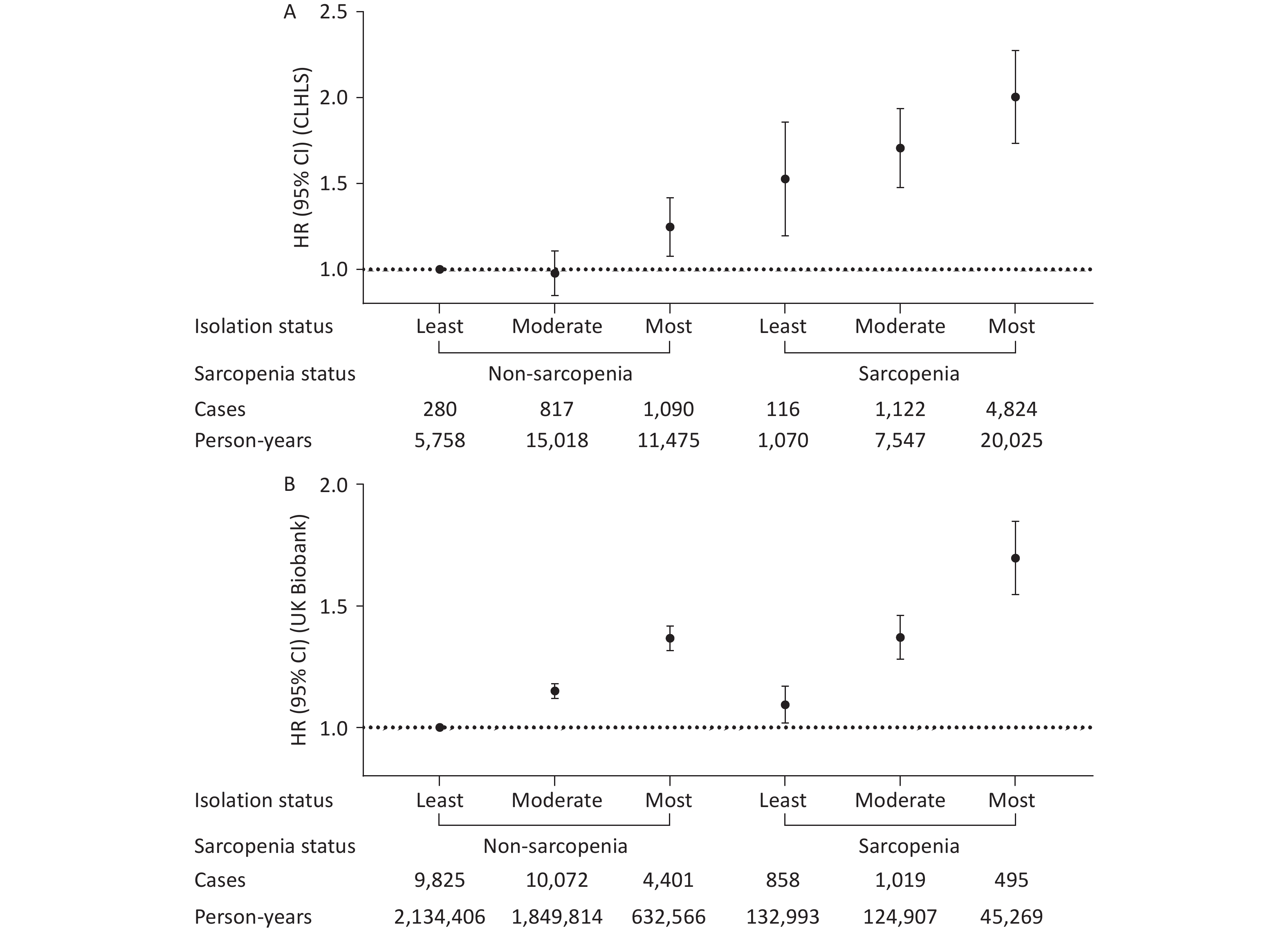
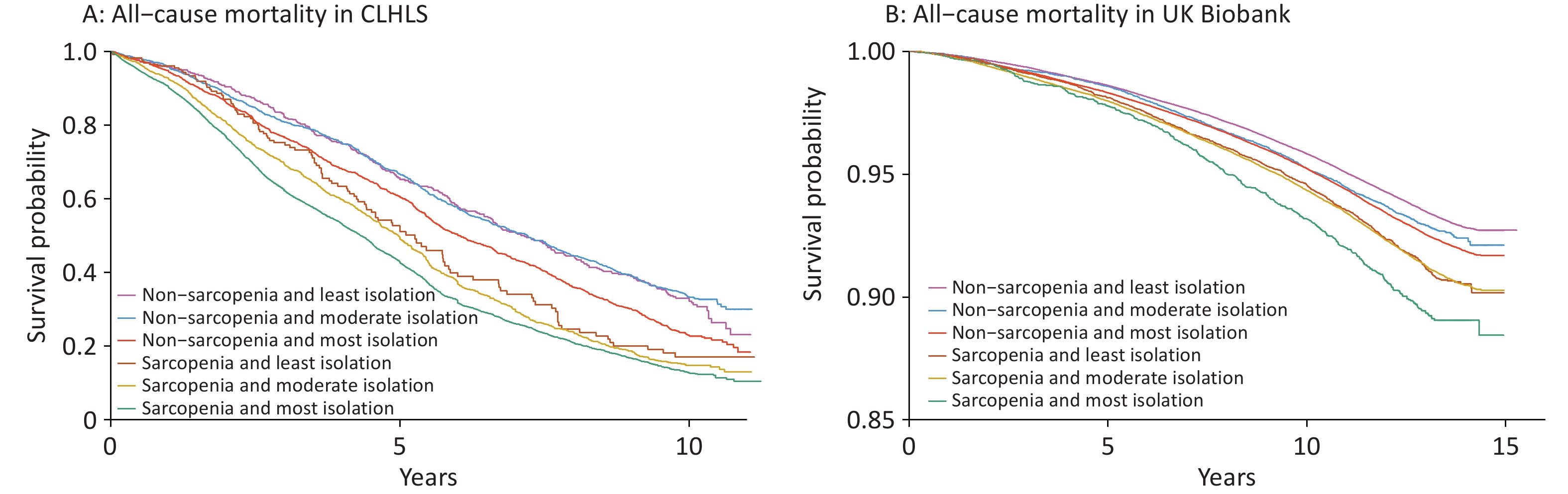
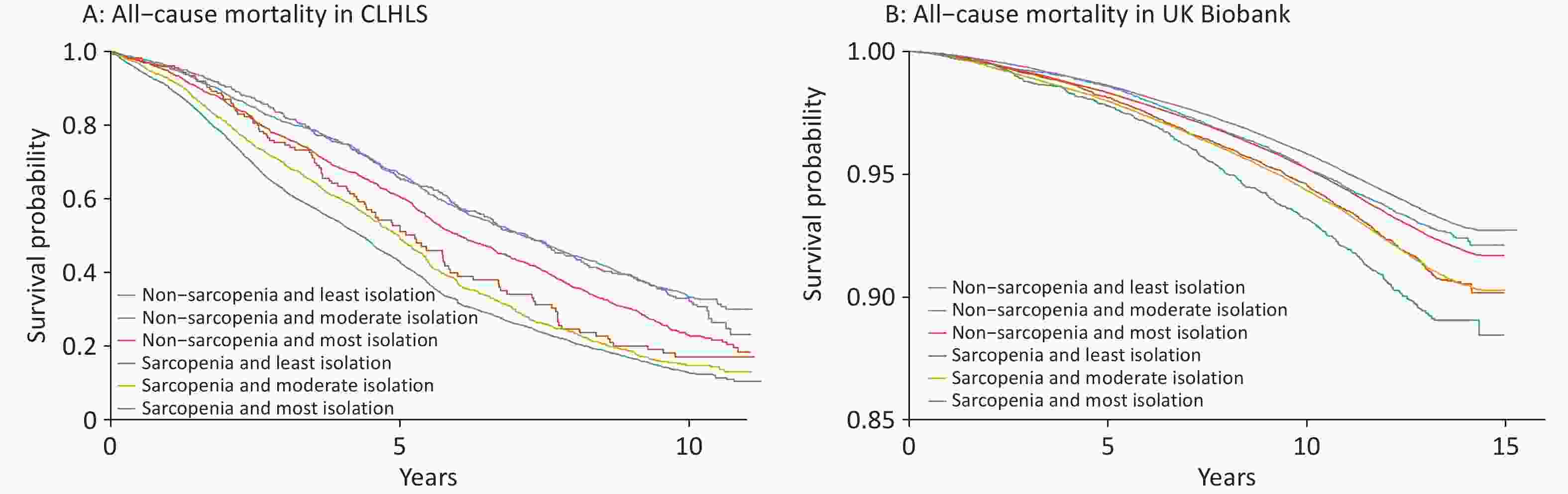
A higher level of social isolation was associated with a higher risk of all-cause mortality, and this association was more pronounced among individuals with sarcopenia in both cohorts (Table 3). For example, the HRs for those with the highest versus lowest social isolation were 1.15 (0.99–1.33) and 1.37 (1.32–1.42) among individuals without sarcopenia, and 1.38 (1.14–1.67) and 1.52 (1.36–1.71) among those with sarcopenia in the CLHLS and UK Biobank, respectively. While no significant interaction was found between sarcopenia and social isolation on all-cause mortality in the CLHLS, both multiplicative and additive interactions were observed in UK Biobank. Specifically, the relative risk due to interaction was 0.23 (95% CI 0.06–0.41) (Figure 2), and P-value for multiplicative interaction was 0.03 (Table 3). Similar patterns were observed for CVD and cancer mortalities; however, no significant