-
Since the 1970s, rare earth elements (REEs) have been widely studied as growth stimulants in agriculture. Furthermore, dual effects of REEs on plant growth are found: low concentrations of REEs can promote crop growth and yield, while high concentrations of REEs appear to be harmful[1]. Moreover, epidemiological investigations indicate that REEs may be neurotoxic; this has also been confirmed by animal studies.
There is a dearth of information in the scientific literature regarding hormesis. To study the hormetic effect of REEs on growth and neurodevelopment, it is necessary to carry out studies in accordance with internationally recognized standard methods. Yttrium (Y) accounts for a relatively high proportion of all rare earth elements. In this paper, the hormetic effects of Y nitrate on male rats were discussed on the basis of Organisation for Economic Co-operation and Development (OECD) developmental neurotoxicity.
The study was in conducted in accordance with OECD Developmental Neurotoxicity Guideline TG 426. One hundred twenty virgin female (to ensure 25 pregnant rats for each dosage group) and 120 male Sprague-Dawley (SD) rats were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). The F1 male offspring selected to continue in the study were identified by body markings after weaning. All studies were conducted according to the laboratory animal management regulations of Beijing regarding animal experimental welfare and ethical inspection and approved by the China National Center for Food Safety Risk Assessment Standing Committee on Ethics in Animal Experimentation.
Yttrium oxide was received as a white, granular solid (purity > 99.99%) from Beijing Research Institute of Nonferrous Metals and dissolved in nitric acid to form Y nitrate. Three different dose of Yttrium nitrate (20, 80, and 320 ppm, calculated based on Y) in dietary and concurrent control (0) were administered to rats by the oral dietary route. Dosing began from the initiation of pregnancy (GD 0), and exposure was continuous throughout the lifetime of pups.
All dams were carefully observed twice during the lactation period and once daily after weaning. Testicle descent was observed, and motor activity and motor and sensory function were monitored. Spatial learning and memory were measured continuously for 6 days from PND 25 to PND 30, and the Morris Water Maze (MWM) for young adults was used from PND 65 to PND 70. The parameters of escape latency (time spent finding the platform), distance (length of the swimming path), percentage of time spent in each of the four quadrants, and swimming speed were measured. TSE VideoMot software (TSE, Germany) was used for recording and analyzing the data.
Ten pups (one per litter) selected for neuropathology on PND 21 and PND 70 were perfused with 4% paraformaldehyde and 1.4% glutaraldehyde. Ultra-microstructural studies were was carried out according to light electron microscopy (CLEM) approaches from the published literature[2]. The images were obtained by using a HITACHI H-7650 transmission electron microscope.
All parameters were presented as means ± standard deviation, and multiple comparison tests for the different dose groups were conducted. Homogeneity of variance was examined using the Levene test. If the Levene test indicated no significant deviation from homogeneity of variances, the data was analyzed by one-way analysis of variance followed by Fisher's least significant difference test; otherwise, the Games-Howell multiple comparison was applied. Differences with P-values < 0.05 were considered significant.
To identify the optimal hormetic dose response, a large dosing range involving many samples and multiple experiments are required. To prevent missing the time window of the hormetic response, multiple kinetic (dose-time) experiments are needed[3]. Body weight and food intake were measured once weekly from PND 4 to PND 63. Table 1 shows the growth curves over the entire study period for male animals. There was no significant change in body weight at any time point with exposure to 80 ppm or 320 ppm doses. However, body weights increased significantly in the 20 ppm group from PND 14 to PND 70 (P < 0.05). There was no difference between food consumption and the utility ratio (P > 0.05).
