-
Prion diseases or transmissible spongiform encephalopathy (TSE), refers to a group of fatal neurodegenerative disorders in humans and various species of animals[1]. Prion diseases in humans mainly include the Creutzfeldt–Jakob disease (CJD), fatal familial insomnia (FFI), and Gerstmann–Sträussler–Scheinker syndrome (GSS). The most common form of human prion disease is sporadic CJD (sCJD), with a worldwide incidence of approximately 1 case per million individuals annually. The definitive diagnosis of sCJD requires neuropathological examination or prion protein (PrPSc) detection in brain tissues. PrPSc is partially protease resistant and can induce its normal cellular isoform (PrPC) to undergo a conformational change. This induction occurs in a self-propagating manner through a seeded aggregation process, resulting in PrPSc accumulation throughout the brain tissues, accompanied by spongiform degeneration, neuronal loss, and gliosis[1]. Besides analysis of brain biopsy specimens, there is lacking of disease-specific premortem diagnostic tests for sCJD currently. Postmortem examination is not only a lagging test but also an unacceptable one in some regions and countries, including China, due to unique culture and traditions; thus, accurate diagnostic methods based on other easily obtainable specimens, such as blood and cerebrospinal fluid (CSF), for sCJD are mostly required.
In the past decades, numerous studies have attempted to identify biomarkers, such as 14-3-3, tau, S100, and neuron specific enolase, in CSF samples for the diagnosis of human prion diseases. However, only CSF 14-3-3 positive by Western blot is included in the diagnostic criteria for probable sCJD[2]. The detection of PrPSc in the CSF of patients with sCJD and other types of human prion diseases is almost impossible with routine methodologies, even with a sensitive technique, such as protein-misfolding cyclic amplification (PMCA).
The development of real-time quaking-induced conversion (RT-QuIC) improves the diagnosis of sCJD, showing good sensitivity and specificity when used in CSF samples[3, 4]. In the Chinese CJD surveillance network, we have established the first-generation (using recombinant hamster PrP aa 23-231 as the substrate) and second-generation (using recombinant hamster PrP aa 90-231 as the substrate) of RT-QuIC assays. These techniques have already been used in prion studies and auxiliary diagnosis for human prion diseases[3, 5]. However, the sensitivity and specificity of the RT-QuIC assays we have established are still not well addressed because CSF samples from definite diagnostic sCJD cases in our CSF bank are insufficient. Recently, we obtained 60 human CSF samples from the US CJD Surveillance Center, Case Western Reserve University. All samples, including 30 definite sCJD cases and 30 non-CJD cases, were analyzed blinded to the diagnosis. The sensitivity and specificity of the established second-generation RT-QuIC assay was evaluated.
The working procedure of the RT-QuIC assay has been described elsewhere[6]. Briefly, the assay was conducted in a black 96-well, optical-bottomed plate (Nunc, 265301) on a BMG FLUOstar plate reader (BMG LABTECH). Fifteen microliters of each CSF sample was mixed with 10 μg of recombinant hamster PrP90-231 in a reaction buffer containing 10 mmol/L PBS, 170 mmol/L NaCl, 10 μmol/L Thioflavin T (ThT), 10 μmol/L EDTA, and 0.002% SDS in final. The final reaction volume was 100 μL. Each tested sample was quadruplicated. Each reaction contained blank (reaction buffer), negative (2 μL of 10% brain homogenate from normal hamster), and positive (2 μL of 10% brain homogenate from scrapie agent 263K-infected hamster) controls. The working conditions are as follows: temperature, 55 °C; shaking speed, 700 rpm; shaking/incubation time, 60/60 s; total reaction time, 60 h. ThT fluorescence (450 nm excitation and 480 nm emission) was automatically measured every 45 min as relative fluorescence units (rfu). The cutoff value was set as the average value of the negative controls plus 10 times of SD. The sample was considered positive when two or more parallel wells revealed positive reactive curves.
Among the 60 tested CSF samples, 29 cases were positive, and 31 were negative in our RT-QuIC tests. Test data were reported to the US CJD Surveillance Center, and the results based on each case were returned to us. As shown in Table 1, all 30 non-CJD cases were negative in CSF RT-QuIC, whereas 29 out 30 definite sCJD cases were positive. Only one sCJD case was negative in CSF RT-QuIC. The sensitivity and specificity of the RT-QuIC assay were estimated as 96.67% and 100%, respectively.
Table 1. RT-QuIC results of 60 CSF samples
Items Definite CJD Non-CJD Total Positive 29 0 29 Negative 1 30 31 Total 30 30 60 In the 29 positive samples, the lag time and the peak of rfu were analyzed. Generally, the average lag time of 29 samples was 8.97 h postreaction (Figure 1A). Regarding the individual samples, the lag time and the peaks of rfu varied largely, that is, from 4 h to 26.33 h postreaction and from 50,000 rfu to 260,000 rfu, respectively (Figure 1B). Majority of the positive samples (28/29, 96.56%) started to convert positively within 14 h. Only one sample showed a relatively long lag time of 26.33 h postreaction with the lowest rfu intensity (50,000 rfu). The relationship between lag time and peak of rfu was evaluated and showed a close correlation (P = 0.001); the shorter the lag time, the higher the peak intensity.
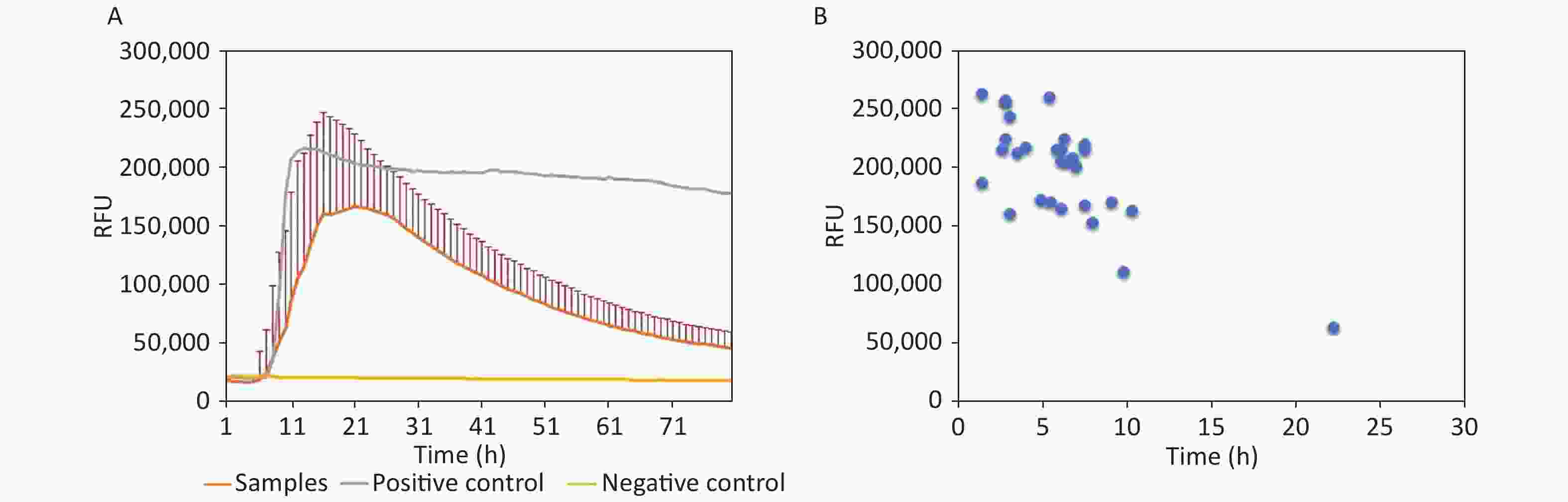
Figure 1. Reactive characteristics of 29 positive samples. (A) Averaged reactive curves of 29 positive samples; mean ± SD of the rfu was calculated. (B) Dot plots of the lag time and peak of 29 positive samples.
Using the testing CSF panel for the sCJD cases supplied by US CJD Surveillance Center, we evaluated the quality of the established RT-QuIC technique in this study. The sensitivity and specificity of our technique are comparable to other published data[6]. We believe that the data of this study can provide a fundamental basis for the usage of RT-QuIC in CJD surveillance in China. A further improvement of the quality control of CSF RT-QuIC (e.g., preparation of rHaPrP90-231, instruments, and reagents) will be beneficial for the clinical diagnosis of suspected sCJD patients.
The CSF RT-QuIC reactivities among the sCJD patients show variation[7]. On the basis of the electrophoretic patterns of PrPSc in brains and the polymorphism of codon 129, sCJD can be divided into six different subtypes[8]. Studies have already proposed that compared with MM1 patients, the MM2 subtypes usually have a longer lag time and a lower rfu intensity in CSF RT-QuIC[7]. Coincidentally, the sCJD case that was negative in RT-QuIC was an MM2 subtype. This result is meaningful for Chinese CJD surveillance because absolute majorities of Han Chinese and Chinese sCJD patients are Met/Met homozygotes at codon 129[9]. In other words, the PrPSc subtypes of Chinese sCJD patients should be MM1 and MM2 predominately. The brain PrPSc subtypes of Chinese CJD patients remain unclear due to a limited implementation of brain autopsy and biopsy in China. The PrPSc subtypes of Japanese sCJD patients have been reported to be 56.82% MM1 and 22.73% MM2[10]. Under the assumption that the profiles of PrPSc subtypes among Chinese patients are similar to those of the Japanese, 22.73% of the probable Chinese sCJD patients may display low reactivity and may even test negative in CSF RT-QuIC. Combining the data of CSF RT-QuIC and that of other routine clinical and laboratory examinations may help speculate the ratio of MM2 subtypes among the probable sCJD patients in China.
-
This study was approved by the Ethical Committee of National Institute for Viral Disease Control and Prevention, China CDC under protocol 2009ZX10004-101.
-
The authors declare that they have no conflict of interest.
-
KX run the RT-QuIC assay, analyzed and interpreted the data, and was a major contributor in writing the manuscript. XHY ran the RT-QuIC assay, analyzed and interpreted the data. WQZ gave advices on the RT-QuIC assay. XPD and QS designed the research and revised the manuscript.
-
We thank Dr. Brian Appleby from US CJD Surveillance Center, Case Western Reserve University for kindly supplying the CSF testing panel for sCJD.
doi: 10.3967/bes2020.081
Assessment of the Sensitivity and Specificity of the Established Real-time Quaking-induced Conversion (RT-QuIC) Technique in Chinese CJD Surveillance
-
Abstract: Real-time quaking-induced conversion (RT-QuIC) assay is a newly established PrPSc-detecting method. The development of RT-QuIC improves the diagnosis of sporadic Creutzfeldt–Jakob disease (sCJD), showing good sensitivity and specificity in many countries when the method was used in cerebrospinal fluid (CSF) samples. However, in China, the sensitivity and specificity of RT-QuIC has yet to be determined due to the lack of definitive diagnosis samples. Recently, 30 definitive sCJD and 30 non-CJD diagnoses were evaluated by RT-QuIC assay. In the 30 sCJD CSF samples, 29 showed positive results. By contrast, all the non-CJD samples were negative. The sensitivity and specificity of our RT-QuIC assay were 96.67% and 100%, respectively, and are comparable to other published data. Results can provide a fundamental basis for the usage of RT-QuIC assay in CJD surveillance in China.
-
Table 1. RT-QuIC results of 60 CSF samples
Items Definite CJD Non-CJD Total Positive 29 0 29 Negative 1 30 31 Total 30 30 60 -
[1] Prusiner SB: Prions. Proceedings of the national academy of sciences of the United States of America, 1998; 95, 13363-83. [2] Collins S, Boyd A, Fletcher A, et al. Creutzfeldt-Jakob disease: diagnostic utility of 14-3-3 protein immunodetection in cerebrospinal fluid. J Clin Neurosci, 2000; 7, 203−8. doi: 10.1054/jocn.1999.0193 [3] McGuire LI, Peden AH, Orru CD, et al. Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann Neurol, 2012; 72, 278−85. doi: 10.1002/ana.23589 [4] Atarashi R, Satoh K, Sano K, et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med, 2011; 17, 175−78. doi: 10.1038/nm.2294 [5] Orru CD, Hughson AG, Groveman BR, et al. Factors that improve RT-QuIC detection of prion seeding activity. Viruses, 2016; 8. [6] Orru CD, Groveman BR, Hughson AG, et al. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. mBio, 2015; 6, e02451–14. [7] Cramm M, Schmitz M, Karch A, et al. Characteristic CSF prion seeding efficiency in humans with prion diseases. Molecular neurobiology, 2015; 51, 396−405. doi: 10.1007/s12035-014-8709-6 [8] Gambetti P, Kong Q, Zou W, et al. Sporadic and familial CJD: classification and characterisation. British Medi Bull, 2003; 66, 213−39. doi: 10.1093/bmb/66.1.213 [9] Shi Q, Zhou W, Chen C, et al. Quality evaluation for the surveillance system of human prion diseases in China based on the data from 2010 to 2016. Prion, 2016; 10, 484−91. doi: 10.1080/19336896.2016.1229731 [10] Nozaki I, Hamaguchi T, Sanjo N, et al. Prospective 10-year surveillance of human prion diseases in Japan. Brain, 2010; 133, 3043−57. doi: 10.1093/brain/awq216 -




 下载:
下载:



 Quick Links
Quick Links