interactions were observed (Supplementary Table S1 and Figure S2). Survival curves clearly demonstrated the lowest survival rate among participants with both sarcopenia and social isolation (Figure 3). Cox regression analysis results confirmed these findings. In the CLHLS, compared with participants without sarcopenia and with the least level of social isolation, those experiencing social isolation (HR: 1.24, 95% CI: 1.08–1.42) or sarcopenia (HR: 1.50, 95% CI: 1.21–1.87) had a higher risk of all-cause mortality. The highest risk was observed among participants with both conditions (HR, 1.99; 95% CI: 1.74–2.28) (Figure 2). Similarly, in the UK Biobank, the highest risk was observed among those with both serious conditions, with HRs (95% CIs) of 1.69 (1.55–1.85) for all-cause mortality, 2.03 (1.67–2.45) for CVD mortality, and 1.36 (1.18–1.56) for cancer mortality (Figure 2; Supplementary Figure S2).
CLHLS UK Biobank Cases/Person-years HR (95% CI) P value Cases/Person-years HR (95% CI) P value Non-sarcopenia Least isolation 280/5,758 1 9,825/2,134,406 1 Moderate isolation 817/15,018 0.96 (0.84–1.10) 0.564 10,072/1,849,814 1.15 (1.12–1.18) <0.001 Most isolation 1,090/11,475 1.15 (0.99–1.33) 0.058 4,401/632,566 1.37 (1.32–1.42) <0.001 Probable and Confirmed Sarcopenia Least isolation 116/1,070 1 858/132,993 1 Moderate isolation 1,122/7,547 1.15 (0.95–1.39) 0.164 1,019/124,907 1.25 (1.14–1.37) <0.001 Most isolation 4,824/20,025 1.38 (1.14–1.67) <0.001 495/45,269 1.52 (1.36–1.71) <0.001 P-interaction 0.56 0.03 Note. Model adjusted for age, sex, ethnicity (UK Biobank only), residence (CLHLS only), Townsend deprivation index (UK Biobank only), economic level (CLHLS only), education level, smoking status, alcohol intake, healthy diet score, physically active, body mass index, and the number of baseline diseases. Multiplicative interactions were evaluated using P value for the product term between the social isolation level (least vs. most) and sarcopenia status (non-sarcopenia vs. sarcopenia). Abbreviations: CLHLS, Chinese Longitudinal