Table 1. Change in Weight of F1 Offspring from PND 4 to PND 70 (mean ± SD, n = 20)
PND 0 ppm 20 ppm 80 ppm 320 ppm 4 11.2 ± 1.4 11.7 ± 1.4 11.8 ± 1.3 12.6 ± 1.4 7 17.5 ± 2.0 18.4 ± 2.1 17.6 ± 1.6 17.4 ± 1.9 14 31.8 ± 2.0 39.5 ± 3.9* 33.4 ± 2.6 29.1 ± 4.8 21 49.8 ± 5.1 56.2 ± 3.5* 52.6 ± 5.8 47.1 ± 11.3 28 86.8 ± 6.0 99.1 ± 6.2* 94.1 ± 7.4 88.6 ± 11.8 35 146.9 ± 9.0 159.1 ± 5.2* 147.7 ± 11.5 148.9 ± 14.3 42 205.4 ± 14.4 235.1 ± 12.0* 209.7 ± 17.3 209.3 ± 13.5 48 262.1 ± 12.4 288.8 ± 11.5* 263.5 ± 12.0 264.8 ± 14.4 56 288.3 ± 17.9 329.2 ± 13.1* 290.7 ± 16.7 294.1 ± 19.8 63 339.1 ± 22.3 395.4 ± 23.3* 341.8 ± 28.9 330.3 ± 24.3 70 402.0 ± 27.2 462.5 ± 29.0* 398.4 ± 32.2 405.2 ± 30.7 Note. *P < 0.05 compared with the control group; 20 rats per group. In this experiment, the 20 ppm group showed an effect similar to hormesis. Also, many pollutants showed hormetic effects, such as a significant body weight increase induced by 30 mg/kg Cd, specific growth rate, and visible alterations to the hepatopancreatic structures of L. vannamei[4]. Low-dose REEs (40 mg/kg) could improve growth performance in broilers without affecting their carcass composition and health[5]. The hormetic doses reported by He[5] were about 20 times higher than those in our study, thus it was suggested that hormetic doses may differ among species.
Anogenital distance (AGD) in males is approximately double that of females. AGD has been discovered to reflect fetal androgen exposure only within a discrete 'masculinization programming window', which also determines the adult size of the testis, prostate, seminal vesicles, and penis[6]. AGD was measured on PND 1. AGD data were divided by body weight for analysis of the AGD index (AGDI). Some studies also suggested that AGD possesses a degree of plasticity in adults. Compared with the control group, AGD at 20 ppm and 80 ppm doses were significantly reduced (3.54 ± 0.53 mm, 3.53 ± 0.48 mm vs. 3.81 ± 0.55 mm, P < 0.05). Also, AGDI was significantly reduced in the 20 and 80 ppm groups (39.9 ± 6.6 mm/g vs. 37.3 ± 5.9 mm/g, 36.6 ± 5.6 mm/g, P < 0.05) and unchanged in the 320 ppm group. A shorter AGD indicates that low-dose Y may be a suspected anti-androgen; this requires further validation in a Hershberger experiment.
For wheel running on PND 22, shock times in the treated groups were significantly lower than those in the control group (P < 0.05), and the shock time was reduced as the dose increased (14.0 ± 11.8, 8.3 ± 7.9, 7.3 ± 5.5 vs. 23.3 ± 14.5, P < 0.05). For wheel running on PND 62, the running distance in the 320 ppm dose group was significantly longer than that of the control group (125.8 ± 48.0 cm vs. 81.7 ± 43.3 cm, P < 0.05). Yttrium improved the motor ability in both PND 22 and PND 62 groups, and the effects appeared to be dose dependent.
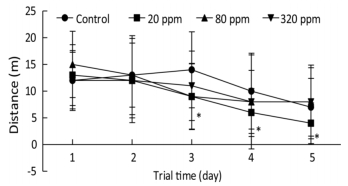
Cognitive endpoints are sensitive to hormetic effects. In the MWM test, changes in the parameters of escape latency, distance, percentage of time spent in the target quadrant, percentage of time spent in the opposite quadrant, and speed were analyzed to evaluate spatial learning and memory[7]. MWM results from PND 25 to PND 30 showed that the percentage of time spent in the target quadrant in the 320 ppm group was significantly lower than that of the control group on the fifth day (0.28 ± 0.11 vs. 0.36 ± 0.18, P < 0.05). MWM results from PND 65 to PND 70 showed that swimming distances in the 20 ppm group were lower than that of control group from the third to the fifth day of the training trial (P < 0.05) (Figure 1). Spatial learning ability was improved in the 20 ppm group but inhibited in the 320 ppm group in the training and probe trials. The results indicated that Y could improve motor ability, which was consistent with the results of wheel running. A shorter swimming distance and a longer percentage target quadrant stay time represents improvement in spatial learning and memory; the opposite represents impairment[8]. Comprehensive analysis of the results from PND 25 and PND 65 showed that learning and memory were improved at low doses and inhibited at high doses, which even caused impairment of learning and memory. Our results suggested that high-dose intrauterine and lactation exposure had an adverse effect on spatial learning and memory.
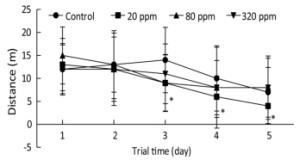
Figure 1. Swimming distance in training trial from PND 65 to PND 70. *P < 0.05 compared with control; n = 10 rats per group.
No treatment-related gross changes were observed in the brains on PND 21 and in the brains and spinal cords on PND 70 in F1 male animals. There were no effects on histopathological alterations in the brains, cerebella, livers, or kidneys on PND 70 in the 320 ppm group. However, we found that in mitochondria of the hippocampus CA3 area in the 320 ppm group, electron density, ambiguous or fracture, and ridge interstitial electron density increased; small black particles were deposited; large black particles were aggregated; and the double membrane was incomplete; The mitochondrial bilayer membrane and inner ridge were intact, and uniform coloring was present in the control group (Figure 2). No significant changes were present at 20 ppm or 80 ppm. It was suggested that oxidative damage to mitochondria was observed at high doses, possibly leading to the decline of spatial learning memory.
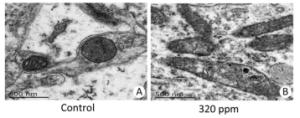
Figure 2. Ultra-microstructure of mitochondria. (A) Control group (6, 000 ×); (B) 320 ppm group (6, 000 ×).
Mitochondrial ultra-microstructure based on this experiment showed oxidative damage to hippocampal organelles in the high-dose group, which was consistent with the results of previous neurobehavioral experiments[9]. Oxidative damage to the mitochondria was induced by high-dose Y, possibly resulting in a decline of spatial learning memory. Kwon and Cha et al.[10] also reported that no oxidative damage was observed at a low-dose in an in vivo mouse model. Such a finding is consistent with our result in the 20 ppm and 80 ppm groups. Therefore, the use of Y as a regulator to promote the growth of animals and plants requires more research.
Extensive evaluations in this developmental neurotoxicity study clearly showed hormetic effects on body weight and cognition. The hormetic dosage is 20 ppm (about 2 mg/kg). Cognition through oxidative damage to the hippocampus was inhibited at high doses (320 ppm). A suspected anti- androgenic effect was induced by the low doses, on which further study is needed.
ZHANG Wen Zhong executed of the whole experiment, SUN Na Na measured parameters, MA Shang Quan was responsible for quality control, CAO Yu was responsible for the system interpretation of the results, and ZHANG Chao was responsible for histopathology and electron microscopy.
doi: 10.3967/bes2018.104
-
Abstract: To evaluate hormesis induced by Yttrium (Y) nitrate in male rats, Y was offered to F0 mother rats and F1 offspring at concentrations of 0, 20, 80, and 320 ppm daily from gestational day (GD) 0 through postnatal day 70 (PND 70). The F1 offspring were evaluated with respect to motor function, learning and memory, and histopathology. Administration of Y improved motor function in a dose dependent manner. In the 20 ppm group, body weight and spatial learning and memory were increased, while the latter was decreased in the 320 ppm group. Additionally, in the 20 ppm and 80 ppm, but not the 320 ppm groups, Y reduced the anogenital distance, which indicated an anti-androgen effect. These results suggest that Y follows a hormetic concentration-related trend with an inverted U-shape.
-
Key words:
- Yttrium /
- Hormesis /
- Anti-androgen /
- Motor activity /
- Cognitive
-
Table 1. Change in Weight of F1 Offspring from PND 4 to PND 70 (mean ± SD, n = 20)
PND 0 ppm 20 ppm 80 ppm 320 ppm 4 11.2 ± 1.4 11.7 ± 1.4 11.8 ± 1.3 12.6 ± 1.4 7 17.5 ± 2.0 18.4 ± 2.1 17.6 ± 1.6 17.4 ± 1.9 14 31.8 ± 2.0 39.5 ± 3.9* 33.4 ± 2.6 29.1 ± 4.8 21 49.8 ± 5.1 56.2 ± 3.5* 52.6 ± 5.8 47.1 ± 11.3 28 86.8 ± 6.0 99.1 ± 6.2* 94.1 ± 7.4 88.6 ± 11.8 35 146.9 ± 9.0 159.1 ± 5.2* 147.7 ± 11.5 148.9 ± 14.3 42 205.4 ± 14.4 235.1 ± 12.0* 209.7 ± 17.3 209.3 ± 13.5 48 262.1 ± 12.4 288.8 ± 11.5* 263.5 ± 12.0 264.8 ± 14.4 56 288.3 ± 17.9 329.2 ± 13.1* 290.7 ± 16.7 294.1 ± 19.8 63 339.1 ± 22.3 395.4 ± 23.3* 341.8 ± 28.9 330.3 ± 24.3 70 402.0 ± 27.2 462.5 ± 29.0* 398.4 ± 32.2 405.2 ± 30.7 Note. *P < 0.05 compared with the control group; 20 rats per group. -
[1] Redling K. Rare earth elements in agriculture with emphasis on animal husbandry. PhD dissertation (Ludwig-Maximilians-Universität München, Munich, Germany). 2006. [2] Begemann I, Galic M. Correlative Light Electron Microscopy:Connecting Synaptic Structure and Function. Front Synaptic Neurosci, 2016; 8, 28. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0227163495/ [3] Calabrese EJ, Mattson MP. How does hormesis impact biology, toxicology, and medicine? NJP Aging Mech Dis, 2017; 3, 13. doi: 10.1038/s41514-017-0013-z [4] Yu YY, Chen SJ, Chen M, et al. Effect of cadmium-polluted diet on growth, salinity stress, hepatotoxicity of juvenile Pacific white shrimp (Litopenaeus vannamei):Protective effect of Zn (Ⅱ)-curcumin. Ecotoxicol Environ Saf, 2016; 125, 176-83. doi: 10.1016/j.ecoenv.2015.11.043 [5] He ML, Wehr U, Rambeck WA. Effect of low doses of dietary rare earth elements on growth performance of broilers. J Anim Physiol Anim Nutr (Berl), 2010; 94, 86-92. doi: 10.1111/jpn.2009.94.issue-1 [6] Vanden DS, Scott HM, MacLeod DJ, et al. Relative importance of prenatal and postnatal androgen action in determining growth of the penis and anogenital distance in the rat before, during and after puberty. Int J Androl, 2011; 34, e578-e586. doi: 10.1111/ija.2011.34.issue-6pt2 [7] Dhingra D, Soni K. Behavioral and biochemical evidences for nootropic activity of boldine in young and aged mice. Biomed Pharmacother, 2017; 97, 895-904. http://www.ncbi.nlm.nih.gov/pubmed/29136766 [8] Yoo DY, Jung HY, Kim JW, et al. Reduction of dynamin 1 in the hippocampus of aged mice is associated with the decline in hippocampal dependent memory. Mol Med Rep, 2016; 14, 4755-60. doi: 10.3892/mmr.2016.5804 [9] Li BH, Zhang LY, Chen C, et al. Effect of developmental histomorphology of rat hippocampus after early stage Yttrium exposure. Chinese Journal of Food Hygiene, 2016; 28, 160-5. (In Chinese) http://d.old.wanfangdata.com.cn/Periodical/zgspwszz201602005 [10] Kwon HJ, Cha MY, Kim D, et al. Mitochondria-targeting ceria nanoparticles as antioxidants for alzheimer's disease. ACS Nano, 2016; 10, 2860-70. doi: 10.1021/acsnano.5b08045 -




 下载:
下载:



 Quick Links
Quick Links