Healthy Longevity Survey; CI, confidence interval; HR, hazard ratio. Table 3. Stratified associations of sarcopenia and social isolation with all-cause mortality
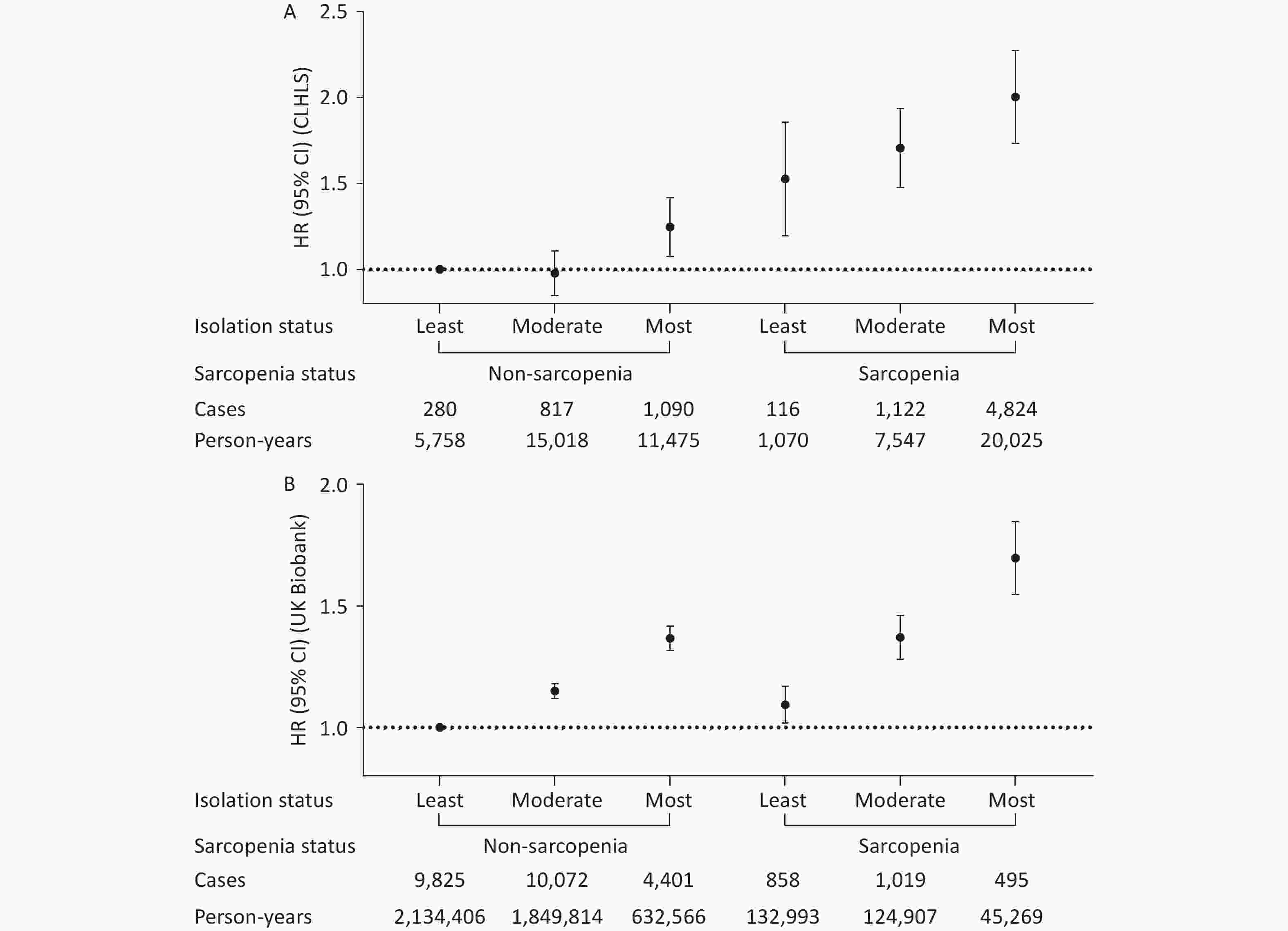
Figure 2. Joint associations of sarcopenia and social isolation with all-cause mortality in the CLHLS (A) and UK Biobank (B). Model adjusted for age, sex, ethnicity (UK Biobank only), residence (CLHLS only), Townsend deprivation index (UK Biobank only), economic level (CLHLS only), education level, smoking status, alcohol intake, healthy diet score, physically active, body mass index, and the number of baseline diseases. RERI (95% CI): 0.25 (-0.06–0.56) in the CLHLS and 0.23 (0.06–0.41) in the UK Biobank. Additive interaction was evaluated using RERI between the social isolation level (least vs. most) and sarcopenia status (non-sarcopenia vs. sarcopenia). Abbreviations: CLHLS, Chinese Longitudinal Healthy Longevity Survey; CI, confidence interval; HR, hazard ratio; RERI, relative excess risk due to interaction.
-
In the CLHLS, the occurrence of few social activities and lack of a spouse were associated with a higher risk of all-cause mortality. Living alone was associated with a lower risk of all-cause mortality, while reduced contact with others was not significantly associated with mortality (Supplementary Table S2). According to the UK Biobank, living alone, reduced contact with others, and limited social activity were associated with an increased risk of all-cause mortality (Supplementary Table S2).
The Cox regression model also suggested significant interactions between sarcopenia and limited social activities or lack of a spouse in the CLHLS (all P-values for interaction < 0.02; Supplementary Tables S3-S4). Less contact with others was significantly associated with sarcopenia in the UK Biobank (P-interaction = 0.007; Supplementary Tables S5-S7). In the joint analysis involving the social isolation component, we found that the presence of any harmful isolation items in individuals with sarcopenia was associated with a significantly increased risk of all-cause mortality (Supplementary Tables S3-S7).
-
The joint associations of sarcopenia and social isolation with all-cause mortality did not change materially in any sensitivity analyses (Supplementary Tables S8-S15).
-
In the two large Chinese and UK cohorts examined in this study, sarcopenia and social isolation were independently associated with an increased risk of all-cause mortality. In the UK Biobank, significant interactions were found among sarcopenia, social isolation, and all-cause mortality. Specifically, the association between social isolation and mortality was stronger in individuals with sarcopenia. Furthermore, participants with both sarcopenia and social isolation exhibited the highest risks of all-cause mortality, CVD mortality, and cancer mortality compared to those with either condition alone.
In line with previous findings, our data showed that sarcopenia and social isolation were independently associated with the risk of all-cause mortality in Chinese and European populations[20,21,31]. Furthermore, we extended these findings by demonstrating that the association between social isolation and mortality is stronger among individuals with sarcopenia. The highest mortality risk was observed among adults with sarcopenia and high levels of social isolation. This is consistent with the Taiwan Longitudinal Study of Aging, which included 3,762 participants aged > 50 years and found that the coexistence of these conditions was related to an increased risk of all-cause mortality[14]. Previous studies have examined the combined effects of social isolation and various risk factors of mortality. For example, a cohort study in the Netherlands with 1,427 participants aged > 65 years found that those with frailty and social isolation had a significantly higher risk of mortality than those without either condition[32]. Frailty, diagnosed on the basis of at least three criteria, i.e., weight loss, low grip strength, exhaustion, slow gait speed, and low physical activity, shares similarities with sarcopenia. However, sarcopenia presents distinct advantages in research and intervention as it can be precisely measured and diagnosed, allowing for early detection and targeted interventions. Moreover, the well-understood risk factors and pathophysiological pathways of sarcopenia facilitate the development of specific treatments, enhancing the effectiveness of public health strategies[1,33]. Additionally, a study using data from the UK Biobank indicated that reducing social isolation and loneliness among people with obesity could decrease the obesity-related excess risk of mortality[34].
The higher prevalence of sarcopenia in the older population in the CLHLS cohort likely reflects an age-related muscle decline, whereas the lower prevalence in the UK Biobank cohort underscores the importance of early detection in midlife. Despite these differences, the consistent direction of the joint associations across cohorts suggests that the interplay between sarcopenia and social isolation contributes to mortality risk independent of age, highlighting the need for life-course interventions targeting both physiological and social health.
Various mechanisms may explain the high mortality risk in adults with sarcopenia and social isolation. Sarcopenia and social isolation are both linked to numerous health issues such as CVD and diabetes, which are well-known risk factors for mortality[5,7,11,12]. However, a lack of social connections might lead to sleep disturbances[35], chronic inflammation[36], and health-risk behaviors such as physical inactivity and poor diet[37], which could exacerbate sarcopenia. Conversely, sarcopenia, a prevalent condition among the elderly, could impair physical function, increase the risk of falls, and reduce social engagement[4,19]. It can also compromise the production and secretion of myokines by skeletal muscles, impacting emotional and motor regulation and leading to cognitive impairment[38], which are risk factors for social isolation[39]. The vicious cycle in which each condition exacerbates the other, significantly increases the mortality risk.
The association between living alone or having less contact with family or friends and all-cause mortality varied with age. Among middle-aged individuals in the UK Biobank, living alone and reduced contact with family or friends were associated with an increased risk of mortality. Conversely, among older adults in the CLHLS, living alone was linked to a reduced risk of mortality, and infrequent contact with family or friends showed no significant association. This discrepancy may be attributed to the increased independence and social engagement among elderly individuals living alone, along with fewer chronic health issues[40,41]. A systematic review and meta-analysis including 18 cohort studies with 62,174 adults reported that living alone was associated with increased mortality among individuals aged < 65 years but not in those aged > 75 years[42]. Regarding social activities, a meta-review of 250,000 elderly individuals highlighted a lower mortality risk among married individuals than among nonmarried individuals, with a relative risk of 0.88[43]. Additionally, frequent participation in social activities was associated with improved longevity among older participants in the CLHLS[44].
This study has several strengths, including the use of large-scale prospective cohorts from China and the UK, allowing for robust analysis across different ethnic and cultural backgrounds. However, the limitations of this study should be acknowledged. First, because the exposure data were collected only at baseline, the findings may not account for changes in the sarcopenia status or social isolation levels over time, and this may have introduced misclassification bias. Second, ASM was estimated using anthropometric equations in the two cohorts. Although this method is not the gold standard for measuring muscle mass, several studies have shown strong agreement between the equations and dual X-ray absorptiometry[25,27]. The absence of recommended physical performance tests (e.g., chair stand, gait speed, and Short Physical Performance Battery score) in the UK Biobank and CLHLS requires the use of alternative measures. Third, residual confounding factors cannot be completely excluded because of the observational nature of the study. Although covariate adjustments were based on previous studies in each cohort, variations in definitions and model adjustments may have influenced the cross-study comparability of the results. Fourth, the CLHLS cohort lacked cause-specific mortality data, precluding any research on cause-specific mortality within this cohort. Finally, differences in exposure definitions were evaluated between the UK Biobank and CLHLS cohorts—for example, marital status was included as part of the social isolation assessment in the CLHLS but not in the UK Biobank. Additionally, substantial differences in sample sizes may have affected the comparability of the findings. However, we believe that the consistent findings from the two diverse cohorts, with different population characteristics and variable definitions, further emphasize the validity and importance of this study.
-
The UK Biobank cohort demonstrated that the co-occurrence of social isolation and sarcopenia was synergistically associated with an increased risk of all-cause and cause-specific mortality, including CVD and cancer, among middle-aged and older adults. Notably, the association between social isolation and mortality was stronger in individuals with sarcopenia. Similarly, individuals experiencing social isolation and sarcopenia had the highest risk of mortality in the CLHLS cohort. The findings from two distinct populations underscore the importance of early identification and targeted interventions addressing social isolation and sarcopenia to reduce mortality risk.
Joint Associations of Sarcopenia and Social Isolation with Mortality: Two Prospective Cohort Studies across Different Cultural Contexts
doi: 10.3967/bes2025.113
- Received Date: 2025-03-10
- Accepted Date: 2025-06-19
-
Key words:
- Sarcopenia /
- Social isolation /
- Mortality /
- UK Biobank /
- CLHLS
Abstract:
No potential conflicts of interest relevant to this article were reported.
The CLHLS and UK Biobank were approved by the Biomedical Ethics Committee of Peking University (IRB00001052-13074) and the North West Multicenter Research Ethics Committee (REC reference numbers 11/NW/0382, 16/NW/0274, and 21/NW/0157). All the participants or their legal representatives provided written informed consent.
| Citation: | Juanjuan Li, Zhe Zhang, Jijuan Zhang, Yuxiang Wang, Hancheng Yu, Gang Liu, An Pan, Yunfei Liao, Tingting Geng. Joint Associations of Sarcopenia and Social Isolation with Mortality: Two Prospective Cohort Studies across Different Cultural Contexts[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2025.113 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: