-
Exercise-induced fatigue is a complex biological phenomenon that significantly impairs athletic performance and health. It is characterized by the inability to sustain a given level of physical activity or maintain a specific exercise intensity, resulting in sluggishness, poor coordination, insensitivity, memory impairment, and difficulty in concentrating. In addition, long-term fatigue has been associated with diseases, such as weakened immunity and cardiovascular diseases. The onset of fatigue is related to several factors, including energy depletion, accumulation of metabolites, oxidative stress, disruption of muscle calcium homeostasis, and central nervous system fatigue[1]. The current research indicates that the major mechanisms for alleviating exercise-induced fatigue involve enhancing energy substrate availability through increased glycogen synthesis, clearing fatigue-causing metabolites, improving mitochondrial function, regulating calcium homeostasis, inhibiting inflammatory responses, regulating neurotransmitters, and maintaining muscle proteins[2]. Several signal transduction pathways mainly MAPK, P13K-AKT, mTOR, AMPK, and NF-κB signaling pathways might be essential elements in the short- and long-term adaptations of the skeletal muscle to exercise-induced fatigue. The current study suggests that an aberrant MAPK signaling pathway impeding muscle regeneration may disrupt biochemical and metabolic processes in muscles, eventually leading to fatigue[3,4]. Additionally, studies have also shown that activation of the PI3K/AKT signaling pathway and increased glucose uptake through the glucose transporter GLUT4 is an effective way to increase energy supply and utilization in muscles[5]. Further research on the complex molecular events underlying fatigue will provide new insights into interventions and countermeasures to enhance exercise performance.
Exercise fatigue is inevitable for professional athletes engaged in competitive sports and individuals who engage in regular exercise. Delaying the onset of exercise fatigue is crucial for improving physical performance, optimizing training outcomes, and reducing the risk of sports injuries. Numerous sports supplements are employed to reduce exercise fatigue and enhance exercise capacity. However, some of these supplements may pose potential health risks, such as gastrointestinal distress caused by sodium bicarbonate. Therefore, it is essential to identify anti-fatigue components or formulations with definite efficacy and fewer adverse effects. Traditional medicines, foods, and naturally active components such as Radix Rehmanniae preparata, Ginseng, and Astragalus membranaceus have been used effectively as healthcare products to relieve exercise fatigue[6,7]. The Homology of Medicine and Food, a special food category in China, can be used in both food and medicine. Compared to medicines, they possess more potent nutritional properties and are primarily used as tonics, which are characterized by a mild flavor and gentle nature. They are known to promote recovery, rehabilitation, and overall health. Fructus lycii (goji berry), a medicinal and food product, is usually preserved and consumed in its dried fruit form. Fructus lycii contains multiple active components, including polysaccharides, carotenoids, flavonoids, and phenolics[8]. Studies have confirmed that Fructus lycii has significant effects on the regulation of immune function, apoptosis, blood lipids, and blood glucose, as well as its anti-tumors, anti-aging, and anti-fatty liver disease properties[9]. Lately, researchers have begun to explore its potential to delay fatigue and enhance athletic performance[10]. Fructus lycii is a complex mixture containing multiple compounds that act on various targets; however, the active compounds and their mechanisms of action remain unclear. Network pharmacology, an emerging branch of pharmacology, provides a means to scrutinize the synergistic effects of multiple components and targets, particularly useful for investigating the potential mechanism of traditional Chinese medicine (TCM) in disease treatment[11].
We hypothesized that the presence of multiple active components of Fructus lycii and their associated mechanisms may contribute to the alleviation of exercise fatigue. Therefore, the main purpose of this study was to explore the complex interplay between Fructus lycii and exercise fatigue using network pharmacology. Subsequently, in vitro assays were performed on C2C12 cells to validate the effects and mechanisms of the representative components of Fructus lycii predicted by network analysis to improve exercise fatigue.
-
The Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) was used to identify the major chemical components and their potential targets (accessed November 22, 2022). Relevant active compounds were screened for oral bioavailability (OB) ≥ 30%[12] and drug like index (DL) ≥ 0.18 of component[13]. In this study, the predicted targets of the main components of Fructus lycii were retrieved from TCMSP. The target protein was mapped to its corresponding gene name using the UniProt human database.
-
The corresponding targets of disease related to fatigue were retrieved from the Therapeutic Target Database (TTD), Online Mendelian Inheritance in Man (OMIM), Drug bank and Gene card using the exercise fatigue-related search terms including “exercise fatigue” “physical fatigue” “fatigue” and “exercise capacity” (accessed on November 22, 2022).
The intersection targets of compounds and disease were considered potential targets of Fructus lycii in the treatment of exercise fatigue, as represented by the Venn diagram in Supplementary Figure S1, available in www.besjournal.com.
-
The intersection targets of the main components of Fructus lycii and exercise fatigue were imported into the Metascape database (http://metascape.org/gp/index.html). The Gene Ontology (GO) enrichment analysis included three aspects: biological process (BP), molecular function (MF), and cellular component (CC) (accessed November 28, 2022). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was used for target pathway enrichment (accessed November 28, 2022). GO enrichment and KEGG pathway analyses were carried out with P < 0.05 as the screening condition. Finally, the top 10 GO terms and the top 20 KEGG terms were screened with the lowest P value and represented as visual graphs using R 4.1.1 software or Bioinformatics (http://www.bioinformatics.com.cn).
-
The “Network Analyzer” function of Cytoscape 3.8.2 was used to calculate the network parameters of each node, protein, gene, active component, or pathway. The effective active compounds and their corresponding intersection targets of exercise fatigue were imported into Cystoscope 3.8.2, to construct an active compound-target network. The intersection targets, effective active compounds, and KEGG pathway enrichment in the Metascape database (P < 0.05) were imported into Cystoscope 3.8.2, to construct an active compound-targets-pathways network. The “degree” value represents the number of one node associated with the other nodes in the network. The larger the degree value, the more important the node is in the network, and the more likely it is to become a core node.
-
Skeletal muscle C2C12 cells (Pythonbio) were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% FBS (Gibco) and 1% penicillin-streptomycin (Gibco). At 90% confluence, the medium was changed to DMEM plus 2% horse serum (Gibco) and 1% penicillin-streptomycin for 6 days to induce C2C12 cells to differentiate into myotube cells, two days after the medium switch. The cells were cultured at 37 °C in a controlled humidified 5% CO2 atmosphere.
-
C2C12 myotubes were seeded in 96-well plates at a density of 10,000 cells per well and incubated overnight. The cells were treated with various concentrations (7.8125–1,000 μmol/L) of quercetin for 24 h. Cell proliferation was measured using the Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Kumamoto, Japan) by adding 10 μL CCK-8 reagent to each well after incubating at 37 °C for 1 h. Optical density (OD) was measured at 450 nm using a plate reader (BioTek, Synergy 4).
-
C2C12 myotubes were washed with phosphate buffer (PBS) and incubated in serum-free DMEM (Gibco) containing FBS for 4 h. After fasting, the cells were treated with 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino)-2-Deoxyglucose (2-NBDG) (ThermoFisher Science) at a concentration of 400 μm at 37 °C for 30 min[14]. The cells were washed twice with PBS and counterstained with Hoechst 33,342 for nucleic acid staining. The mean stained area was analyzed using Image Xpress software (Molecular Devices, San Jose, CA, USA).
-
After treatment, C2C12 myotubes were cultured with 100 μmol/L tert-butyl hydroperoxide (TBHP) as an ROS inducer for 30 min before labeling with CellRox Green reagent (ThermoFisher Science)[14]. Then, the cells were stained with CellRox Green reagent for 30 min at 37 °C. The subsequent procedure was similar to that described previously.
-
Mitochondrial membrane potential and mitochondrial mass were measured using the Image-iT™ TMRM reagent (ThermoFisher Scientific) and MitoTracker™ Green FM (Thermo Fisher Scientific), respectively, according to the manufacturer’s instructions[15]. Cells were washed twice with PBS, then loaded with TMRM staining or MitoTracker and Hoechst 33342 at 37 °C avoiding light for 30 min. The subsequent procedure was similar to that described previously.
-
C2C12 cells were fixed with fixative buffer (eBioscienceTM Fixation: Fixation/Permeabilization = 1:3) from the eBioscience™ Foxp3 (ThermoFisher Science) at 18–22 °C for 30 min. The cells were then washed twice with the permeabilization buffer. The cells were then incubated with the following primary antibodies: p-p38 MAPK (1:200, CST), p-MAPK (Erk1/2, 1:200, CST), p-PI3K (1:200, Affinity), p-AKT (1:400, CST), and p-JNK (1:200, CST) at 18–22 °C for 30 min. After washing twice with PBS, the cells were probed with the following secondary antibodies: Anti-mouse IgG or Anti-rabbit IgG (1:1,000, CST) for 1 h at 18–22 °C. After washing twice, the cells were stained with Hoechst 33,342 for 5 min, and the mean stained area was assessed using Image Xpress software[16].
-
The results are expressed as mean and standard deviation (SD). Statistical analyses were performed using GraphPad Prism 7.0 (GraphPad Software Inc., San Diego, CA, USA). Differences were analyzed using one-way analysis of variance (ANOVA), followed by Fisher’s least significant difference (LSD) multiple comparisons. Statistical significance was considered at P-values less than 0.05.
-
A total of 188 chemical components of Fructus lycii were identified, of which 45 had OB > 30% and DL > 0.18. After eliminating the duplicate targets, 45 components had 188 potential targets. The results are presented in Supplementary Table S1, available in www.besjournal.com.
Table S1. Potential active components of Fructus Lycii
Molecule ID Molecule name OB (%) DL MOL001323 Sitosterol alpha1 43.2813 0.78354 MOL003578 Cycloartenol 38.6857 0.78093 MOL001494 Mandenol 41.9962 0.19321 MOL001495 Ethyl linolenate 46.101 0.19716 MOL001979 LAN 42.1192 0.74787 MOL000449 Stigmasterol 43.8299 0.75665 MOL000358 beta-sitosterol 36.9139 0.75123 MOL005406 atropine 45.9706 0.19328 MOL005438 campesterol 37.5768 0.71488 MOL006209 cyanin 47.4209 0.75918 MOL007449 24-methylidenelophenol 44.1926 0.7533 MOL008173 daucosterol_qt 36.9139 0.75316 MOL008400 glycitein 50.4789 0.23826 MOL010234 delta-Carotene 31.8009 0.54639 MOL000953 CLR 37.8739 0.67677 MOL009604 14b-pregnane 34.7792 0.33723 MOL009612 (24R)-4alpha-Methyl-24-ethylcholesta-7,25-dien-3beta-ylacetate 46.3575 0.8398 MOL009615 24-Methylenecycloartan-3beta,21-diol 37.3173 0.79751 MOL009617 24-ethylcholest-22-enol 37.0945 0.7511 MOL009618 24-ethylcholesta-5,22-dienol 43.8299 0.75636 MOL009620 24-methyl-31-norlanost-9(11)-enol 37.9997 0.75092 MOL009621 24-methylenelanost-8-enol 42.3682 0.76769 MOL009622 Fucosterol 43.7764 0.75668 MOL009631 31-Norcyclolaudenol 38.6821 0.81391 MOL009633 31-norlanost-9(11)-enol 38.3539 0.7249 MOL009634 31-norlanosterol 42.2046 0.73012 MOL009635 4,24-methyllophenol 37.8347 0.74999 MOL009639 Lophenol 38.1294 0.714 MOL009640 4alpha,14alpha,24-trimethylcholesta-8,24-dienol 38.9099 0.75772 MOL009641 4alpha,24-dimethylcholesta-7,24-dienol 42.653 0.75297 MOL009642 4alpha-methyl-24-ethylcholesta-7,24-dienol 42.2951 0.78304 MOL009644 6-Fluoroindole-7-Dehydrocholesterol 43.726 0.72224 MOL009646 7-O-Methylluteolin-6-C-beta-glucoside_qt 40.7737 0.30497 MOL009650 Atropine 42.159 0.19299 MOL009651 Cryptoxanthin monoepoxide 46.9537 0.56103 MOL009653 Cycloeucalenol 39.7265 0.79446 MOL009656 (E,E)-1-ethyl octadeca-3,13-dienoate 41.9962 0.19364 MOL009660 methyl (1R,4aS,7R,7aS)-4a,7-dihydroxy-7-methyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1,5,6,7a-tetrahydrocyclopenta[d]pyran-4-carboxylate 39.4285 0.46558 MOL009662 Lantadene A 38.6794 0.57405 MOL009664 Physalin A 91.7065 0.27207 MOL009665 Physcion-8-O-beta-D-gentiobioside 43.9036 0.62426 MOL009677 lanost-8-en-3beta-ol 34.2263 0.74036 MOL009678 lanost-8-enol 34.2263 0.74167 MOL009681 Obtusifoliol 42.552 0.7565 MOL000098 quercetin 46.4333 0.27525 Note. DL, Drug Like Index; OB, Oral Bioavailability. -
There were 6,490 potential targets associated with exercise fatigue in the TTD, OMIM, Drug Bank, and Gene Card databases.
-
By considering the intersection targets of Fructus lycii components associated with exercise fatigue, a total of 144 targets were obtained, as shown in Supplementary Figure S1 and Supplementary Table S2 (available in www.besjournal.com).
Table S2. Information of intersection target of components and exercise-induced fatigue
ID Molecule name Target name Gene name GQ1 Sitosterol alpha1 Progesterone receptor PGR GQ1 Sitosterol alpha1 Prostaglandin G/H synthase 2 PTGS2 GQ1 Sitosterol alpha1 Mineralocorticoid receptor NR3C2 GQ2 Cycloartenol Mineralocorticoid receptor NR3C2 GQ3 Mandenol Prostaglandin G/H synthase 1 PTGS1 GQ3 Mandenol Prostaglandin G/H synthase 2 PTGS2 GQ4 Ethyl linolenate Prostaglandin G/H synthase 1 PTGS1 GQ5 LAN Progesterone receptor PGR GQ5 LAN Mineralocorticoid receptor NR3C2 GQ6 Stigmasterol Progesterone receptor PGR GQ6 Stigmasterol Mineralocorticoid receptor NR3C2 GQ6 Stigmasterol Ig gamma-1 chain C region IGHG1 GQ6 Stigmasterol Retinoic acid receptor RXR-alpha RXRA GQ6 Stigmasterol Prostaglandin G/H synthase 1 PTGS1 GQ6 Stigmasterol Prostaglandin G/H synthase 2 PTGS2 GQ6 Stigmasterol Alpha-2A adrenergic receptor ADRA2A GQ6 Stigmasterol Sodium-dependent noradrenaline transporter SLC6A2 GQ6 Stigmasterol Sodium-dependent dopamine transporter SLC6A3 GQ6 Stigmasterol Beta-2 adrenergic receptor ADRB2 GQ6 Stigmasterol Aldose reductase AKR1B1 GQ6 Stigmasterol Urokinase-type plasminogen activator PLAU GQ6 Stigmasterol Leukotriene A-4 hydrolase LTA4H GQ6 Stigmasterol Amine oxidase [flavin-containing] B MAOB GQ6 Stigmasterol Amine oxidase [flavin-containing] A MAOA GQ6 Stigmasterol Muscarinic acetylcholine receptor M3 CHRM3 GQ6 Stigmasterol Beta-1 adrenergic receptor ADRB1 GQ6 Stigmasterol Sodium channel protein type 5 subunit alpha SCN5A GQ6 Stigmasterol 5-hydroxytryptamine 2A receptor HTR2A GQ6 Stigmasterol Gamma-aminobutyric-acid receptor subunit alpha-3 GABRA3 GQ6 Stigmasterol Muscarinic acetylcholine receptor M2 CHRM2 GQ6 Stigmasterol Alpha-1B adrenergic receptor ADRA1B GQ6 Stigmasterol Neuronal acetylcholine receptor subunit alpha-7 CHRNA7 GQ7 beta-sitosterol Progesterone receptor PGR GQ7 beta-sitosterol Prostaglandin G/H synthase 1 PTGS1 GQ7 beta-sitosterol Prostaglandin G/H synthase 2 PTGS2 GQ7 beta-sitosterol Heat shock protein HSP 90-alpha HSP90AA1 GQ7 beta-sitosterol Potassium voltage-gated channel subfamily H member 2 KCNH2 GQ7 beta-sitosterol D(1A) dopamine receptor DRD1 GQ7 beta-sitosterol Muscarinic acetylcholine receptor M3 CHRM3 GQ7 beta-sitosterol Sodium channel protein type 5 subunit alpha SCN5A GQ7 beta-sitosterol cGMP-inhibited 3',5'-cyclic phosphodiesterase A PDE3A GQ7 beta-sitosterol 5-hydroxytryptamine 2A receptor HTR2A GQ7 beta-sitosterol Gamma-aminobutyric-acid receptor subunit alpha-5 GABRA5 GQ7 beta-sitosterol Gamma-aminobutyric-acid receptor subunit alpha-3 GABRA3 GQ7 beta-sitosterol Muscarinic acetylcholine receptor M2 CHRM2 GQ7 beta-sitosterol Alpha-1B adrenergic receptor ADRA1B GQ7 beta-sitosterol Beta-2 adrenergic receptor ADRB2 GQ7 beta-sitosterol Neuronal acetylcholine receptor subunit alpha-2 CHRNA2 GQ7 beta-sitosterol Sodium-dependent serotonin transporter SLC6A4 GQ7 beta-sitosterol Mu-type opioid receptor OPRM1 GQ7 beta-sitosterol Neuronal acetylcholine receptor subunit alpha-7 CHRNA7 GQ7 beta-sitosterol Apoptosis regulator Bcl-2 BCL2 GQ7 beta-sitosterol Apoptosis regulator BAX BAX GQ7 beta-sitosterol Caspase-9 CASP9 GQ7 beta-sitosterol Transcription factor AP-1 JUN GQ7 beta-sitosterol Caspase-3 CASP3 GQ7 beta-sitosterol Caspase-8 CASP8 GQ7 beta-sitosterol Protein kinase C alpha type PRKCA GQ7 beta-sitosterol Transforming growth factor beta-1 TGFB1 GQ7 beta-sitosterol Serum paraoxonase/arylesterase 1 PON1 GQ7 beta-sitosterol Microtubule-associated protein 2 MAP2 GQ8 (-)-Hyoscyamine D(1A) dopamine receptor DRD1 GQ8 (-)-Hyoscyamine Muscarinic acetylcholine receptor M3 CHRM3 GQ8 (-)-Hyoscyamine Beta-1 adrenergic receptor ADRB1 GQ8 (-)-Hyoscyamine Alpha-2A adrenergic receptor ADRA2A GQ8 (-)-Hyoscyamine Alpha-2C adrenergic receptor ADRA2C GQ8 (-)-Hyoscyamine Delta-type opioid receptor OPRD1 GQ8 (-)-Hyoscyamine 5-hydroxytryptamine 2A receptor HTR2A GQ8 (-)-Hyoscyamine Sodium-dependent noradrenaline transporter SLC6A2 GQ8 (-)-Hyoscyamine Muscarinic acetylcholine receptor M2 CHRM2 GQ8 (-)-Hyoscyamine Alpha-2B adrenergic receptor ADRA2B GQ8 (-)-Hyoscyamine Alpha-1B adrenergic receptor ADRA1B GQ8 (-)-Hyoscyamine Sodium-dependent dopamine transporter SLC6A3 GQ8 (-)-Hyoscyamine Beta-2 adrenergic receptor ADRB2 GQ8 (-)-Hyoscyamine Sodium-dependent serotonin transporter SLC6A4 GQ8 (-)-Hyoscyamine D(2) dopamine receptor DRD5 GQ8 (-)-Hyoscyamine Mu-type opioid receptor OPRM1 GQ8 (-)-Hyoscyamine 5-hydroxytryptamine 1B receptor HTR1B GQ8 (-)-Hyoscyamine Histamine H1 receptor HRH1 GQ8 (-)-Hyoscyamine 5-hydroxytryptamine 1A receptor HTR1A GQ9 campesterol Progesterone receptor PGR GQ10 cyanin Prostaglandin G/H synthase 2 PTGS2 GQ10 cyanin Heat shock protein HSP 90-alpha HSP90AA1 GQ11 24-methylidenelophenol Progesterone receptor PGR GQ11 24-methylidenelophenol Mineralocorticoid receptor NR3C2 GQ12 daucosterol_qt Progesterone receptor PGR GQ13 glycitein Prostaglandin G/H synthase 1 PTGS1 GQ13 glycitein Estrogen receptor ESR1 GQ13 glycitein Androgen receptor AR GQ13 glycitein Peroxisome proliferator-activated receptor gamma PPARG GQ13 glycitein Prostaglandin G/H synthase 2 PTGS2 GQ13 glycitein Retinoic acid receptor RXR-alpha RXRA GQ13 glycitein cGMP-inhibited 3',5'-cyclic phosphodiesterase A PDE3A GQ13 glycitein Estrogen receptor beta ESR2 GQ13 glycitein Mitogen-activated protein kinase 14 MAPK14 GQ13 glycitein Heat shock protein HSP 90-alpha HSP90AA1 GQ13 glycitein Trypsin-1 PRSS1 GQ13 glycitein Cyclin-A2 CCNA2 GQ13 glycitein Nitric oxide synthase, inducible NOS2 GQ13 glycitein Collagenase 3 MMP13 GQ13 glycitein Neutrophil collagenase MMP8 GQ14 CLR Progesterone receptor PGR GQ14 CLR Mineralocorticoid receptor NR3C2 GQ15 14b-pregnane Prostaglandin G/H synthase 2 PTGS2 GQ15 14b-pregnane Progesterone receptor PGR GQ16 24-ethylcholesta-5,22-dienol Progesterone receptor PGR GQ16 24-ethylcholesta-5,22-dienol Mineralocorticoid receptor NR3C2 GQ17 Fucosterol Progesterone receptor PGR GQ17 Fucosterol Mineralocorticoid receptor NR3C2 GQ18 31-norlanosterol Progesterone receptor PGR GQ18 31-norlanosterol Mineralocorticoid receptor NR3C2 GQ19 4,24-methyllophenol Progesterone receptor PGR GQ20 Lophenol Progesterone receptor PGR GQ21 4alpha,14alpha,24-trimethylcholesta-8,24-dienol Progesterone receptor PGR GQ22 4alpha,24-dimethylcholesta-7,24-dienol Progesterone receptor PGR GQ22 4alpha,24-dimethylcholesta-7,24-dienol Mineralocorticoid receptor NR3C2 GQ23 4alpha-methyl-24-ethylcholesta-7,24-dienol Progesterone receptor PGR GQ24 6-Fluoroindole-7-Dehydrocholesterol Progesterone receptor PGR GQ24 6-Fluoroindole-7-Dehydrocholesterol Mineralocorticoid receptor NR3C2 GQ24 6-Fluoroindole-7-Dehydrocholesterol Glucocorticoid receptor NR3C1 GQ25 7-O-Methylluteolin-6-C-beta-glucoside_qt Prostaglandin G/H synthase 2 PTGS2 GQ25 7-O-Methylluteolin-6-C-beta-glucoside_qt DNA topoisomerase 2-alpha TOP2A GQ25 7-O-Methylluteolin-6-C-beta-glucoside_qt Heat shock protein HSP 90-alpha HSP90AA1 GQ26 Atropine D(1A) dopamine receptor DRD1 GQ26 Atropine Muscarinic acetylcholine receptor M3 CHRM3 GQ26 Atropine D(1B) dopamine receptor DRD5 GQ26 Atropine Beta-1 adrenergic receptor ADRB1 GQ26 Atropine Sodium channel protein type 5 subunit alpha SCN5A GQ26 Atropine Alpha-2A adrenergic receptor ADRA2A GQ26 Atropine 5-hydroxytryptamine 1A receptor HTR1A GQ26 Atropine Alpha-2C adrenergic receptor ADRA2C GQ26 Atropine Delta-type opioid receptor OPRD1 GQ26 Atropine Histamine H1 receptor HRH1 GQ26 Atropine 5-hydroxytryptamine 2A receptor HTR2A GQ26 Atropine Sodium-dependent noradrenaline transporter SLC6A2 GQ26 Atropine Muscarinic acetylcholine receptor M2 CHRM2 GQ26 Atropine Alpha-2B adrenergic receptor ADRA2B GQ26 Atropine Alpha-1B adrenergic receptor ADRA1B GQ26 Atropine Sodium-dependent dopamine transporter SLC6A3 GQ26 Atropine Beta-2 adrenergic receptor ADRB2 GQ26 Atropine Sodium-dependent serotonin transporter SLC6A4 GQ26 Atropine D(2) dopamine receptor DRD5 GQ26 Atropine Mu-type opioid receptor OPRM1 GQ26 Atropine 5-hydroxytryptamine 1B receptor HTR1B GQ27 Physcion-8-O-beta-D-gentiobioside DNA topoisomerase 2-alpha TOP2A GQ28 lanost-8-en-3beta-ol Progesterone receptor PGR GQ28 lanost-8-en-3beta-ol Mineralocorticoid receptor NR3C2 GQ29 Obtusifoliol Progesterone receptor PGR GQ29 Obtusifoliol Mineralocorticoid receptor NR3C2 GQ30 quercetin Prostaglandin G/H synthase 1 PTGS1 GQ30 quercetin Androgen receptor AR GQ30 quercetin Peroxisome proliferator-activated receptor gamma PPARG GQ30 quercetin Prostaglandin G/H synthase 2 PTGS2 GQ30 quercetin Heat shock protein HSP 90-alpha HSP90AA1 GQ30 quercetin Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma isoform PIK3CG GQ30 quercetin Dipeptidyl peptidase 4 DPP4 GQ30 quercetin Aldose reductase AKR1B1 GQ30 quercetin Trypsin-1 PRSS1 GQ30 quercetin DNA topoisomerase 2-alpha TOP2A GQ30 quercetin Prothrombin F2 GQ30 quercetin Potassium voltage-gated channel subfamily H member 2 KCNH2 GQ30 quercetin Sodium channel protein type 5 subunit alpha SCN5A GQ30 quercetin Coagulation factor X F10 GQ30 quercetin Beta-2 adrenergic receptor ADRB2 GQ30 quercetin Stromelysin-1 MMP3 GQ30 quercetin Coagulation factor VII F7 GQ30 quercetin Nitric-oxide synthase, endothelial NOS3 GQ30 quercetin Retinoic acid receptor RXR-alpha RXRA GQ30 quercetin Acetylcholinesterase ACHE GQ30 quercetin Amine oxidase [flavin-containing] B MAOB GQ30 quercetin Transcription factor p65 RELA GQ30 quercetin Epidermal growth factor receptor EGFR GQ30 quercetin RAC-alpha serine/threonine-protein kinase AKT1 GQ30 quercetin G1/S-specific cyclin-D1 CCND1 GQ30 quercetin Apoptosis regulator Bcl-2 BCL2 GQ30 quercetin Bcl-2-like protein 1 BCL2L1 GQ30 quercetin Proto-oncogene c-Fos FOS GQ30 quercetin Cyclin-dependent kinase inhibitor 1 CDKN1A GQ30 quercetin Apoptosis regulator BAX BAX GQ30 quercetin Caspase-9 CASP9 GQ30 quercetin Urokinase-type plasminogen activator PLAU GQ30 quercetin 72 kDa type IV collagenase MMP2 GQ30 quercetin Matrix metalloproteinase-9 MMP9 GQ30 quercetin Mitogen-activated protein kinase 1 MAPK1 GQ30 quercetin Interleukin-10 IL10 GQ30 quercetin Retinoblastoma-associated protein RB1 GQ30 quercetin Tumor necrosis factor TNF GQ30 quercetin Transcription factor AP-1 JUN GQ30 quercetin Interleukin-6 IL6 GQ30 quercetin Caspase-3 CASP3 GQ30 quercetin Cellular tumor antigen p53 TP53 GQ30 quercetin NF-kappa-B inhibitor alpha NFKBIA GQ30 quercetin Xanthine dehydrogenase/oxidase XDH GQ30 quercetin Caspase-8 CASP8 GQ30 quercetin RAF proto-oncogene serine/threonine-protein kinase RAF1 GQ30 quercetin Superoxide dismutase [Cu-Zn] SOD1 GQ30 quercetin Protein kinase C alpha type PRKCA GQ30 quercetin Interstitial collagenase MMP1 GQ30 quercetin Hypoxia-inducible factor 1-alpha HIF1A GQ30 quercetin Signal transducer and activator of transcription 1-alpha/beta STAT1 GQ30 quercetin Cell division control protein 2 homolog CDK1 GQ30 quercetin Peroxisome proliferator-activated receptor gamma PPARG GQ30 quercetin Heme oxygenase 1 HMOX1 GQ30 quercetin Cytochrome P450 3A4 CYP3A4 GQ30 quercetin Caveolin-1 CAV1 GQ30 quercetin Myc proto-oncogene protein MYC GQ30 quercetin Tissue factor F3 GQ30 quercetin Gap junction alpha-1 protein GJA1 GQ30 quercetin Cytochrome P450 1A1 CYP1A1 GQ30 quercetin Intercellular adhesion molecule 1 ICAM1 GQ30 quercetin Interleukin-1 beta IL1B GQ30 quercetin Small inducible cytokine A2 CCL2 GQ30 quercetin E-selectin SELE GQ30 quercetin Vascular cell adhesion protein 1 VCAM1 GQ30 quercetin Prostaglandin E2 receptor, EP3 subtype PTGER3 GQ30 quercetin Interleukin-8 CXCL8 GQ30 quercetin Nitric oxide synthase, endothelial NOS3 GQ30 quercetin Heat shock protein beta-1 HSPB1 GQ30 quercetin Transforming growth factor beta-1 TGFB1 GQ30 quercetin Maltase-glucoamylase, intestinal MGAM GQ30 quercetin Interleukin-2 IL2 GQ30 quercetin Cytochrome P450 1B1 CYP1B1 GQ30 quercetin Tissue-type plasminogen activator PLAT GQ30 quercetin Thrombomodulin THBD GQ30 quercetin Plasminogen activator inhibitor 1 SERPINE1 GQ30 quercetin Interferon gamma IFNG GQ30 quercetin Arachidonate 5-lipoxygenase ALOX5 GQ30 quercetin Phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN PTEN GQ30 quercetin Interleukin-1 alpha IL1A GQ30 quercetin Myeloperoxidase MPO GQ30 quercetin DNA topoisomerase 2-alpha TOP2A GQ30 quercetin Neutrophil cytosol factor 1 NCF1 GQ30 quercetin Nuclear factor erythroid 2-related factor 2 NFE2L2 GQ30 quercetin NAD(P)H dehydrogenase [quinone] 1 NQO1 GQ30 quercetin Poly [ADP-ribose] polymerase 1 PARP1 GQ30 quercetin Aryl hydrocarbon receptor AHR GQ30 quercetin Solute carrier family 2, facilitated glucose transporter member 4 SLC2A4 GQ30 quercetin Collagen alpha-1(III) chain COL3A1 GQ30 quercetin C-X-C motif chemokine 11 CXCL11 GQ30 quercetin C-X-C motif chemokine 2 CXCL2 GQ30 quercetin Serine/threonine-protein kinase Chk2 CHEK2 GQ30 quercetin Insulin receptor INSR GQ30 quercetin Peroxisome proliferator-activated receptor alpha PPARA GQ30 quercetin Peroxisome proliferator-activated receptor delta PPARD GQ30 quercetin C-reactive protein CRP GQ30 quercetin C-X-C motif chemokine 10 CXCL10 GQ30 quercetin Inhibitor of nuclear factor kappa-B kinase subunit alpha CHUK GQ30 quercetin Osteopontin SPP1 GQ30 quercetin Runt-related transcription factor 2 RUNX2 GQ30 quercetin Ras association domain-containing protein 1 RASSF1 GQ30 quercetin Cathepsin D CTSD GQ30 quercetin Insulin-like growth factor-binding protein 3 IGFBP3 GQ30 quercetin Insulin-like growth factor II IGF2 GQ30 quercetin CD40 ligand CD40LG GQ30 quercetin Receptor tyrosine-protein kinase erbB-3 ERBB3 GQ30 quercetin Serum paraoxonase/arylesterase 1 PON1 GQ30 quercetin Type I iodothyronine deiodinase DIO1 GQ30 quercetin Ras GTPase-activating protein 1 RASA1 GQ30 quercetin Glutathione S-transferase Mu 1 GSTM1 -
The 144 potential targets of the components of Fructus lycii associated with exercise fatigue were imported into Cystoscope 3.8.2 to construct an active compound-targets network, as shown in Figure 1. There were 174 nodes, including 30 nodes for chemical components, 144 nodes for targets, and 530 edges for interactions between nodes. Quercetin (degree = 110), β-sitosterol (degree = 29), stigmasterol (degree = 23), 7-O-methylluteolin-6-C-beta-glucoside_qt (degree = 21), atropine (degree = 19), and glycitein (degree = 15) of Fructus lycii were identified as the main active components with an anti-exercise fatigue effect. Quercetin was selected as the representative component for further studies.
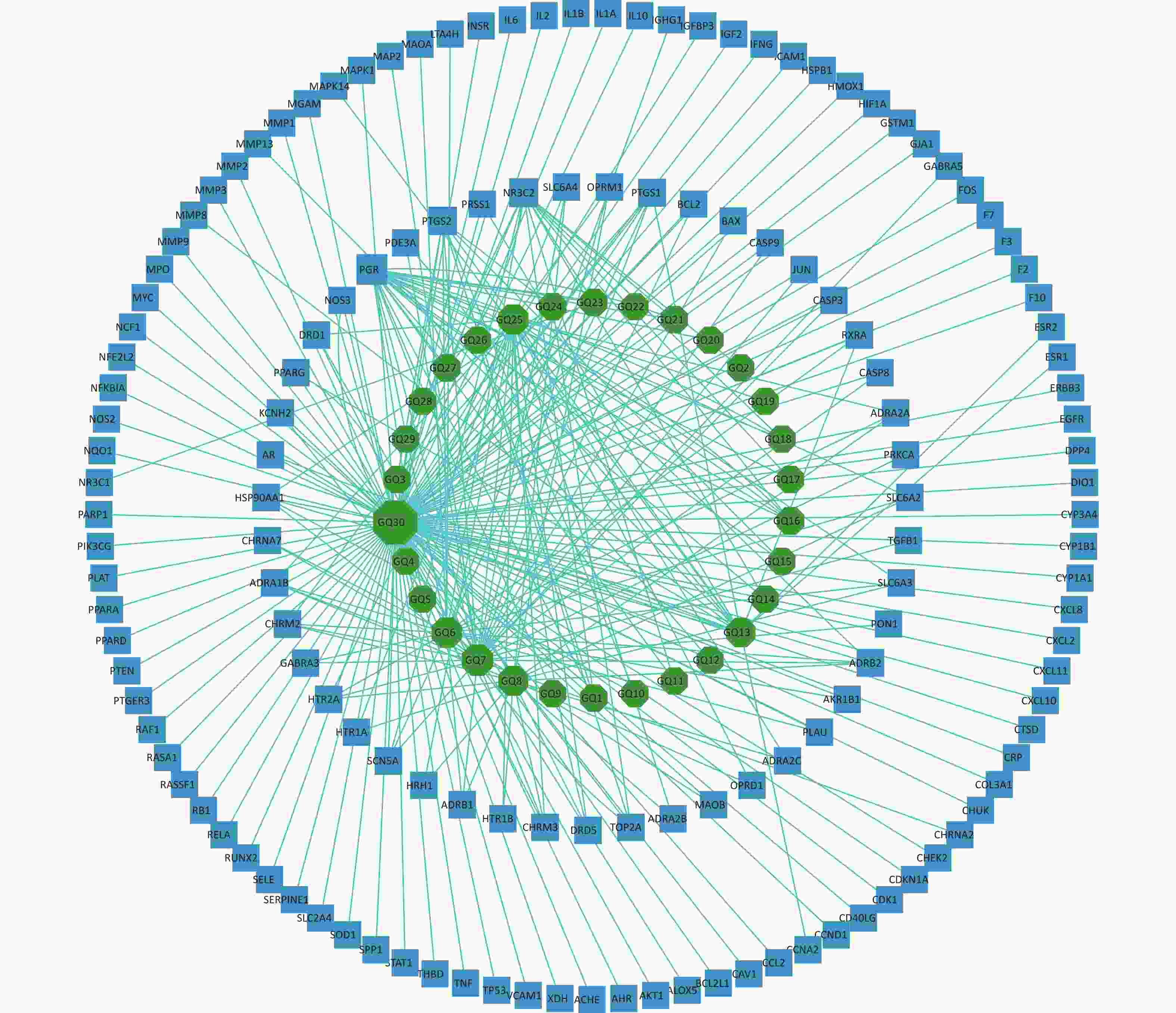
Figure 1. Compound-targets network. GQ1, Sitosterol alpha1; GQ2, Cycloartenol; GQ3, Mandenol; GQ4, Ethyl linolenate; GQ5, LAN; GQ6, Stigmasterol; GQ7, Beta-sitosterol; GQ8, (-)-Hyoscyamine; GQ9, Campesterol; GQ10, Cyanin; GQ11, 24-methylidenelophenol; GQ12, Daucosterol qt; GQ13, Glycitein; GQ14, CLR; GQ15, 14b-pregnane; GQ16, 24-ethylcholesta-5,22-dienol; GQ17, Fucosterol; GQ18, 31-norlanosterol; GQ19, 4,24-methyllophenol; GQ20, Lophenol; GGQ21, 4alpha,14alpha,24-trimethylcholesta-8,24-dienol; GQ22, 4alpha,24-dimethylcholesta-7,24-dienol; GQ23, 4alpha-methyl-24-ethylcholesta-7,24-dienol; GQ24, 6-Fluoroindole-7-Dehydrocholesterol; GQ25, 7-O-methylluteolin-6-C-beta-glucoside_qt; GQ26, Atropine; GQ27, Physcion-8-O-beta-D-gentiobioside; GQ28, Lanost-8-en-3beta-ol; GQ29, Obtusifoliol; GQ30, Quercetin.
-
The Mediascape database was utilized to analyze 144 key targets, and the results of the GO enrichment analysis were divided into three categories: BP, CC, and MF, as shown in Figure 2A. These targets are highly related to cellular components, including the synaptic membrane. Responses to nitrogen compounds, hormones, lipids, and xenobiotic stimuli are primarily involved in biological processes. In addition, G protein-coupled amine receptor activity, transcription co-regulator binding, DNA-binding transcription factor binding, nuclear receptor activity, and ligand-activated transcription factor activity of the molecular function were closely related to these targets.
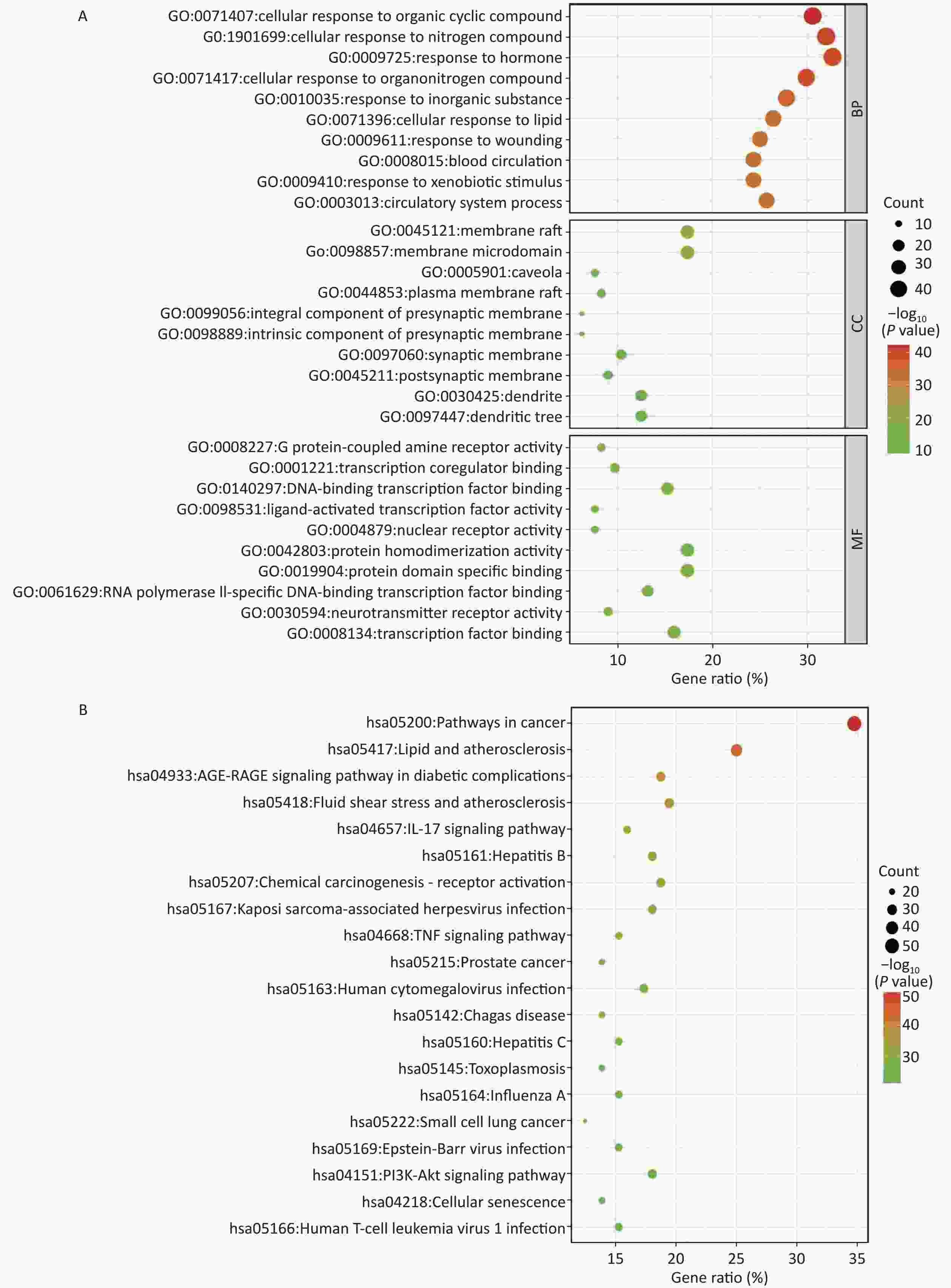
Figure 2. Enrichment analysis. (A) GO enrichment analysis; (B) KEGG enrichment analysis. BP, biological process; CC, cellular component; MF, molecular function. P < 0.05.
A total of 198 pathways with P < 0.05 were identified, and the top 20 pathways are illustrated as enriched dot bubbles in Figure 2B. The top three pathways were hsa05200 (degree = 50), hsa05417 (degree = 36), and hsa04933 (degree = 27), all of which were highly associated with cancer-, inflammation-, immune-, and apoptosis-related pathways, as revealed by enrichment analysis.
-
To explain the pharmacological mechanism of anti-exercise fatigue in Fructus lycii in detail, a compound-target-pathway network was constructed. As shown in Figure 3 and Supplementary Tables S3–S4 (available in www.besjournal.com), there were 198 pathways related to 136 of the 144 key targets and eight targets without pathways. The average degrees of the targets, pathways, and components were 17.35, 12.28, and 8.83, respectively. Based on the double median degree (degree ≥ 18), 39 targets were selected. Targets with the highest degrees were MAPK1 (117), AKT1 (98), RAF1 (84), RELA (77), TNF (68), PRKCA (66), MAPK14 (62), JUN (57), CHUK (54), IL6 (52), and BAX (52). The top signaling pathways included hsa05200 (pathways in cancer), hsa04933 (AGE-RAGE signaling pathway in diabetic complications, degree = 28), hsa04151 (PI3K-Akt signaling pathway, degree = 27), hsa04080 (neuroactive ligand-receptor interaction pathway, degree = 25), hsa04657 (IL-17 signaling pathway, degree = 24), hsa04668 (TNF signaling pathway, degree = 23), hsa05022 (pathways of neurodegeneration - multiple diseases) and hsa04010 (MAPK signaling pathway, degree = 23). Among these, the PI3K-Akt and MAPK signaling pathways are closely related to exercise fatigue[17]. Moreover, most of the core target proteins identified by network pharmacology analysis were associated with the PI3K-Akt and MAPK signaling pathways. Therefore, in the present study, we first focused on the PI3K-Akt and MAPK signaling pathways.
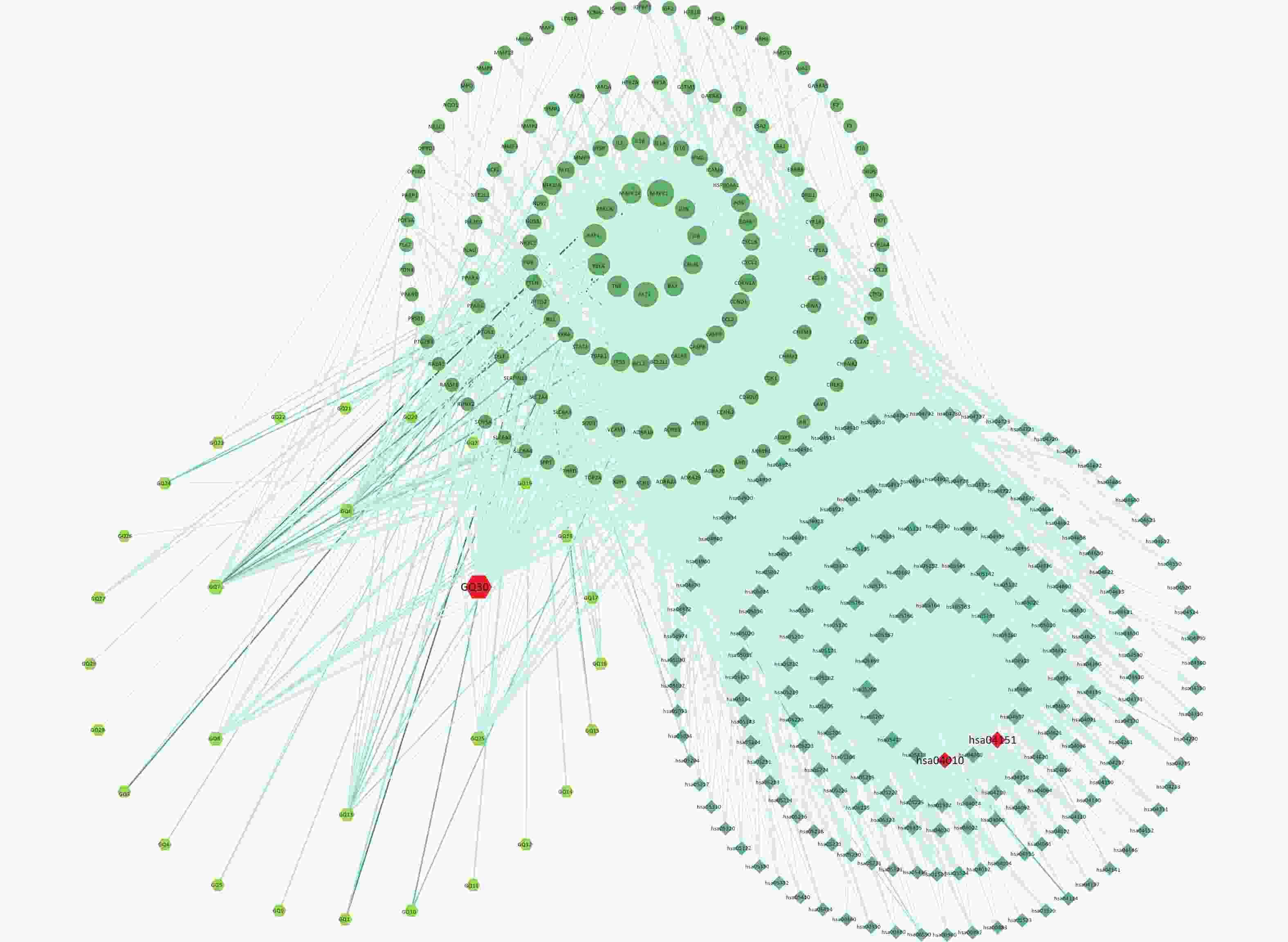
Figure 3. Compound-targets-pathways network. GQ1, Sitosterol alpha1; GQ2, Cycloartenol; GQ3, Mandenol; GQ4, Ethyl linolenate; GQ5, LAN; GQ6, Stigmasterol; GQ7, Beta-sitosterol; GQ8, (-)-Hyoscyamine; GQ9, Campesterol; GQ10, Cyanin; GQ11, 24-methylidenelophenol; GQ12, Daucosterol_qt; GQ13, Glycitein; GQ14, CLR; GQ15, 14b-pregnane; GQ16, 24-ethylcholesta-5,22-dienol; GQ17, Fucosterol; GQ18, 31-norlanosterol; GQ19, 4,24-methyllophenol; GQ20, Lophenol; GGQ21, 4alpha,14alpha,24-trimethylcholesta-8,24-dienol; GQ22, 4alpha,24-dimethylcholesta-7,24-dienol; GQ23, 4alpha-methyl-24-ethylcholesta-7,24-dienol; GQ24, 6-Fluoroindole-7-Dehydrocholesterol; GQ25, 7-O-Methylluteolin-6-C-beta-glucoside_qt; GQ26, Atropine; GQ27, Physcion-8-O-beta-D-gentiobioside; GQ28, Lanost-8-en-3beta-ol; GQ29, Obtusifoliol; GQ30, Quercetin.
-
Upon treating C2C12 cells with or without quercetin for 24 h, we observed that quercetin reduced cell viability in a dose-dependent manner, as shown by the CCK-8 assay (Supplementary Figure S2, available in www.besjournal.com). Quercetin at 125 μmol/L reduced the viability of C2C12 cells to approximately 80%. The viability of cells treated with 453.5 μmol/L was 50%, which was defined as inhibitory concentration 50 (IC50) of quercetin. We chose a concentration that was around 1/10 of the IC50 as the highest concentration of quercetin, 50 μmol/L. This concentration exhibited no discernible toxicity and was also consistent with commonly used concentration of quercetin in other related experiments.
-
To assess the effect of quercetin treatment on glucose uptake, C2C12 myotubes were treated with different concentrations of quercetin (50, 25, 12.5, 6.25 μmol/L) and then exposed to 2-NBDG, a fluorescently labeled glucose analog. Compared to the control, the glucose uptake of C2C12 myotubes was significantly increased by quercetin (P < 0.01), as shown in Figure 4A.
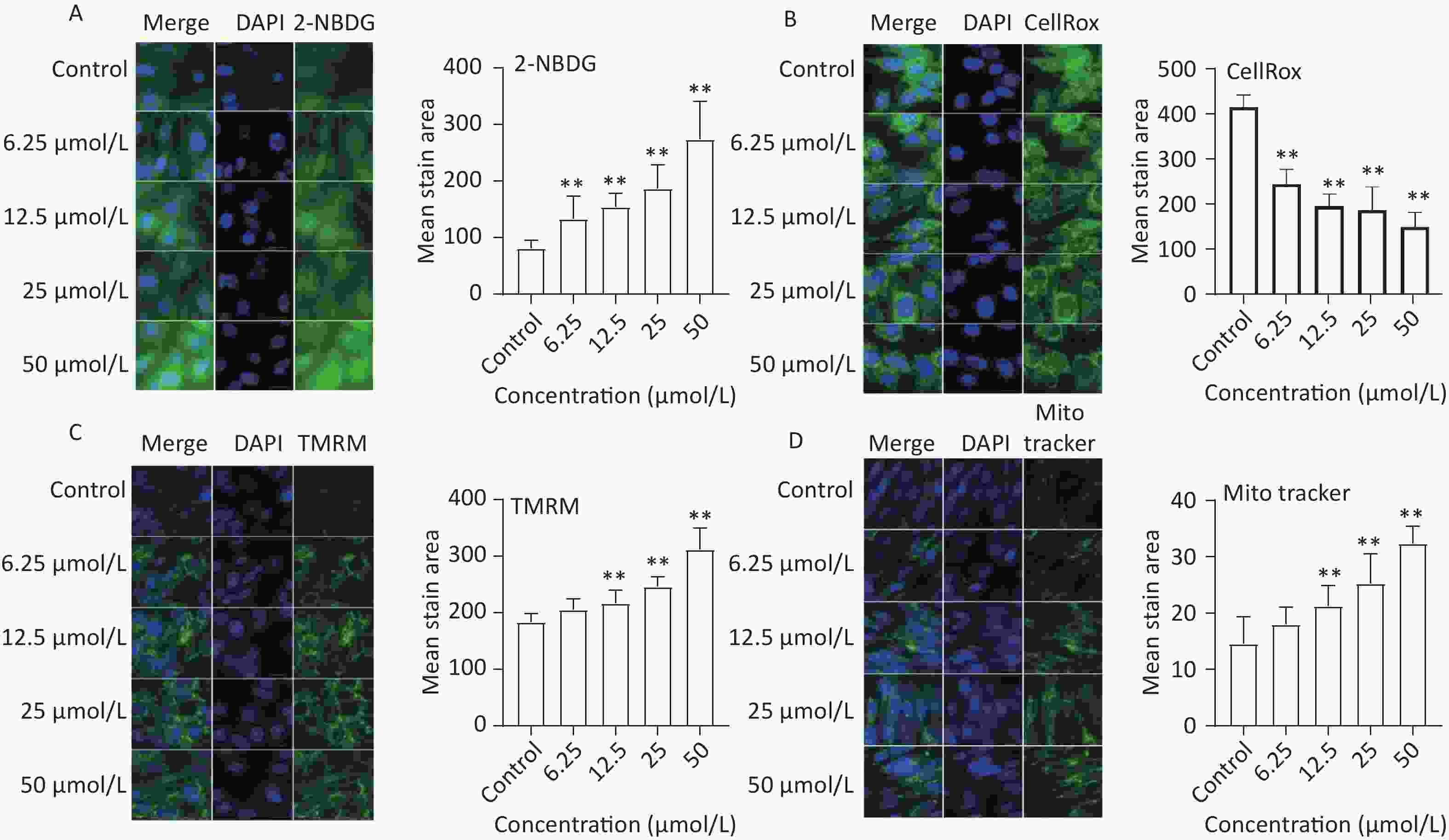
Figure 4. The effect of quercetin on glucose uptake, ROS generation, and mitochondria function in C2C12 cells. The 2-NBDG (A), CellRox (B), TMRM (C), and MitoTracker (D) probes were detected by a high content imaging analysis system; Left panel: representative images. Data presented were from three biological replicates; Scale bar size: 50 μm; *P < 0.05; **P < 0.01.
-
The effects of quercetin on ROS production were analyzed in TBHP-treated C2C12 myotube cells. Quercetin significantly reduced ROS production in a concentration-dependent manner (P < 0.01; Figure 4B).
-
Mitochondrial biogenesis is intricately linked to multiple physiological processes and is a crucial factor influencing skeletal muscle function[18]. In our study, mitochondrial mass and membrane potential were evaluated using MitoTracker and TMRM, respectively. The results showed that quercetin significantly increased the mitochondrial membrane potential and the mean stained area of TMRM-and Mito Tracker-stained mitochondria in a concentration-dependent manner (Figure 4C and Figure 4D).
-
The expression of key targets in the MAPK (p-38 MAPK, p-MAPK, and p-JNK) and PI3K-Akt (p-PI3K and p-AKT) signaling pathways was analyzed based on the results of network pharmacology. The immunofluorescence results (Figure 5A) revealed increased protein expression of p-P38 MAPK, p-MAPK, and p-JNK in the quercetin group compared to the control group. Moreover, the protein levels of p-PI3K and p-AKT markedly increased after quercetin treatment (Figure 5B).
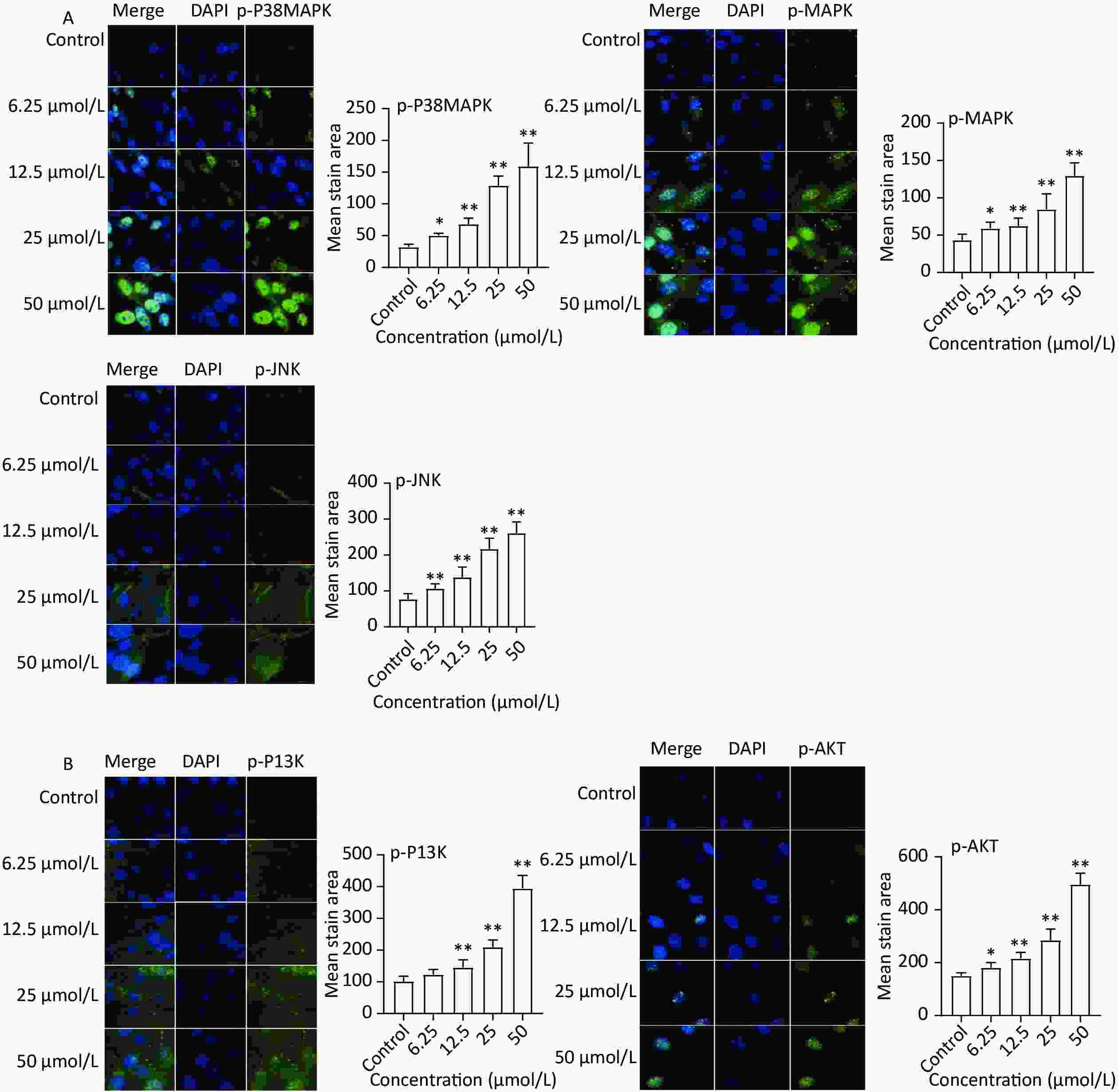
Figure 5. Protein expression of MAPK and PI3K-AKT signaling pathways. (A) MAPK signaling pathways, the protein expression of p-P38 MAPK, p-MAPK, and p-JNK was detected by a high content imaging analysis system; (B) PI3K-AKT signaling pathway, p-PI3K and p-AKT was detected by a high content imaging analysis system; Left panel: representative images. Data presented were from three biological replicates; Scale bar size: 50 μm; *P < 0.05; **P < 0.01.
-
A meta-analysis was conducted to investigate the effect of Fructus lycii on exercise fatigue. The findings indicated that Fructus lycii extended the duration of exhaustive exercise and stored muscle and liver glycogen reserves while decreasing the levels of BUN and lactic acid (Supplementary Tables S6–S7 and Figure S3–S4, available in www.besjournal.com). These indicators are important for evaluating exercise fatigue[19,20], suggesting that Fructus lycii might have the potential to improve exercise performance. To further investigate the mechanism and identify the primary components responsible for this effect, we conducted a network pharmacology analysis. Six potential active components in Fructus lycii as predicted by network pharmacology, emerged, with quercetin exhibiting the highest degree of influence. Other key components included β-sitosterol, stigmasterol, 7-O-methylluteolin-6-C-beta-glucoside_qt, atropine, and glycitein. Multiple pathways have been identified to be related to anti-exercise fatigue, including the AGE-RAGE signaling pathway in diabetic complications, PI3K-Akt signaling pathway, neuroactive ligand-receptor interaction pathway, IL-17 signaling pathway, TNF signaling pathway, and MAPK signaling pathway. Furthermore, quercetin, one of the main active components of Fructus lycii[21], and its two signaling pathways (MAPK and PI3K-Akt) closely related to exercise fatigue were investigated in an in vitro study to clarify its function and mechanism.
Table S6. Methodological quality assessment of the studies included
Quality score criterion Peer-reviewed publication Control of temperature Random allocation to treatment or control Blinded induction of model Blinded assessment of outcome Use of anesthetic without significant intrinsic neuroprotective activity Appropriate animal model Sample size calculation Compliance with animal welfare regulations Statement of potential conflict of interests Total Yang 2019 (30) 0 0 1 0 0 0 1 0 1 0 3 Cao 2018 (17) 0 0 1 0 0 0 1 0 1 0 3 Ji et al. 2011 (18) 1 1 1 0 0 1 1 0 1 0 6 Ding et al. 2001 (29) 1 0 1 0 0 0 1 0 1 0 4 Qin et al. 2009 (27) 1 0 1 0 0 0 1 0 1 0 4 Hu et al. 2008 (21) 1 1 1 0 0 0 1 0 1 0 5 Liu et al. 2011 (19) 1 0 1 0 0 0 1 0 1 0 4 Yi et al. 2010 (26) 1 0 1 0 0 0 1 0 1 0 4 Wang et al. 2002 (24) 1 0 1 0 0 0 1 0 1 0 4 Liu 2019 (22) 0 1 1 0 0 0 1 0 1 0 4 Yang et al. 2018 (20) 1 1 1 0 0 0 1 0 1 0 5 Ma 2019 (23) 1 0 1 0 0 0 1 0 1 0 4 Niu et al. 1994 (25) 1 0 1 0 0 0 1 0 1 0 4 Wu et al. 2008 (9) 1 1 1 0 0 0 1 0 1 0 5 Wang et al. 2017 (28) 1 0 1 0 0 0 1 0 1 0 4 Note. As showed in Supplementary Table S6, the quality score of the included studies ranged from 3 to 5 and the average quality score was 4.2 points. All studies randomly allocated animals to the control group and the treatment group, adopted appropriate animal models and compliance with animal welfare regulations. However, the method of blinded induction of model and blinded assessment of outcome were not involved in those included studies. In addition, there was no statement of potential conflict of interests in those articles. Table S7. Meta-analysis for each sub-outcome measure
Outcome Meta-analysis of outcome Test of heterogeneity Model used SMD 95% CI P I2 P Blood lactates −1.58 −1.58 to −0.97 < 0.01 80% < 0.01 Random-effects Muscle glycogen 0.85 0.04 to 1.67 < 0.01 76% < 0.01 Random-effects Liver glycogen 0.91 0.40 to 1.41 < 0.01 60% < 0.01 Random-effects SOD 1.30 0.45 to 2.14 < 0.01 78% < 0.01 Random-effects MAD −0.84 −1.18 to −0.05 < 0.01 32% 0.18 Fixed-effects Note. SMD, standardized mean difference; I2, I-squared statistic; CI, confidence interval; SOD, superoxide dismutase; MAD, malondialdehyde. Quercetin, a flavonoid, possesses unique biological properties that hold potential for improving mental and physical performance, as well as conferring benefits to overall health and disease resistance. It exhibits anti-inflammatory, antiviral, antioxidant, anticarcinogenic, and psychostimulant activities[22]. Several studies have shown that quercetin inhibits LPS-induced mRNA expression of TNF-α, IL-8 and IL-1α[23]. In addition, quercetin is widely used as a nutritional supplement and phytochemical therapy for the treatment of various diseases, such as diabetes, obesity, and cardiovascular diseases[24]. Recent studies have reported that dietary quercetin supplementation can ameliorate exercise fatigue and enhance performance[25]; however, the underlying mechanism is still unclear. Our study demonstrated that quercetin significantly increased glucose uptake, enhanced mitochondrial function, and decreased ROS levels. Increasing the capacity for glucose uptake and mitochondrial energy production has been suggested as an effective approach to delay exercise fatigue[26,27]. The skeletal muscles play an important role in exercise and energy balance. Recently, increasing evidence has revealed that mitochondria are essential for maintaining energy homeostasis, which is highly correlated with muscle function and energy production, resulting in enhanced exercise performance[28,29]. Excessive ROS production during exercise can impair mitochondrial and nuclear DNA, and induce mitochondrial dysfunction, which appears to be a key issue during exhaustive exercise. Similar to our findings, quercetin improves mitochondrial regeneration, enhances energy metabolism, and improves exercise endurance by regulating mitochondrial gene expression in mouse muscles[30]. In addition, glucose is initially phosphorylated by hexokinase, which traps glucose intracellularly in the skeletal muscle and is then utilized to produce energy[31]. Quercetin may facilitate glucose uptake to exert beneficial effects on maintaining glucose homeostasis. This glucose can be stored as glycogen or utilized to produce energy during exercise. Notably, research has underscored quercetin’s role in elevating insulin-stimulated glucose uptake and coupled with an improved replenishment function, which is conducive to the rapid restoration of muscle glycogen. This synergistic effect serves to improve time-to-exhaustion during exercise[32].
Previous reports have indicated that the MAPK and PI3K-Akt signaling pathways play crucial roles in various cellular processes, including cell survival, proliferation, differentiation, motility, and apoptosis. Our in vitro experiments revealed that quercetin exerts its biological functions by regulating the MAPK and PI3K-Akt signaling pathways. The MAPK signaling pathway regulates energy metabolism by enhancing mitochondrial function and glucose uptake[33,34]. The glucose transporter GLUT4 is critical for skeletal muscle glucose uptake and the maintenance of whole-body glucose homeostasis. Similarly, black rice extracts were found to stimulate GLUT4 glucose uptake through the activation of PI3K-Akt and MAPK signaling in C2C12 myotubes[5]. Previous studies have shown that activation of the PI3K-Akt and MAPK signaling pathways may help reduce oxidative damage[35,36]. Consistent with our results, ginsenoside Rb1 has also been shown to alleviate fatigue syndrome by reducing skeletal muscle oxidative stress through the activation of the PI3K-Akt pathway[37]. Adaptation and maintenance of the oxidative-antioxidant balance depend heavily on the MAPK signaling pathway. Moreover, the MAPK signaling pathway contributes to antioxidant activity by regulating the levels of SOD, which plays a critical role in anti-fatigue effects[38]. Overall, our findings suggest that quercetin exerts its effects via the PI3K-Akt and MAPK signaling pathways.
It is also noteworthy that Fructus lycii regulates the inflammatory pathways that are involved in exercise fatigue. Exercise fatigue is often accompanied by increased levels of pro-inflammatory cytokines[39]. It has also been suggested that cytokine release may result in prolonged sickness symptoms or behavioral stillness, which can sometimes be observed in cases of overtraining syndrome in athletes, as well as in a variety of systemic immune and inflammatory conditions. For example, TNF-α, IL-1β, and IL-6 also increased as a result of muscle fatigue[40]. Additionally, post-exercise mobilization of T-lymphocytes, particularly CD4+ and CD8+ lymphocytes, from peripheral lymphoid compartments into the blood has been observed[41]. The IL-17, TNF, and MAPK signaling pathways are the main pathway-linked inflammatory responses, as shown in our study[42]. Studies have suggested that regulating the expression of pro-inflammatory and anti-inflammatory cytokines by regulating signaling pathways involved in anti-inflammatory was an effective way to relieve exercise fatigue[2]. Hence, future research endeavors should focus on extensively investigating Fructus lycii’s potential in combating to exercise fatigue.
Our study lays the groundwork for potential avenues of deeper exploration into the mechanisms underlying the impacts of Fructus lycii on exercise fatigue. Nonetheless, there exist certain limitations. First, we focused on Fructus lycii and its energy metabolism and antioxidant mechanisms. Moving forward, it is imperative to pay continuous attention to other active ingredients and mechanisms, especially the functions of the inflammatory pathways identified in our study. Second, although we used immunofluorescence staining combined with high-content imaging to display the phosphorylation levels of key target proteins associated with the identified signaling pathways, western blotting can also be used as an auxiliary method to verify the expression and phosphorylation levels of key target proteins. In addition, the mRNA expression should be measured during follow-up. Finally, the study used cultured cells as a model, which does not fully represent in vivo physiological conditions. Therefore, in vivo studies are needed to confirm the anti-fatigue activity of Fructus lycii and its components.
In summary, we conducted a multi-component, multi-target, and multi-pathway exploration of the anti-exercise-fatigue effects of Fructus lycii. The main active components and possible molecular mechanisms underlying the anti-exercise-fatigue activity of Fructus lycii should be further investigated. Quercetin, a crucial active component in Fructus lycii, was shown to enhance energy metabolism and reduce oxidative stress, thereby delaying exercise fatigue. The PI3K-Akt and MAPK signaling pathways might be involved in this process. These results pave the way for subsequent research endeavors aimed at deeper explorations of the mechanisms underlying the effects of Fructus lycii on exercise fatigue.
Fructus lycii significantly alleviated exercise fatigue. Six potential active components, including quercetin, sitosterol, stigmasterol, 7-O-methylluteolin-6-C-beta-glucoside_qt, atropine, and glycitein, may contribute to the improvement of exercise fatigue via multiple pathways, including the PI3K-Akt, neuroactive ligand-receptor interaction, IL-17, TNF, and MAPK signaling pathways. Quercetin may play important roles in reducing oxidative stress, boosting glucose uptake, and enhancing mitochondrial function. The underlying mechanism may involve the PI3K-AKT and MAPK signaling pathways.
-
JI Xiao Ning: methodology, formal analysis, validation, investigation, and writing - original draft; LIU Zhao Ping: conceptualization, methodology, writing - review and editing; ZHANG Chao Zheng: formal analysis, investigation, data curation, writing - review and editing; CHEN Min: conceptualization, investigation, writing - review and editing; LIANG Jiang: conceptualization, writing - review and editing. ZHANG Lei: conceptualization, resources, writing - review and editing; LU Jiang: conceptualization, supervision, writing - review and editing, and project administration. All authors have approved the final version of the manuscript submitted for publication.
-
Table S3. Information of targets of exercise-induced fatigue
Degree Name Neighborhood connectivity 117 MAPK1 15.31624 98 AKT1 16.81633 84 RAF1 15.54762 77 RELA 19.09091 68 TNF 16.77941 66 PRKCA 15.04545 62 MAPK14 16.27419 57 JUN 19.52632 54 CHUK 20.48148 52 BAX 19.34615 52 IL6 19.55769 51 TP53 19.23529 50 NFKBIA 20.4 48 EGFR 17.8125 47 CCND1 18.76596 46 CASP3 21.08696 46 FOS 20.28261 45 IL1B 18.93333 44 BCL2 20.56818 42 CDKN1A 19.33333 38 CASP8 22.10526 37 CASP9 21.48649 36 TGFB1 19.75 35 MYC 20.45714 34 CXCL8 21.73529 32 IFNG 18.5625 30 PGR 6.625 30 PTGS2 18.43333 30 STAT1 22.1 29 BCL2L1 21.2069 26 RB1 23 26 PTEN 20.80769 23 INSR 15.65217 22 IL10 17.18182 22 IL1A 21.04545 21 IL2 20.33333 20 HSP90AA1 23.8 19 RXRA 24.36842 19 NOS3 22.33333 18 NOS2 17.61111 18 CCL2 25.5 17 MMP9 27.76471 17 ICAM1 23.70588 15 NR3C2 3.714286 15 CXCL2 23.86667 14 ADRB2 23.57143 14 ADRB1 14.85714 14 HIF1A 23.78571 14 CD40LG 18.71429 13 CHRM3 15.92308 13 DRD1 14.53846 13 PPARG 25.16667 13 CCNA2 19.23077 13 PIK3CG 22.84615 13 CXCL10 24.46154 12 MMP2 28.16667 12 NCF1 24.75 11 MAOB 18.45455 11 GSTM1 26.45455 10 PTGS1 21.3 10 SLC6A3 13.2 10 MAOA 9.6 10 CHRM2 19.6 10 ADRA1B 17 10 VCAM1 27.6 10 PPARA 25.3 9 HTR2A 18.11111 9 CHRNA7 19.66667 9 ESR1 21.44444 9 F2 28.44444 9 SOD1 22.55556 9 MMP1 32.66667 9 CDK1 23.44444 9 CYP1A1 24.44444 9 CYP1B1 22.44444 9 SERPINE1 26 8 PLAU 29.5 8 GABRA3 13.125 8 ESR2 20.5 8 MMP3 33.375 8 SELE 31.5 8 NFE2L2 35.375 8 SLC2A4 22.125 8 ERBB3 29.625 7 GABRA5 11.71429 7 DRD5 18.16667 7 HMOX1 37.71429 7 CYP3A4 22.85714 7 PTGER3 36 7 COL3A1 28.42857 7 SPP1 28.85714 7 RASSF1 32 7 CTSD 29.28571 7 IGF2 37.57143 6 PDE3A 14.66667 6 OPRM1 19.5 6 HTR1B 17 6 HTR1A 17 6 AR 38 6 PRSS1 29.66667 6 CAV1 31.83333 6 PLAT 32.66667 6 ALOX5 26.83333 5 ADRA2A 20.4 5 SCN5A 38 5 SLC6A4 17.2 5 OPRD1 18.8 5 HRH1 17.4 5 TOP2A 31 5 MPO 30.2 5 NQO1 45.8 5 PARP1 34 5 AHR 36.4 5 CXCL11 33 5 CHEK2 35.2 5 PPARD 36.4 5 IGFBP3 34.2 4 SLC6A2 16.5 4 ADRA2C 19.75 4 ADRA2B 19.75 4 MMP13 17.25 4 HSPB1 38.75 4 THBD 43.25 4 RASA1 37 3 XDH 38.66667 3 F3 48 3 RUNX2 45.66667 2 AKR1B1 65 2 LTA4H 14 2 KCNH2 68 2 CHRNA2 27 2 PON1 68 2 NR3C1 14 2 DPP4 55.5 2 F10 58 2 F7 58 2 ACHE 59 2 GJA1 58.5 2 DIO1 60.5 1 IGHG1 23 1 MAP2 29 1 MMP8 15 1 MGAM 107 1 CRP 107 Table S4. Information of pathways of exercise-induced fatigue
Pathway Description Degree Neighborhood connectivity hsa05200 Pathways in cancer 51 35.80392 hsa05417 Lipid and atherosclerosis 37 41.48649 hsa05418 Fluid shear stress and atherosclerosis 29 35.62069 hsa04933 AGE-RAGE signaling pathway in diabetic complications 28 44.89286 hsa05207 Chemical carcinogenesis - receptor activation 28 39.89286 hsa05161 Hepatitis B 27 56.66667 hsa05167 Kaposi sarcoma-associated herpesvirus infection 27 52.66667 hsa04151 PI3K-Akt signaling pathway 27 46.33333 hsa05163 Human cytomegalovirus infection 26 57 hsa04080 Neuroactive ligand-receptor interaction 25 15.12 hsa04657 IL-17 signaling pathway 24 46.25 hsa04668 TNF signaling pathway 23 49.73913 hsa05160 Hepatitis C 23 55.30435 hsa05164 Influenza A 23 54.52174 hsa05169 Epstein-Barr virus infection 23 52.47826 hsa05166 Human T-cell leukemia virus 1 infection 23 52.91304 hsa04010 MAPK signaling pathway 23 54.6087 hsa05205 Proteoglycans in cancer 22 49.04545 hsa05208 Chemical carcinogenesis - reactive oxygen species 22 45.72727 hsa05022 Pathways of neurodegeneration - multiple diseases 22 51.18182 hsa05215 Prostate cancer 21 50.66667 hsa05142 Chagas disease 21 54.85714 hsa05145 Toxoplasmosis 21 51.85714 hsa04218 Cellular senescence 21 49.90476 hsa05152 Tuberculosis 21 56.42857 hsa05171 Coronavirus disease - COVID-19 21 51.52381 hsa05132 Salmonella infection 21 61.42857 hsa05162 Measles 20 54.7 hsa05225 Hepatocellular carcinoma 20 49.65 hsa05165 Human papillomavirus infection 20 57.6 hsa05222 Small cell lung cancer 19 51.52632 hsa04926 Relaxin signaling pathway 19 53.89474 hsa04210 Apoptosis 19 60.94737 hsa04932 Non-alcoholic fatty liver disease 19 52.36842 hsa05202 Transcriptional misregulation in cancer 19 35.05263 hsa05170 Human immunodeficiency virus 1 infection 19 64.84211 hsa05206 MicroRNAs in cancer 19 46.84211 hsa01522 Endocrine resistance 18 56.55556 hsa04620 Toll-like receptor signaling pathway 18 58.38889 hsa04659 Th17 cell differentiation 18 51.94444 hsa04621 NOD-like receptor signaling pathway 18 56 hsa04024 cAMP signaling pathway 18 45.5 hsa05010 Alzheimer disease 18 57.16667 hsa05168 Herpes simplex virus 1 infection 18 56.61111 hsa05140 Leishmaniasis 17 54.23529 hsa04625 C-type lectin receptor signaling pathway 17 64.88235 hsa04380 Osteoclast differentiation 17 59.76471 hsa04915 Estrogen signaling pathway 17 47.82353 hsa05224 Breast cancer 17 56.94118 hsa05130 Pathogenic Escherichia coli infection 17 61.29412 hsa05415 Diabetic cardiomyopathy 17 40.11765 hsa04020 Calcium signaling pathway 17 27.41176 hsa05212 Pancreatic cancer 16 64.125 hsa05210 Colorectal cancer 16 64.875 hsa04064 NF-kappa B signaling pathway 16 43.625 hsa04066 HIF-1 signaling pathway 16 53.9375 hsa05135 Yersinia infection 16 63.6875 hsa05131 Shigellosis 16 67.75 hsa04115 p53 signaling pathway 15 42.53333 hsa05220 Chronic myeloid leukemia 15 66.4 hsa05235 PD-L1 expression and PD-1 checkpoint pathway in cancer 15 66.2 hsa05323 Rheumatoid arthritis 15 43.8 hsa05146 Amoebiasis 15 48 hsa04660 T cell receptor signaling pathway 15 66.66667 hsa04071 Sphingolipid signaling pathway 15 64.86667 hsa04068 FoxO signaling pathway 15 61.13333 hsa04936 Alcoholic liver disease 15 59.6 hsa04022 cGMP-PKG signaling pathway 15 40.86667 hsa04062 Chemokine signaling pathway 15 54.53333 hsa04060 Cytokine-cytokine receptor interaction 15 39.66667 hsa05219 Bladder cancer 14 51.92857 hsa05223 Non-small cell lung cancer 14 63.71429 hsa05133 Pertussis 14 61.71429 hsa04726 Serotonergic synapse 14 43.14286 hsa04919 Thyroid hormone signaling pathway 14 57.64286 hsa05226 Gastric cancer 14 64.07143 hsa04630 JAK-STAT signaling pathway 14 55.85714 hsa05203 Viral carcinogenesis 14 58.78571 hsa05144 Malaria 13 42.61538 hsa01524 Platinum drug resistance 13 59 hsa04014 Ras signaling pathway 13 62.46154 hsa05020 Prion disease 13 55.84615 hsa05213 Endometrial cancer 12 69.58333 hsa05321 Inflammatory bowel disease 12 55 hsa05214 Glioma 12 71.25 hsa01521 EGFR tyrosine kinase inhibitor resistance 12 68.5 hsa04921 Oxytocin signaling pathway 12 63.83333 hsa04510 Focal adhesion 12 66.5 hsa05012 Parkinson disease 12 39.5 hsa05134 Legionellosis 11 60 hsa05221 Acute myeloid leukemia 11 66.63636 hsa04917 Prolactin signaling pathway 11 70.54545 hsa05218 Melanoma 11 71.72727 hsa04658 Th1 and Th2 cell differentiation 11 67.63636 hsa04928 Parathyroid hormone synthesis, secretion and action 11 61 hsa04931 Insulin resistance 11 57.09091 hsa04725 Cholinergic synapse 11 56 hsa04722 Neurotrophin signaling pathway 11 80.90909 hsa04371 Apelin signaling pathway 11 55.90909 hsa04261 Adrenergic signaling in cardiomyocytes 11 58.27273 hsa04217 Necroptosis 11 50.36364 hsa05014 Amyotrophic lateral sclerosis 11 55.81818 hsa05143 African trypanosomiasis 10 51.8 hsa04370 VEGF signaling pathway 10 71.4 hsa05120 Epithelial cell signaling in Helicobacter pylori infection 10 64.1 hsa04012 ErbB signaling pathway 10 75.3 hsa04540 Gap junction 10 56 hsa04061 Viral protein interaction with cytokine and cytokine receptor 10 44.6 hsa04110 Cell cycle 10 46.2 hsa04728 Dopaminergic synapse 10 52 hsa04072 Phospholipase D signaling pathway 10 69 hsa05216 Thyroid cancer 9 63.66667 hsa04920 Adipocytokine signaling pathway 9 64.66667 hsa04622 RIG-I-like receptor signaling pathway 9 66 hsa05230 Central carbon metabolism in cancer 9 74.55556 hsa04662 B cell receptor signaling pathway 9 86.77778 hsa04610 Complement and coagulation cascades 9 26.77778 hsa05231 Choline metabolism in cancer 9 80.88889 hsa04914 Progesterone-mediated oocyte maturation 9 69.44444 hsa04935 Growth hormone synthesis, secretion and action 9 78.44444 hsa04611 Platelet activation 9 59.11111 hsa04140 Autophagy - animal 9 68.55556 hsa04150 mTOR signaling pathway 9 81.55556 hsa04613 Neutrophil extracellular trap formation 9 79.88889 hsa04923 Regulation of lipolysis in adipocytes 8 49.25 hsa05416 Viral myocarditis 8 50.375 hsa04664 Fc epsilon RI signaling pathway 8 87.375 hsa05211 Renal cell carcinoma 8 80.75 hsa05031 Amphetamine addiction 8 51.375 hsa04912 GnRH signaling pathway 8 80.5 hsa04670 Leukocyte transendothelial migration 8 49.25 hsa04650 Natural killer cell mediated cytotoxicity 8 78.5 hsa04015 Rap1 signaling pathway 8 87 hsa05016 Huntington disease 8 55.375 hsa04215 Apoptosis - multiple species 7 63.42857 hsa05332 Graft-versus-host disease 7 62.57143 hsa05030 Cocaine addiction 7 53.71429 hsa04623 Cytosolic DNA-sensing pathway 7 69.85714 hsa04929 GnRH secretion 7 82.57143 hsa04211 Longevity regulating pathway 7 73 hsa05032 Morphine addiction 7 43.42857 hsa04152 AMPK signaling pathway 7 57 hsa04723 Retrograde endocannabinoid signaling 7 69.71429 hsa04934 Cushing syndrome 7 69 hsa04310 Wnt signaling pathway 7 65.57143 hsa05034 Alcoholism 7 63.28571 hsa04810 Regulation of actin cytoskeleton 7 68.42857 hsa01523 Antifolate resistance 6 82.33333 hsa05330 Allograft rejection 6 59.16667 hsa04940 Type I diabetes mellitus 6 64.33333 hsa04672 Intestinal immune network for IgA production 6 57.16667 hsa04913 Ovarian steroidogenesis 6 45.83333 hsa05204 Chemical carcinogenesis - DNA adducts 6 44 hsa04137 Mitophagy - animal 6 71 hsa03320 PPAR signaling pathway 6 42.16667 hsa04742 Taste transduction 6 39.66667 hsa04970 Salivary secretion 6 52.5 hsa04350 TGF-beta signaling pathway 6 81 hsa04666 Fc gamma R-mediated phagocytosis 6 95.83333 hsa04750 Inflammatory mediator regulation of TRP channels 6 64.16667 hsa04114 Oocyte meiosis 6 69.33333 hsa04910 Insulin signaling pathway 6 88 hsa04550 Signaling pathways regulating pluripotency of stem cells 6 99 hsa04390 Hippo signaling pathway 6 55.33333 hsa04960 Aldosterone-regulated sodium reabsorption 5 83.6 hsa00380 Tryptophan metabolism 5 47.4 hsa04930 Type II diabetes mellitus 5 82.8 hsa00330 Arginine and proline metabolism 5 51 hsa00590 Arachidonic acid metabolism 5 49.2 hsa00982 Drug metabolism - cytochrome P450 5 47.4 hsa00980 Metabolism of xenobiotics by cytochrome P450 5 46.8 hsa00983 Drug metabolism - other enzymes 5 44.8 hsa04640 Hematopoietic cell lineage 5 77 hsa04270 Vascular smooth muscle contraction 5 95 hsa05322 Systemic lupus erythematosus 5 66.8 hsa04514 Cell adhesion molecules 5 49.4 hsa04141 Protein processing in endoplasmic reticulum 5 64.4 hsa04360 Axon guidance 5 93.8 hsa05310 Asthma 4 75.5 hsa05033 Nicotine addiction 4 55.5 hsa05320 Autoimmune thyroid disease 4 63.75 hsa04730 Long-term depression 4 116.25 hsa00140 Steroid hormone biosynthesis 4 55.75 hsa04213 Longevity regulating pathway - multiple species 4 82 hsa05217 Basal cell carcinoma 4 85.75 hsa04720 Long-term potentiation 4 116.25 hsa04924 Renin secretion 4 58 hsa04520 Adherens junction 4 96.5 hsa04612 Antigen processing and presentation 4 79.5 hsa04721 Synaptic vesicle cycle 4 54.25 hsa04146 Peroxisome 4 57 hsa04727 GABAergic synapse 4 69.75 hsa05410 Hypertrophic cardiomyopathy 4 88.5 hsa05414 Dilated cardiomyopathy 4 79 hsa04713 Circadian entrainment 4 106.75 hsa04916 Melanogenesis 4 116.25 hsa04972 Pancreatic secretion 4 70.75 hsa04974 Protein digestion and absorption 4 53.25 Table S5. Summary of studies included in the systematic review
Study Information of aninmals Type of Fructus Lycii Intervention vs. control Time of intervention Outcome measures used Yang 2019 Kunming mice, weighing 12–22 g, half-male and half-female, SPF Decoction of Fructus Lycii 3 mg/(g∙d) (n = 10) vs. 6 mg/(g∙d) (n = 10) vs. Placebo (distilled water 0.02 mL/(g∙d), n = 10) 30 days MDA, ROS, SOD, CAT, GSH-Px Cao 2018 Kunming mice, weighing 18–22 g, half-male and half-female, SPF Decoction of Fructus Lycii 3 mg/(g∙d) (n = 10) vs. 6 mg/(g∙d) (n = 10) vs. Placebo (distilled water 0.02 mL/(g∙d), n = 10) 30 days The time of exhaustive swimming, blood glucose, muscle glycogen and liver glycogen, BUN and lactic acid Ji et al 2011 Eight-week-old Wistar rats, weighing 220 ± 23.19 g, female Decoction of Fructus Lycii Decoction of Fructus Lycii (n = 8) vs. Placebo (n = 8) 30 days The time of exhaustive swimming, SOD, MAD Ding et al 2001 Kunming mice, weighing 20–24 g, male Decoction of Fructus Lycii Decoction of Fructus Lycii (n = 10) vs. Placebo (n = 10) 2 weeks The time of exhaustive swimming, SOD, MAD Qin et al 2009 mice Extract of Fructus Lycii 5 mg/(g∙d) (n = 10) vs. 10 mg/(g∙d) (n = 10) vs. 20 mg/(g∙d) (n = 10) vs. Placebo (Equivalent saline, n = 10) 2 weeks The time of exhaustive swimming Hu et al 2008 Kunming mice, weighing 18–22 g, female Raw juice of Fructus Lycii 10 mL/(kg∙d) (n = 10) vs. 20 mL/(kg∙d) (n = 10) vs. 30 mL/(kg∙d) (n = 10) vs. Placebo (Equivalent saline, n = 10) 3 weeks The time of exhaustive swimming, and liver glycogen,BUN Liu et al 2011 Kunming mice, weighing 24 ± 5 g, female Decoction of Fructus Lycii 5 mg/(g∙d) (n = 10) vs. Placebo (Equivalent distilled water, n = 10) 10 days The time of exhaustive swimming Yi et al 2010 Kunming mice, weighing 24 ± 5 g, half-male and half-female Decoction of Fructus Lycii 5 mg/(g∙d) (n = 10) vs. 2.5 mg/(g∙d) (n = 10) vs. Placebo(Equivalent running water, n = 10) 2 weeks The time of exhaustive swimming Wang et al 2002 Kunming mice, weighing 20 ± 2 g, male and female Decoction of Fructus Lycii 0.2 mL/10 (g∙d) (n = 10) vs. 0.1 mL/10 (g∙d) (n = 10) vs. Placebo (Equivalent saline, n = 10) 1 week The time of exhaustive swimming Liu 2019 Kunming mice, 6 week, male Extract of Fructus Lycii 0.3 mg/(g∙d) (n = 10) vs. 0.6 mg/(g∙d) (n = 10) vs. 0.9 mg/(g∙d) (n = 10) vs. Placebo (n = 10) 2 weeks The time of exhaustive swimming, muscle glycogen and liver glycogen, BUN and lactic acid, SOD, MDA Yang et al 2018 Kunming mice, weighing 18–22 g, male, SPF Extract of Fructus Lycii 0.5 mg/(g∙d) (n = 10) vs. 1 mg/g/d (n = 10) vs. 1.5 mg/(g∙d) (n = 10) vs. Placebo (n = 10) 30 days The time of exhaustive swimming, muscle glycogen and liver glycogen, BUN and lactic acid Ma 2019 Rats, SPF Fruit of Fructus Lycii 0.5g/(kg∙d) (n = 10) vs. 3g/(kg∙d) (n = 15) vs. Placebo (5 mL/kg saline, n = 15) 6 weeks The time of exhaustive swimming Niu et al. 1994 Kunming mice, weighing 18–24 g, half-male and half-female, SPF Decoction of Fructus Lycii 5g/(kg∙d) (n = 10) vs. 2.5/(kg∙d) (n = 10) vs. Placebo (Equivalent running water, n = 10) 2 weeks The time of exhaustive swimming, blood glucose, and lactic acid Wu et al. 2008 Kunming mice, 3 week, weighing 18–22 g,female Raw juice of Fructus Lycii 0.2 mL/10 (g∙d) (n = 10) vs. 0.25 mL/10 (g∙d) (n = 10) vs. Placebo (Equivalent saline, n = 10) 3 weeks The time of exhaustive swimming, BUN and lactic acid Wang et al. 2017 Kunming mice, weighing 60–80 g, female Extract of Fructus Lycii 0.2 mL/10 (g∙d) (n = 10) vs. Placebo (n = 10) 3 weeks The time of exhaustive swimming Note. MDA, malondialdehyde; ROS, reactive oxygen species; SOD, superoxide dismutase; CAT, catalase; GSH-Px, glutathione peroxidase; BUN, blood urea nitrogen. A total 15 RCTs studies included, the information in detail were showed in the Supplementary Table S5. The exhaustive time, which the main parameter of anti-exercise-fatigue ability, were assessed in 14 articles. blood lactate (included in 6 articles) and BUN (included in 5 articles) were the main product of exercise metabolism and important marker for the evaluation of exercise fatigue. As the important energy source, muscle glycogen was included in 2 articles and liver glycogen in 3 articles. SOD (in 3 articles) and MAD (in 4 articles) also were the important outcomes included in our study. Based on the placebo group, the experimental group received decoction of Fructus Lycii (8 articles), extract of Fructus Lycii (4 articles), raw juice of Fructus Lycii (2 articles) and fruit of Fructus Lycii (1 article). Treatment duration ranged from 10 to 42 days. 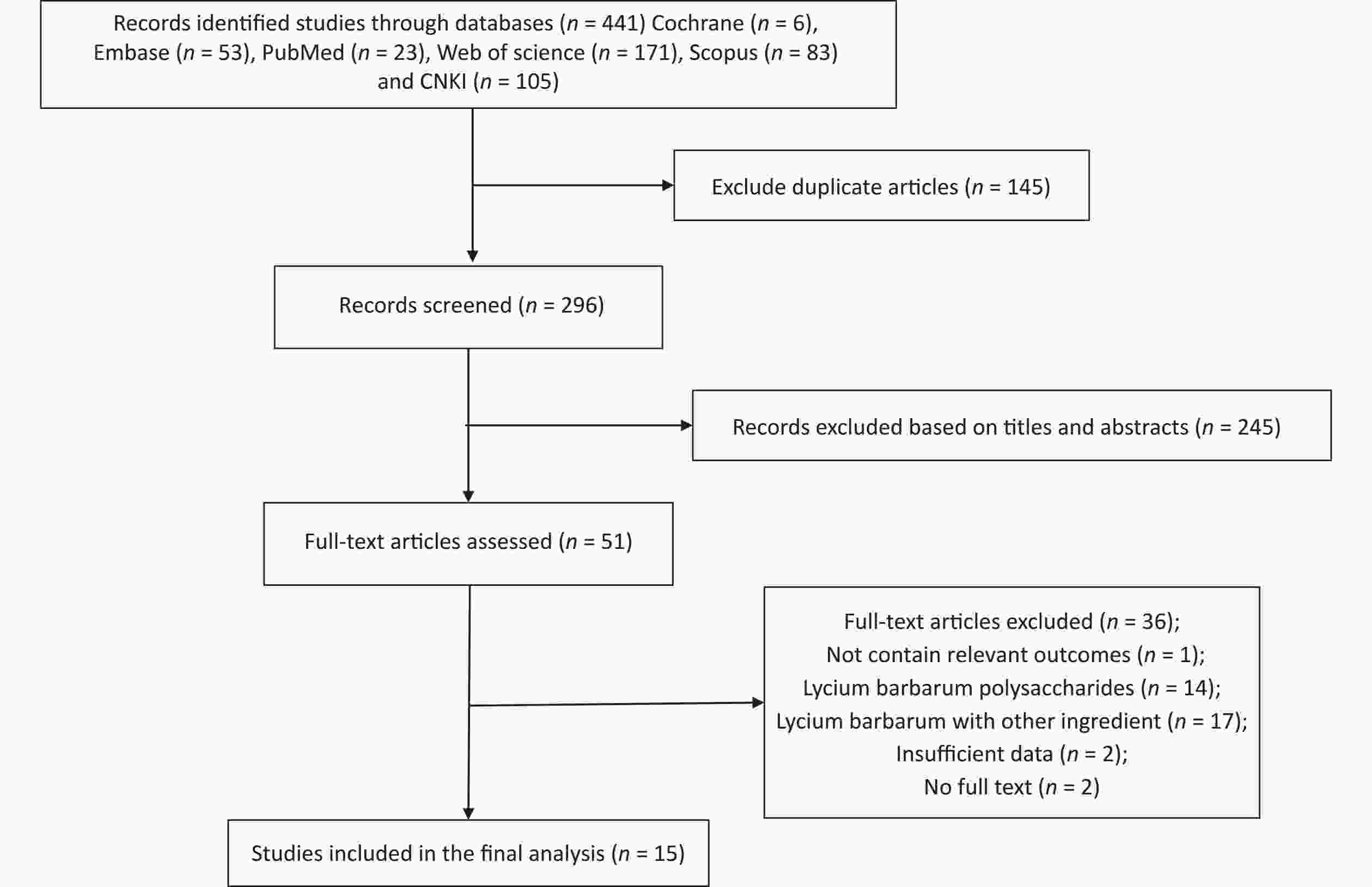
Figure S3. Flow diagram of the study selection. Supplementary Figure S3 showed that a total of 441 relevant literature sources were identified through the search strategy. After removing 145 duplicate articles, the titles and abstracts of the remaining papers were screened to exclude those that were not related to Fructus Lycii and exercise-induced fatigue. And then, we reviewed 51 articles with full texts and 15 randomized controlled trials (RCTs) involving animals were included in the final analysis.
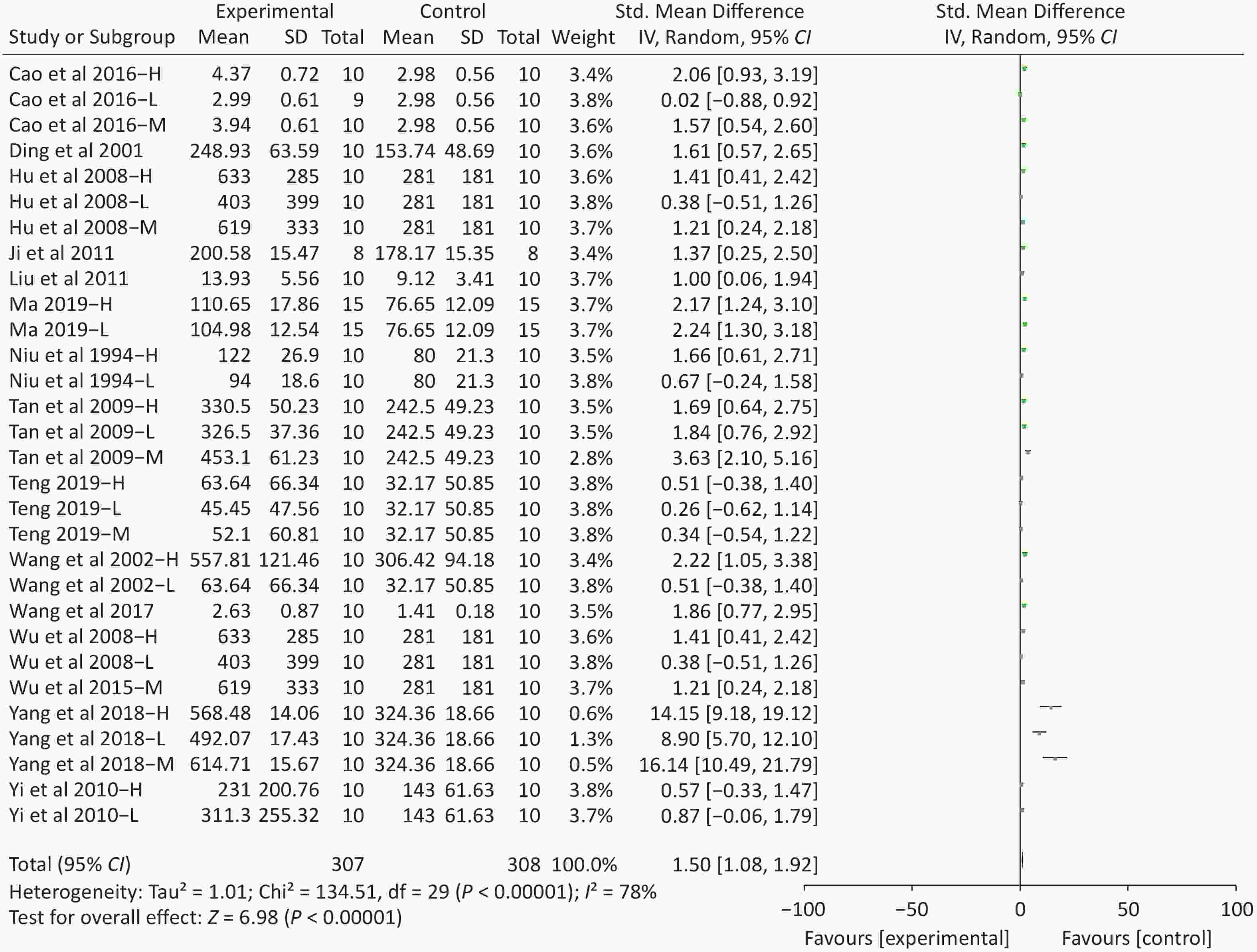
Figure S4. Forest plot of s standardized mean differences in exhausted time between Fructus Lycii and placebo. Weights have been calculated using random effects model. Degree of heterogeneity in the pooled estimates is represented at I2 statistic. SMD, standardized mean difference; Chi2, Chi-square test; df, degrees of freedom; I2, I-squared statistic; Z, Z-test; CI, confidence interval; H, high-dose intervention in each study; M, median-dose intervention in each study; L, low-dose intervention in each study. The exhausted time was the primary outcome. There was significant heterogeneity among the 14 studies (I2 = 78%, P < 0.01) and therefore a random effect model was used as shown in the Supplementary Figure S4. Meta-analysis of 14 studies showed significant effects of Fructus Lycii on increasing the time to exhaustion compared with control groups (SMD 1.5; 95% CI 1.08 to 1.92; P < 0.01).
doi: 10.3967/bes2024.005
The Effect and Mechanism of Fructus lycii on Improvement of Exercise Fatigue Using a Network Pharmacological Approach with in vitro Experimental Verification
-
Abstract:
Objective This study aimed to investigate the effect and underlying mechanism of Fructus lycii in improving exercise fatigue. Methods A network pharmacological approach was used to explore potential mechanisms of action of Fructus lycii. Skeletal muscle C2C12 cells and immunofluorescence were employed to verify the effect and mechanism of the representative components in Fructus lycii predicted by network pharmacological analysis. Results Six potential active components, namely quercetin, β-sitosterol, stigmasterol, 7-O-methylluteolin-6-C-beta-glucoside_qt, atropine, and glycitein, were identified to have potency in improving exercise fatigue via multiple pathways, such as the PI3K-Akt, neuroactive ligand-receptor interaction, IL-17, TNF, and MAPK signaling pathways. The immunofluorescence results indicated that quercetin, a significant active component in Fructus lycii, increased the mean staining area of 2-NBDG, TMRM, and MitoTracker, and decreased the area of CellRox compared to the control. Furthermore, the protein expression levels of p-38 MAPK, p-MAPK, p-JNK, p-PI3K, and p-AKT markedly increased after quercetin treatment. Conclusion Fructus lycii might alleviate exercise fatigue through multiple components and pathways. Among these, quercetin appears to improve exercise fatigue by enhancing energy metabolism and reducing oxidative stress. The PI3K-AKT and MAPK signaling pathways also appear to play a role in this process. -
Key words:
- Fructus lycii /
- Exercise fatigue /
- Network pharmacology
注释:1) CONFLICTS OF INTEREST: -
Figure 1. Compound-targets network. GQ1, Sitosterol alpha1; GQ2, Cycloartenol; GQ3, Mandenol; GQ4, Ethyl linolenate; GQ5, LAN; GQ6, Stigmasterol; GQ7, Beta-sitosterol; GQ8, (-)-Hyoscyamine; GQ9, Campesterol; GQ10, Cyanin; GQ11, 24-methylidenelophenol; GQ12, Daucosterol qt; GQ13, Glycitein; GQ14, CLR; GQ15, 14b-pregnane; GQ16, 24-ethylcholesta-5,22-dienol; GQ17, Fucosterol; GQ18, 31-norlanosterol; GQ19, 4,24-methyllophenol; GQ20, Lophenol; GGQ21, 4alpha,14alpha,24-trimethylcholesta-8,24-dienol; GQ22, 4alpha,24-dimethylcholesta-7,24-dienol; GQ23, 4alpha-methyl-24-ethylcholesta-7,24-dienol; GQ24, 6-Fluoroindole-7-Dehydrocholesterol; GQ25, 7-O-methylluteolin-6-C-beta-glucoside_qt; GQ26, Atropine; GQ27, Physcion-8-O-beta-D-gentiobioside; GQ28, Lanost-8-en-3beta-ol; GQ29, Obtusifoliol; GQ30, Quercetin.
Figure 3. Compound-targets-pathways network. GQ1, Sitosterol alpha1; GQ2, Cycloartenol; GQ3, Mandenol; GQ4, Ethyl linolenate; GQ5, LAN; GQ6, Stigmasterol; GQ7, Beta-sitosterol; GQ8, (-)-Hyoscyamine; GQ9, Campesterol; GQ10, Cyanin; GQ11, 24-methylidenelophenol; GQ12, Daucosterol_qt; GQ13, Glycitein; GQ14, CLR; GQ15, 14b-pregnane; GQ16, 24-ethylcholesta-5,22-dienol; GQ17, Fucosterol; GQ18, 31-norlanosterol; GQ19, 4,24-methyllophenol; GQ20, Lophenol; GGQ21, 4alpha,14alpha,24-trimethylcholesta-8,24-dienol; GQ22, 4alpha,24-dimethylcholesta-7,24-dienol; GQ23, 4alpha-methyl-24-ethylcholesta-7,24-dienol; GQ24, 6-Fluoroindole-7-Dehydrocholesterol; GQ25, 7-O-Methylluteolin-6-C-beta-glucoside_qt; GQ26, Atropine; GQ27, Physcion-8-O-beta-D-gentiobioside; GQ28, Lanost-8-en-3beta-ol; GQ29, Obtusifoliol; GQ30, Quercetin.
Figure 4. The effect of quercetin on glucose uptake, ROS generation, and mitochondria function in C2C12 cells. The 2-NBDG (A), CellRox (B), TMRM (C), and MitoTracker (D) probes were detected by a high content imaging analysis system; Left panel: representative images. Data presented were from three biological replicates; Scale bar size: 50 μm; *P < 0.05; **P < 0.01.
Figure 5. Protein expression of MAPK and PI3K-AKT signaling pathways. (A) MAPK signaling pathways, the protein expression of p-P38 MAPK, p-MAPK, and p-JNK was detected by a high content imaging analysis system; (B) PI3K-AKT signaling pathway, p-PI3K and p-AKT was detected by a high content imaging analysis system; Left panel: representative images. Data presented were from three biological replicates; Scale bar size: 50 μm; *P < 0.05; **P < 0.01.
S3. Flow diagram of the study selection. Supplementary Figure S3 showed that a total of 441 relevant literature sources were identified through the search strategy. After removing 145 duplicate articles, the titles and abstracts of the remaining papers were screened to exclude those that were not related to Fructus Lycii and exercise-induced fatigue. And then, we reviewed 51 articles with full texts and 15 randomized controlled trials (RCTs) involving animals were included in the final analysis.
S4. Forest plot of s standardized mean differences in exhausted time between Fructus Lycii and placebo. Weights have been calculated using random effects model. Degree of heterogeneity in the pooled estimates is represented at I2 statistic. SMD, standardized mean difference; Chi2, Chi-square test; df, degrees of freedom; I2, I-squared statistic; Z, Z-test; CI, confidence interval; H, high-dose intervention in each study; M, median-dose intervention in each study; L, low-dose intervention in each study. The exhausted time was the primary outcome. There was significant heterogeneity among the 14 studies (I2 = 78%, P < 0.01) and therefore a random effect model was used as shown in the Supplementary Figure S4. Meta-analysis of 14 studies showed significant effects of Fructus Lycii on increasing the time to exhaustion compared with control groups (SMD 1.5; 95% CI 1.08 to 1.92; P < 0.01).
S1. Potential active components of Fructus Lycii
Molecule ID Molecule name OB (%) DL MOL001323 Sitosterol alpha1 43.2813 0.78354 MOL003578 Cycloartenol 38.6857 0.78093 MOL001494 Mandenol 41.9962 0.19321 MOL001495 Ethyl linolenate 46.101 0.19716 MOL001979 LAN 42.1192 0.74787 MOL000449 Stigmasterol 43.8299 0.75665 MOL000358 beta-sitosterol 36.9139 0.75123 MOL005406 atropine 45.9706 0.19328 MOL005438 campesterol 37.5768 0.71488 MOL006209 cyanin 47.4209 0.75918 MOL007449 24-methylidenelophenol 44.1926 0.7533 MOL008173 daucosterol_qt 36.9139 0.75316 MOL008400 glycitein 50.4789 0.23826 MOL010234 delta-Carotene 31.8009 0.54639 MOL000953 CLR 37.8739 0.67677 MOL009604 14b-pregnane 34.7792 0.33723 MOL009612 (24R)-4alpha-Methyl-24-ethylcholesta-7,25-dien-3beta-ylacetate 46.3575 0.8398 MOL009615 24-Methylenecycloartan-3beta,21-diol 37.3173 0.79751 MOL009617 24-ethylcholest-22-enol 37.0945 0.7511 MOL009618 24-ethylcholesta-5,22-dienol 43.8299 0.75636 MOL009620 24-methyl-31-norlanost-9(11)-enol 37.9997 0.75092 MOL009621 24-methylenelanost-8-enol 42.3682 0.76769 MOL009622 Fucosterol 43.7764 0.75668 MOL009631 31-Norcyclolaudenol 38.6821 0.81391 MOL009633 31-norlanost-9(11)-enol 38.3539 0.7249 MOL009634 31-norlanosterol 42.2046 0.73012 MOL009635 4,24-methyllophenol 37.8347 0.74999 MOL009639 Lophenol 38.1294 0.714 MOL009640 4alpha,14alpha,24-trimethylcholesta-8,24-dienol 38.9099 0.75772 MOL009641 4alpha,24-dimethylcholesta-7,24-dienol 42.653 0.75297 MOL009642 4alpha-methyl-24-ethylcholesta-7,24-dienol 42.2951 0.78304 MOL009644 6-Fluoroindole-7-Dehydrocholesterol 43.726 0.72224 MOL009646 7-O-Methylluteolin-6-C-beta-glucoside_qt 40.7737 0.30497 MOL009650 Atropine 42.159 0.19299 MOL009651 Cryptoxanthin monoepoxide 46.9537 0.56103 MOL009653 Cycloeucalenol 39.7265 0.79446 MOL009656 (E,E)-1-ethyl octadeca-3,13-dienoate 41.9962 0.19364 MOL009660 methyl (1R,4aS,7R,7aS)-4a,7-dihydroxy-7-methyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1,5,6,7a-tetrahydrocyclopenta[d]pyran-4-carboxylate 39.4285 0.46558 MOL009662 Lantadene A 38.6794 0.57405 MOL009664 Physalin A 91.7065 0.27207 MOL009665 Physcion-8-O-beta-D-gentiobioside 43.9036 0.62426 MOL009677 lanost-8-en-3beta-ol 34.2263 0.74036 MOL009678 lanost-8-enol 34.2263 0.74167 MOL009681 Obtusifoliol 42.552 0.7565 MOL000098 quercetin 46.4333 0.27525 Note. DL, Drug Like Index; OB, Oral Bioavailability. S2. Information of intersection target of components and exercise-induced fatigue
ID Molecule name Target name Gene name GQ1 Sitosterol alpha1 Progesterone receptor PGR GQ1 Sitosterol alpha1 Prostaglandin G/H synthase 2 PTGS2 GQ1 Sitosterol alpha1 Mineralocorticoid receptor NR3C2 GQ2 Cycloartenol Mineralocorticoid receptor NR3C2 GQ3 Mandenol Prostaglandin G/H synthase 1 PTGS1 GQ3 Mandenol Prostaglandin G/H synthase 2 PTGS2 GQ4 Ethyl linolenate Prostaglandin G/H synthase 1 PTGS1 GQ5 LAN Progesterone receptor PGR GQ5 LAN Mineralocorticoid receptor NR3C2 GQ6 Stigmasterol Progesterone receptor PGR GQ6 Stigmasterol Mineralocorticoid receptor NR3C2 GQ6 Stigmasterol Ig gamma-1 chain C region IGHG1 GQ6 Stigmasterol Retinoic acid receptor RXR-alpha RXRA GQ6 Stigmasterol Prostaglandin G/H synthase 1 PTGS1 GQ6 Stigmasterol Prostaglandin G/H synthase 2 PTGS2 GQ6 Stigmasterol Alpha-2A adrenergic receptor ADRA2A GQ6 Stigmasterol Sodium-dependent noradrenaline transporter SLC6A2 GQ6 Stigmasterol Sodium-dependent dopamine transporter SLC6A3 GQ6 Stigmasterol Beta-2 adrenergic receptor ADRB2 GQ6 Stigmasterol Aldose reductase AKR1B1 GQ6 Stigmasterol Urokinase-type plasminogen activator PLAU GQ6 Stigmasterol Leukotriene A-4 hydrolase LTA4H GQ6 Stigmasterol Amine oxidase [flavin-containing] B MAOB GQ6 Stigmasterol Amine oxidase [flavin-containing] A MAOA GQ6 Stigmasterol Muscarinic acetylcholine receptor M3 CHRM3 GQ6 Stigmasterol Beta-1 adrenergic receptor ADRB1 GQ6 Stigmasterol Sodium channel protein type 5 subunit alpha SCN5A GQ6 Stigmasterol 5-hydroxytryptamine 2A receptor HTR2A GQ6 Stigmasterol Gamma-aminobutyric-acid receptor subunit alpha-3 GABRA3 GQ6 Stigmasterol Muscarinic acetylcholine receptor M2 CHRM2 GQ6 Stigmasterol Alpha-1B adrenergic receptor ADRA1B GQ6 Stigmasterol Neuronal acetylcholine receptor subunit alpha-7 CHRNA7 GQ7 beta-sitosterol Progesterone receptor PGR GQ7 beta-sitosterol Prostaglandin G/H synthase 1 PTGS1 GQ7 beta-sitosterol Prostaglandin G/H synthase 2 PTGS2 GQ7 beta-sitosterol Heat shock protein HSP 90-alpha HSP90AA1 GQ7 beta-sitosterol Potassium voltage-gated channel subfamily H member 2 KCNH2 GQ7 beta-sitosterol D(1A) dopamine receptor DRD1 GQ7 beta-sitosterol Muscarinic acetylcholine receptor M3 CHRM3 GQ7 beta-sitosterol Sodium channel protein type 5 subunit alpha SCN5A GQ7 beta-sitosterol cGMP-inhibited 3',5'-cyclic phosphodiesterase A PDE3A GQ7 beta-sitosterol 5-hydroxytryptamine 2A receptor HTR2A GQ7 beta-sitosterol Gamma-aminobutyric-acid receptor subunit alpha-5 GABRA5 GQ7 beta-sitosterol Gamma-aminobutyric-acid receptor subunit alpha-3 GABRA3 GQ7 beta-sitosterol Muscarinic acetylcholine receptor M2 CHRM2 GQ7 beta-sitosterol Alpha-1B adrenergic receptor ADRA1B GQ7 beta-sitosterol Beta-2 adrenergic receptor ADRB2 GQ7 beta-sitosterol Neuronal acetylcholine receptor subunit alpha-2 CHRNA2 GQ7 beta-sitosterol Sodium-dependent serotonin transporter SLC6A4 GQ7 beta-sitosterol Mu-type opioid receptor OPRM1 GQ7 beta-sitosterol Neuronal acetylcholine receptor subunit alpha-7 CHRNA7 GQ7 beta-sitosterol Apoptosis regulator Bcl-2 BCL2 GQ7 beta-sitosterol Apoptosis regulator BAX BAX GQ7 beta-sitosterol Caspase-9 CASP9 GQ7 beta-sitosterol Transcription factor AP-1 JUN GQ7 beta-sitosterol Caspase-3 CASP3 GQ7 beta-sitosterol Caspase-8 CASP8 GQ7 beta-sitosterol Protein kinase C alpha type PRKCA GQ7 beta-sitosterol Transforming growth factor beta-1 TGFB1 GQ7 beta-sitosterol Serum paraoxonase/arylesterase 1 PON1 GQ7 beta-sitosterol Microtubule-associated protein 2 MAP2 GQ8 (-)-Hyoscyamine D(1A) dopamine receptor DRD1 GQ8 (-)-Hyoscyamine Muscarinic acetylcholine receptor M3 CHRM3 GQ8 (-)-Hyoscyamine Beta-1 adrenergic receptor ADRB1 GQ8 (-)-Hyoscyamine Alpha-2A adrenergic receptor ADRA2A GQ8 (-)-Hyoscyamine Alpha-2C adrenergic receptor ADRA2C GQ8 (-)-Hyoscyamine Delta-type opioid receptor OPRD1 GQ8 (-)-Hyoscyamine 5-hydroxytryptamine 2A receptor HTR2A GQ8 (-)-Hyoscyamine Sodium-dependent noradrenaline transporter SLC6A2 GQ8 (-)-Hyoscyamine Muscarinic acetylcholine receptor M2 CHRM2 GQ8 (-)-Hyoscyamine Alpha-2B adrenergic receptor ADRA2B GQ8 (-)-Hyoscyamine Alpha-1B adrenergic receptor ADRA1B GQ8 (-)-Hyoscyamine Sodium-dependent dopamine transporter SLC6A3 GQ8 (-)-Hyoscyamine Beta-2 adrenergic receptor ADRB2 GQ8 (-)-Hyoscyamine Sodium-dependent serotonin transporter SLC6A4 GQ8 (-)-Hyoscyamine D(2) dopamine receptor DRD5 GQ8 (-)-Hyoscyamine Mu-type opioid receptor OPRM1 GQ8 (-)-Hyoscyamine 5-hydroxytryptamine 1B receptor HTR1B GQ8 (-)-Hyoscyamine Histamine H1 receptor HRH1 GQ8 (-)-Hyoscyamine 5-hydroxytryptamine 1A receptor HTR1A GQ9 campesterol Progesterone receptor PGR GQ10 cyanin Prostaglandin G/H synthase 2 PTGS2 GQ10 cyanin Heat shock protein HSP 90-alpha HSP90AA1 GQ11 24-methylidenelophenol Progesterone receptor PGR GQ11 24-methylidenelophenol Mineralocorticoid receptor NR3C2 GQ12 daucosterol_qt Progesterone receptor PGR GQ13 glycitein Prostaglandin G/H synthase 1 PTGS1 GQ13 glycitein Estrogen receptor ESR1 GQ13 glycitein Androgen receptor AR GQ13 glycitein Peroxisome proliferator-activated receptor gamma PPARG GQ13 glycitein Prostaglandin G/H synthase 2 PTGS2 GQ13 glycitein Retinoic acid receptor RXR-alpha RXRA GQ13 glycitein cGMP-inhibited 3',5'-cyclic phosphodiesterase A PDE3A GQ13 glycitein Estrogen receptor beta ESR2 GQ13 glycitein Mitogen-activated protein kinase 14 MAPK14 GQ13 glycitein Heat shock protein HSP 90-alpha HSP90AA1 GQ13 glycitein Trypsin-1 PRSS1 GQ13 glycitein Cyclin-A2 CCNA2 GQ13 glycitein Nitric oxide synthase, inducible NOS2 GQ13 glycitein Collagenase 3 MMP13 GQ13 glycitein Neutrophil collagenase MMP8 GQ14 CLR Progesterone receptor PGR GQ14 CLR Mineralocorticoid receptor NR3C2 GQ15 14b-pregnane Prostaglandin G/H synthase 2 PTGS2 GQ15 14b-pregnane Progesterone receptor PGR GQ16 24-ethylcholesta-5,22-dienol Progesterone receptor PGR GQ16 24-ethylcholesta-5,22-dienol Mineralocorticoid receptor NR3C2 GQ17 Fucosterol Progesterone receptor PGR GQ17 Fucosterol Mineralocorticoid receptor NR3C2 GQ18 31-norlanosterol Progesterone receptor PGR GQ18 31-norlanosterol Mineralocorticoid receptor NR3C2 GQ19 4,24-methyllophenol Progesterone receptor PGR GQ20 Lophenol Progesterone receptor PGR GQ21 4alpha,14alpha,24-trimethylcholesta-8,24-dienol Progesterone receptor PGR GQ22 4alpha,24-dimethylcholesta-7,24-dienol Progesterone receptor PGR GQ22 4alpha,24-dimethylcholesta-7,24-dienol Mineralocorticoid receptor NR3C2 GQ23 4alpha-methyl-24-ethylcholesta-7,24-dienol Progesterone receptor PGR GQ24 6-Fluoroindole-7-Dehydrocholesterol Progesterone receptor PGR GQ24 6-Fluoroindole-7-Dehydrocholesterol Mineralocorticoid receptor NR3C2 GQ24 6-Fluoroindole-7-Dehydrocholesterol Glucocorticoid receptor NR3C1 GQ25 7-O-Methylluteolin-6-C-beta-glucoside_qt Prostaglandin G/H synthase 2 PTGS2 GQ25 7-O-Methylluteolin-6-C-beta-glucoside_qt DNA topoisomerase 2-alpha TOP2A GQ25 7-O-Methylluteolin-6-C-beta-glucoside_qt Heat shock protein HSP 90-alpha HSP90AA1 GQ26 Atropine D(1A) dopamine receptor DRD1 GQ26 Atropine Muscarinic acetylcholine receptor M3 CHRM3 GQ26 Atropine D(1B) dopamine receptor DRD5 GQ26 Atropine Beta-1 adrenergic receptor ADRB1 GQ26 Atropine Sodium channel protein type 5 subunit alpha SCN5A GQ26 Atropine Alpha-2A adrenergic receptor ADRA2A GQ26 Atropine 5-hydroxytryptamine 1A receptor HTR1A GQ26 Atropine Alpha-2C adrenergic receptor ADRA2C GQ26 Atropine Delta-type opioid receptor OPRD1 GQ26 Atropine Histamine H1 receptor HRH1 GQ26 Atropine 5-hydroxytryptamine 2A receptor HTR2A GQ26 Atropine Sodium-dependent noradrenaline transporter SLC6A2 GQ26 Atropine Muscarinic acetylcholine receptor M2 CHRM2 GQ26 Atropine Alpha-2B adrenergic receptor ADRA2B GQ26 Atropine Alpha-1B adrenergic receptor ADRA1B GQ26 Atropine Sodium-dependent dopamine transporter SLC6A3 GQ26 Atropine Beta-2 adrenergic receptor ADRB2 GQ26 Atropine Sodium-dependent serotonin transporter SLC6A4 GQ26 Atropine D(2) dopamine receptor DRD5 GQ26 Atropine Mu-type opioid receptor OPRM1 GQ26 Atropine 5-hydroxytryptamine 1B receptor HTR1B GQ27 Physcion-8-O-beta-D-gentiobioside DNA topoisomerase 2-alpha TOP2A GQ28 lanost-8-en-3beta-ol Progesterone receptor PGR GQ28 lanost-8-en-3beta-ol Mineralocorticoid receptor NR3C2 GQ29 Obtusifoliol Progesterone receptor PGR GQ29 Obtusifoliol Mineralocorticoid receptor NR3C2 GQ30 quercetin Prostaglandin G/H synthase 1 PTGS1 GQ30 quercetin Androgen receptor AR GQ30 quercetin Peroxisome proliferator-activated receptor gamma PPARG GQ30 quercetin Prostaglandin G/H synthase 2 PTGS2 GQ30 quercetin Heat shock protein HSP 90-alpha HSP90AA1 GQ30 quercetin Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma isoform PIK3CG GQ30 quercetin Dipeptidyl peptidase 4 DPP4 GQ30 quercetin Aldose reductase AKR1B1 GQ30 quercetin Trypsin-1 PRSS1 GQ30 quercetin DNA topoisomerase 2-alpha TOP2A GQ30 quercetin Prothrombin F2 GQ30 quercetin Potassium voltage-gated channel subfamily H member 2 KCNH2 GQ30 quercetin Sodium channel protein type 5 subunit alpha SCN5A GQ30 quercetin Coagulation factor X F10 GQ30 quercetin Beta-2 adrenergic receptor ADRB2 GQ30 quercetin Stromelysin-1 MMP3 GQ30 quercetin Coagulation factor VII F7 GQ30 quercetin Nitric-oxide synthase, endothelial NOS3 GQ30 quercetin Retinoic acid receptor RXR-alpha RXRA GQ30 quercetin Acetylcholinesterase ACHE GQ30 quercetin Amine oxidase [flavin-containing] B MAOB GQ30 quercetin Transcription factor p65 RELA GQ30 quercetin Epidermal growth factor receptor EGFR GQ30 quercetin RAC-alpha serine/threonine-protein kinase AKT1 GQ30 quercetin G1/S-specific cyclin-D1 CCND1 GQ30 quercetin Apoptosis regulator Bcl-2 BCL2 GQ30 quercetin Bcl-2-like protein 1 BCL2L1 GQ30 quercetin Proto-oncogene c-Fos FOS GQ30 quercetin Cyclin-dependent kinase inhibitor 1 CDKN1A GQ30 quercetin Apoptosis regulator BAX BAX GQ30 quercetin Caspase-9 CASP9 GQ30 quercetin Urokinase-type plasminogen activator PLAU GQ30 quercetin 72 kDa type IV collagenase MMP2 GQ30 quercetin Matrix metalloproteinase-9 MMP9 GQ30 quercetin Mitogen-activated protein kinase 1 MAPK1 GQ30 quercetin Interleukin-10 IL10 GQ30 quercetin Retinoblastoma-associated protein RB1 GQ30 quercetin Tumor necrosis factor TNF GQ30 quercetin Transcription factor AP-1 JUN GQ30 quercetin Interleukin-6 IL6 GQ30 quercetin Caspase-3 CASP3 GQ30 quercetin Cellular tumor antigen p53 TP53 GQ30 quercetin NF-kappa-B inhibitor alpha NFKBIA GQ30 quercetin Xanthine dehydrogenase/oxidase XDH GQ30 quercetin Caspase-8 CASP8 GQ30 quercetin RAF proto-oncogene serine/threonine-protein kinase RAF1 GQ30 quercetin Superoxide dismutase [Cu-Zn] SOD1 GQ30 quercetin Protein kinase C alpha type PRKCA GQ30 quercetin Interstitial collagenase MMP1 GQ30 quercetin Hypoxia-inducible factor 1-alpha HIF1A GQ30 quercetin Signal transducer and activator of transcription 1-alpha/beta STAT1 GQ30 quercetin Cell division control protein 2 homolog CDK1 GQ30 quercetin Peroxisome proliferator-activated receptor gamma PPARG GQ30 quercetin Heme oxygenase 1 HMOX1 GQ30 quercetin Cytochrome P450 3A4 CYP3A4 GQ30 quercetin Caveolin-1 CAV1 GQ30 quercetin Myc proto-oncogene protein MYC GQ30 quercetin Tissue factor F3 GQ30 quercetin Gap junction alpha-1 protein GJA1 GQ30 quercetin Cytochrome P450 1A1 CYP1A1 GQ30 quercetin Intercellular adhesion molecule 1 ICAM1 GQ30 quercetin Interleukin-1 beta IL1B GQ30 quercetin Small inducible cytokine A2 CCL2 GQ30 quercetin E-selectin SELE GQ30 quercetin Vascular cell adhesion protein 1 VCAM1 GQ30 quercetin Prostaglandin E2 receptor, EP3 subtype PTGER3 GQ30 quercetin Interleukin-8 CXCL8 GQ30 quercetin Nitric oxide synthase, endothelial NOS3 GQ30 quercetin Heat shock protein beta-1 HSPB1 GQ30 quercetin Transforming growth factor beta-1 TGFB1 GQ30 quercetin Maltase-glucoamylase, intestinal MGAM GQ30 quercetin Interleukin-2 IL2 GQ30 quercetin Cytochrome P450 1B1 CYP1B1 GQ30 quercetin Tissue-type plasminogen activator PLAT GQ30 quercetin Thrombomodulin THBD GQ30 quercetin Plasminogen activator inhibitor 1 SERPINE1 GQ30 quercetin Interferon gamma IFNG GQ30 quercetin Arachidonate 5-lipoxygenase ALOX5 GQ30 quercetin Phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN PTEN GQ30 quercetin Interleukin-1 alpha IL1A GQ30 quercetin Myeloperoxidase MPO GQ30 quercetin DNA topoisomerase 2-alpha TOP2A GQ30 quercetin Neutrophil cytosol factor 1 NCF1 GQ30 quercetin Nuclear factor erythroid 2-related factor 2 NFE2L2 GQ30 quercetin NAD(P)H dehydrogenase [quinone] 1 NQO1 GQ30 quercetin Poly [ADP-ribose] polymerase 1 PARP1 GQ30 quercetin Aryl hydrocarbon receptor AHR GQ30 quercetin Solute carrier family 2, facilitated glucose transporter member 4 SLC2A4 GQ30 quercetin Collagen alpha-1(III) chain COL3A1 GQ30 quercetin C-X-C motif chemokine 11 CXCL11 GQ30 quercetin C-X-C motif chemokine 2 CXCL2 GQ30 quercetin Serine/threonine-protein kinase Chk2 CHEK2 GQ30 quercetin Insulin receptor INSR GQ30 quercetin Peroxisome proliferator-activated receptor alpha PPARA GQ30 quercetin Peroxisome proliferator-activated receptor delta PPARD GQ30 quercetin C-reactive protein CRP GQ30 quercetin C-X-C motif chemokine 10 CXCL10 GQ30 quercetin Inhibitor of nuclear factor kappa-B kinase subunit alpha CHUK GQ30 quercetin Osteopontin SPP1 GQ30 quercetin Runt-related transcription factor 2 RUNX2 GQ30 quercetin Ras association domain-containing protein 1 RASSF1 GQ30 quercetin Cathepsin D CTSD GQ30 quercetin Insulin-like growth factor-binding protein 3 IGFBP3 GQ30 quercetin Insulin-like growth factor II IGF2 GQ30 quercetin CD40 ligand CD40LG GQ30 quercetin Receptor tyrosine-protein kinase erbB-3 ERBB3 GQ30 quercetin Serum paraoxonase/arylesterase 1 PON1 GQ30 quercetin Type I iodothyronine deiodinase DIO1 GQ30 quercetin Ras GTPase-activating protein 1 RASA1 GQ30 quercetin Glutathione S-transferase Mu 1 GSTM1 S6. Methodological quality assessment of the studies included
Quality score criterion Peer-reviewed publication Control of temperature Random allocation to treatment or control Blinded induction of model Blinded assessment of outcome Use of anesthetic without significant intrinsic neuroprotective activity Appropriate animal model Sample size calculation Compliance with animal welfare regulations Statement of potential conflict of interests Total Yang 2019 (30) 0 0 1 0 0 0 1 0 1 0 3 Cao 2018 (17) 0 0 1 0 0 0 1 0 1 0 3 Ji et al. 2011 (18) 1 1 1 0 0 1 1 0 1 0 6 Ding et al. 2001 (29) 1 0 1 0 0 0 1 0 1 0 4 Qin et al. 2009 (27) 1 0 1 0 0 0 1 0 1 0 4 Hu et al. 2008 (21) 1 1 1 0 0 0 1 0 1 0 5 Liu et al. 2011 (19) 1 0 1 0 0 0 1 0 1 0 4 Yi et al. 2010 (26) 1 0 1 0 0 0 1 0 1 0 4 Wang et al. 2002 (24) 1 0 1 0 0 0 1 0 1 0 4 Liu 2019 (22) 0 1 1 0 0 0 1 0 1 0 4 Yang et al. 2018 (20) 1 1 1 0 0 0 1 0 1 0 5 Ma 2019 (23) 1 0 1 0 0 0 1 0 1 0 4 Niu et al. 1994 (25) 1 0 1 0 0 0 1 0 1 0 4 Wu et al. 2008 (9) 1 1 1 0 0 0 1 0 1 0 5 Wang et al. 2017 (28) 1 0 1 0 0 0 1 0 1 0 4 Note. As showed in Supplementary Table S6, the quality score of the included studies ranged from 3 to 5 and the average quality score was 4.2 points. All studies randomly allocated animals to the control group and the treatment group, adopted appropriate animal models and compliance with animal welfare regulations. However, the method of blinded induction of model and blinded assessment of outcome were not involved in those included studies. In addition, there was no statement of potential conflict of interests in those articles. S7. Meta-analysis for each sub-outcome measure
Outcome Meta-analysis of outcome Test of heterogeneity Model used SMD 95% CI P I2 P Blood lactates −1.58 −1.58 to −0.97 < 0.01 80% < 0.01 Random-effects Muscle glycogen 0.85 0.04 to 1.67 < 0.01 76% < 0.01 Random-effects Liver glycogen 0.91 0.40 to 1.41 < 0.01 60% < 0.01 Random-effects SOD 1.30 0.45 to 2.14 < 0.01 78% < 0.01 Random-effects MAD −0.84 −1.18 to −0.05 < 0.01 32% 0.18 Fixed-effects Note. SMD, standardized mean difference; I2, I-squared statistic; CI, confidence interval; SOD, superoxide dismutase; MAD, malondialdehyde. S3. Information of targets of exercise-induced fatigue
Degree Name Neighborhood connectivity 117 MAPK1 15.31624 98 AKT1 16.81633 84 RAF1 15.54762 77 RELA 19.09091 68 TNF 16.77941 66 PRKCA 15.04545 62 MAPK14 16.27419 57 JUN 19.52632 54 CHUK 20.48148 52 BAX 19.34615 52 IL6 19.55769 51 TP53 19.23529 50 NFKBIA 20.4 48 EGFR 17.8125 47 CCND1 18.76596 46 CASP3 21.08696 46 FOS 20.28261 45 IL1B 18.93333 44 BCL2 20.56818 42 CDKN1A 19.33333 38 CASP8 22.10526 37 CASP9 21.48649 36 TGFB1 19.75 35 MYC 20.45714 34 CXCL8 21.73529 32 IFNG 18.5625 30 PGR 6.625 30 PTGS2 18.43333 30 STAT1 22.1 29 BCL2L1 21.2069 26 RB1 23 26 PTEN 20.80769 23 INSR 15.65217 22 IL10 17.18182 22 IL1A 21.04545 21 IL2 20.33333 20 HSP90AA1 23.8 19 RXRA 24.36842 19 NOS3 22.33333 18 NOS2 17.61111 18 CCL2 25.5 17 MMP9 27.76471 17 ICAM1 23.70588 15 NR3C2 3.714286 15 CXCL2 23.86667 14 ADRB2 23.57143 14 ADRB1 14.85714 14 HIF1A 23.78571 14 CD40LG 18.71429 13 CHRM3 15.92308 13 DRD1 14.53846 13 PPARG 25.16667 13 CCNA2 19.23077 13 PIK3CG 22.84615 13 CXCL10 24.46154 12 MMP2 28.16667 12 NCF1 24.75 11 MAOB 18.45455 11 GSTM1 26.45455 10 PTGS1 21.3 10 SLC6A3 13.2 10 MAOA 9.6 10 CHRM2 19.6 10 ADRA1B 17 10 VCAM1 27.6 10 PPARA 25.3 9 HTR2A 18.11111 9 CHRNA7 19.66667 9 ESR1 21.44444 9 F2 28.44444 9 SOD1 22.55556 9 MMP1 32.66667 9 CDK1 23.44444 9 CYP1A1 24.44444 9 CYP1B1 22.44444 9 SERPINE1 26 8 PLAU 29.5 8 GABRA3 13.125 8 ESR2 20.5 8 MMP3 33.375 8 SELE 31.5 8 NFE2L2 35.375 8 SLC2A4 22.125 8 ERBB3 29.625 7 GABRA5 11.71429 7 DRD5 18.16667 7 HMOX1 37.71429 7 CYP3A4 22.85714 7 PTGER3 36 7 COL3A1 28.42857 7 SPP1 28.85714 7 RASSF1 32 7 CTSD 29.28571 7 IGF2 37.57143 6 PDE3A 14.66667 6 OPRM1 19.5 6 HTR1B 17 6 HTR1A 17 6 AR 38 6 PRSS1 29.66667 6 CAV1 31.83333 6 PLAT 32.66667 6 ALOX5 26.83333 5 ADRA2A 20.4 5 SCN5A 38 5 SLC6A4 17.2 5 OPRD1 18.8 5 HRH1 17.4 5 TOP2A 31 5 MPO 30.2 5 NQO1 45.8 5 PARP1 34 5 AHR 36.4 5 CXCL11 33 5 CHEK2 35.2 5 PPARD 36.4 5 IGFBP3 34.2 4 SLC6A2 16.5 4 ADRA2C 19.75 4 ADRA2B 19.75 4 MMP13 17.25 4 HSPB1 38.75 4 THBD 43.25 4 RASA1 37 3 XDH 38.66667 3 F3 48 3 RUNX2 45.66667 2 AKR1B1 65 2 LTA4H 14 2 KCNH2 68 2 CHRNA2 27 2 PON1 68 2 NR3C1 14 2 DPP4 55.5 2 F10 58 2 F7 58 2 ACHE 59 2 GJA1 58.5 2 DIO1 60.5 1 IGHG1 23 1 MAP2 29 1 MMP8 15 1 MGAM 107 1 CRP 107 S4. Information of pathways of exercise-induced fatigue
Pathway Description Degree Neighborhood connectivity hsa05200 Pathways in cancer 51 35.80392 hsa05417 Lipid and atherosclerosis 37 41.48649 hsa05418 Fluid shear stress and atherosclerosis 29 35.62069 hsa04933 AGE-RAGE signaling pathway in diabetic complications 28 44.89286 hsa05207 Chemical carcinogenesis - receptor activation 28 39.89286 hsa05161 Hepatitis B 27 56.66667 hsa05167 Kaposi sarcoma-associated herpesvirus infection 27 52.66667 hsa04151 PI3K-Akt signaling pathway 27 46.33333 hsa05163 Human cytomegalovirus infection 26 57 hsa04080 Neuroactive ligand-receptor interaction 25 15.12 hsa04657 IL-17 signaling pathway 24 46.25 hsa04668 TNF signaling pathway 23 49.73913 hsa05160 Hepatitis C 23 55.30435 hsa05164 Influenza A 23 54.52174 hsa05169 Epstein-Barr virus infection 23 52.47826 hsa05166 Human T-cell leukemia virus 1 infection 23 52.91304 hsa04010 MAPK signaling pathway 23 54.6087 hsa05205 Proteoglycans in cancer 22 49.04545 hsa05208 Chemical carcinogenesis - reactive oxygen species 22 45.72727 hsa05022 Pathways of neurodegeneration - multiple diseases 22 51.18182 hsa05215 Prostate cancer 21 50.66667 hsa05142 Chagas disease 21 54.85714 hsa05145 Toxoplasmosis 21 51.85714 hsa04218 Cellular senescence 21 49.90476 hsa05152 Tuberculosis 21 56.42857 hsa05171 Coronavirus disease - COVID-19 21 51.52381 hsa05132 Salmonella infection 21 61.42857 hsa05162 Measles 20 54.7 hsa05225 Hepatocellular carcinoma 20 49.65 hsa05165 Human papillomavirus infection 20 57.6 hsa05222 Small cell lung cancer 19 51.52632 hsa04926 Relaxin signaling pathway 19 53.89474 hsa04210 Apoptosis 19 60.94737 hsa04932 Non-alcoholic fatty liver disease 19 52.36842 hsa05202 Transcriptional misregulation in cancer 19 35.05263 hsa05170 Human immunodeficiency virus 1 infection 19 64.84211 hsa05206 MicroRNAs in cancer 19 46.84211 hsa01522 Endocrine resistance 18 56.55556 hsa04620 Toll-like receptor signaling pathway 18 58.38889 hsa04659 Th17 cell differentiation 18 51.94444 hsa04621 NOD-like receptor signaling pathway 18 56 hsa04024 cAMP signaling pathway 18 45.5 hsa05010 Alzheimer disease 18 57.16667 hsa05168 Herpes simplex virus 1 infection 18 56.61111 hsa05140 Leishmaniasis 17 54.23529 hsa04625 C-type lectin receptor signaling pathway 17 64.88235 hsa04380 Osteoclast differentiation 17 59.76471 hsa04915 Estrogen signaling pathway 17 47.82353 hsa05224 Breast cancer 17 56.94118 hsa05130 Pathogenic Escherichia coli infection 17 61.29412 hsa05415 Diabetic cardiomyopathy 17 40.11765 hsa04020 Calcium signaling pathway 17 27.41176 hsa05212 Pancreatic cancer 16 64.125 hsa05210 Colorectal cancer 16 64.875 hsa04064 NF-kappa B signaling pathway 16 43.625 hsa04066 HIF-1 signaling pathway 16 53.9375 hsa05135 Yersinia infection 16 63.6875 hsa05131 Shigellosis 16 67.75 hsa04115 p53 signaling pathway 15 42.53333 hsa05220 Chronic myeloid leukemia 15 66.4 hsa05235 PD-L1 expression and PD-1 checkpoint pathway in cancer 15 66.2 hsa05323 Rheumatoid arthritis 15 43.8 hsa05146 Amoebiasis 15 48 hsa04660 T cell receptor signaling pathway 15 66.66667 hsa04071 Sphingolipid signaling pathway 15 64.86667 hsa04068 FoxO signaling pathway 15 61.13333 hsa04936 Alcoholic liver disease 15 59.6 hsa04022 cGMP-PKG signaling pathway 15 40.86667 hsa04062 Chemokine signaling pathway 15 54.53333 hsa04060 Cytokine-cytokine receptor interaction 15 39.66667 hsa05219 Bladder cancer 14 51.92857 hsa05223 Non-small cell lung cancer 14 63.71429 hsa05133 Pertussis 14 61.71429 hsa04726 Serotonergic synapse 14 43.14286 hsa04919 Thyroid hormone signaling pathway 14 57.64286 hsa05226 Gastric cancer 14 64.07143 hsa04630 JAK-STAT signaling pathway 14 55.85714 hsa05203 Viral carcinogenesis 14 58.78571 hsa05144 Malaria 13 42.61538 hsa01524 Platinum drug resistance 13 59 hsa04014 Ras signaling pathway 13 62.46154 hsa05020 Prion disease 13 55.84615 hsa05213 Endometrial cancer 12 69.58333 hsa05321 Inflammatory bowel disease 12 55 hsa05214 Glioma 12 71.25 hsa01521 EGFR tyrosine kinase inhibitor resistance 12 68.5 hsa04921 Oxytocin signaling pathway 12 63.83333 hsa04510 Focal adhesion 12 66.5 hsa05012 Parkinson disease 12 39.5 hsa05134 Legionellosis 11 60 hsa05221 Acute myeloid leukemia 11 66.63636 hsa04917 Prolactin signaling pathway 11 70.54545 hsa05218 Melanoma 11 71.72727 hsa04658 Th1 and Th2 cell differentiation 11 67.63636 hsa04928 Parathyroid hormone synthesis, secretion and action 11 61 hsa04931 Insulin resistance 11 57.09091 hsa04725 Cholinergic synapse 11 56 hsa04722 Neurotrophin signaling pathway 11 80.90909 hsa04371 Apelin signaling pathway 11 55.90909 hsa04261 Adrenergic signaling in cardiomyocytes 11 58.27273 hsa04217 Necroptosis 11 50.36364 hsa05014 Amyotrophic lateral sclerosis 11 55.81818 hsa05143 African trypanosomiasis 10 51.8 hsa04370 VEGF signaling pathway 10 71.4 hsa05120 Epithelial cell signaling in Helicobacter pylori infection 10 64.1 hsa04012 ErbB signaling pathway 10 75.3 hsa04540 Gap junction 10 56 hsa04061 Viral protein interaction with cytokine and cytokine receptor 10 44.6 hsa04110 Cell cycle 10 46.2 hsa04728 Dopaminergic synapse 10 52 hsa04072 Phospholipase D signaling pathway 10 69 hsa05216 Thyroid cancer 9 63.66667 hsa04920 Adipocytokine signaling pathway 9 64.66667 hsa04622 RIG-I-like receptor signaling pathway 9 66 hsa05230 Central carbon metabolism in cancer 9 74.55556 hsa04662 B cell receptor signaling pathway 9 86.77778 hsa04610 Complement and coagulation cascades 9 26.77778 hsa05231 Choline metabolism in cancer 9 80.88889 hsa04914 Progesterone-mediated oocyte maturation 9 69.44444 hsa04935 Growth hormone synthesis, secretion and action 9 78.44444 hsa04611 Platelet activation 9 59.11111 hsa04140 Autophagy - animal 9 68.55556 hsa04150 mTOR signaling pathway 9 81.55556 hsa04613 Neutrophil extracellular trap formation 9 79.88889 hsa04923 Regulation of lipolysis in adipocytes 8 49.25 hsa05416 Viral myocarditis 8 50.375 hsa04664 Fc epsilon RI signaling pathway 8 87.375 hsa05211 Renal cell carcinoma 8 80.75 hsa05031 Amphetamine addiction 8 51.375 hsa04912 GnRH signaling pathway 8 80.5 hsa04670 Leukocyte transendothelial migration 8 49.25 hsa04650 Natural killer cell mediated cytotoxicity 8 78.5 hsa04015 Rap1 signaling pathway 8 87 hsa05016 Huntington disease 8 55.375 hsa04215 Apoptosis - multiple species 7 63.42857 hsa05332 Graft-versus-host disease 7 62.57143 hsa05030 Cocaine addiction 7 53.71429 hsa04623 Cytosolic DNA-sensing pathway 7 69.85714 hsa04929 GnRH secretion 7 82.57143 hsa04211 Longevity regulating pathway 7 73 hsa05032 Morphine addiction 7 43.42857 hsa04152 AMPK signaling pathway 7 57 hsa04723 Retrograde endocannabinoid signaling 7 69.71429 hsa04934 Cushing syndrome 7 69 hsa04310 Wnt signaling pathway 7 65.57143 hsa05034 Alcoholism 7 63.28571 hsa04810 Regulation of actin cytoskeleton 7 68.42857 hsa01523 Antifolate resistance 6 82.33333 hsa05330 Allograft rejection 6 59.16667 hsa04940 Type I diabetes mellitus 6 64.33333 hsa04672 Intestinal immune network for IgA production 6 57.16667 hsa04913 Ovarian steroidogenesis 6 45.83333 hsa05204 Chemical carcinogenesis - DNA adducts 6 44 hsa04137 Mitophagy - animal 6 71 hsa03320 PPAR signaling pathway 6 42.16667 hsa04742 Taste transduction 6 39.66667 hsa04970 Salivary secretion 6 52.5 hsa04350 TGF-beta signaling pathway 6 81 hsa04666 Fc gamma R-mediated phagocytosis 6 95.83333 hsa04750 Inflammatory mediator regulation of TRP channels 6 64.16667 hsa04114 Oocyte meiosis 6 69.33333 hsa04910 Insulin signaling pathway 6 88 hsa04550 Signaling pathways regulating pluripotency of stem cells 6 99 hsa04390 Hippo signaling pathway 6 55.33333 hsa04960 Aldosterone-regulated sodium reabsorption 5 83.6 hsa00380 Tryptophan metabolism 5 47.4 hsa04930 Type II diabetes mellitus 5 82.8 hsa00330 Arginine and proline metabolism 5 51 hsa00590 Arachidonic acid metabolism 5 49.2 hsa00982 Drug metabolism - cytochrome P450 5 47.4 hsa00980 Metabolism of xenobiotics by cytochrome P450 5 46.8 hsa00983 Drug metabolism - other enzymes 5 44.8 hsa04640 Hematopoietic cell lineage 5 77 hsa04270 Vascular smooth muscle contraction 5 95 hsa05322 Systemic lupus erythematosus 5 66.8 hsa04514 Cell adhesion molecules 5 49.4 hsa04141 Protein processing in endoplasmic reticulum 5 64.4 hsa04360 Axon guidance 5 93.8 hsa05310 Asthma 4 75.5 hsa05033 Nicotine addiction 4 55.5 hsa05320 Autoimmune thyroid disease 4 63.75 hsa04730 Long-term depression 4 116.25 hsa00140 Steroid hormone biosynthesis 4 55.75 hsa04213 Longevity regulating pathway - multiple species 4 82 hsa05217 Basal cell carcinoma 4 85.75 hsa04720 Long-term potentiation 4 116.25 hsa04924 Renin secretion 4 58 hsa04520 Adherens junction 4 96.5 hsa04612 Antigen processing and presentation 4 79.5 hsa04721 Synaptic vesicle cycle 4 54.25 hsa04146 Peroxisome 4 57 hsa04727 GABAergic synapse 4 69.75 hsa05410 Hypertrophic cardiomyopathy 4 88.5 hsa05414 Dilated cardiomyopathy 4 79 hsa04713 Circadian entrainment 4 106.75 hsa04916 Melanogenesis 4 116.25 hsa04972 Pancreatic secretion 4 70.75 hsa04974 Protein digestion and absorption 4 53.25 S5. Summary of studies included in the systematic review
Study Information of aninmals Type of Fructus Lycii Intervention vs. control Time of intervention Outcome measures used Yang 2019 Kunming mice, weighing 12–22 g, half-male and half-female, SPF Decoction of Fructus Lycii 3 mg/(g∙d) (n = 10) vs. 6 mg/(g∙d) (n = 10) vs. Placebo (distilled water 0.02 mL/(g∙d), n = 10) 30 days MDA, ROS, SOD, CAT, GSH-Px Cao 2018 Kunming mice, weighing 18–22 g, half-male and half-female, SPF Decoction of Fructus Lycii 3 mg/(g∙d) (n = 10) vs. 6 mg/(g∙d) (n = 10) vs. Placebo (distilled water 0.02 mL/(g∙d), n = 10) 30 days The time of exhaustive swimming, blood glucose, muscle glycogen and liver glycogen, BUN and lactic acid Ji et al 2011 Eight-week-old Wistar rats, weighing 220 ± 23.19 g, female Decoction of Fructus Lycii Decoction of Fructus Lycii (n = 8) vs. Placebo (n = 8) 30 days The time of exhaustive swimming, SOD, MAD Ding et al 2001 Kunming mice, weighing 20–24 g, male Decoction of Fructus Lycii Decoction of Fructus Lycii (n = 10) vs. Placebo (n = 10) 2 weeks The time of exhaustive swimming, SOD, MAD Qin et al 2009 mice Extract of Fructus Lycii 5 mg/(g∙d) (n = 10) vs. 10 mg/(g∙d) (n = 10) vs. 20 mg/(g∙d) (n = 10) vs. Placebo (Equivalent saline, n = 10) 2 weeks The time of exhaustive swimming Hu et al 2008 Kunming mice, weighing 18–22 g, female Raw juice of Fructus Lycii 10 mL/(kg∙d) (n = 10) vs. 20 mL/(kg∙d) (n = 10) vs. 30 mL/(kg∙d) (n = 10) vs. Placebo (Equivalent saline, n = 10) 3 weeks The time of exhaustive swimming, and liver glycogen,BUN Liu et al 2011 Kunming mice, weighing 24 ± 5 g, female Decoction of Fructus Lycii 5 mg/(g∙d) (n = 10) vs. Placebo (Equivalent distilled water, n = 10) 10 days The time of exhaustive swimming Yi et al 2010 Kunming mice, weighing 24 ± 5 g, half-male and half-female Decoction of Fructus Lycii 5 mg/(g∙d) (n = 10) vs. 2.5 mg/(g∙d) (n = 10) vs. Placebo(Equivalent running water, n = 10) 2 weeks The time of exhaustive swimming Wang et al 2002 Kunming mice, weighing 20 ± 2 g, male and female Decoction of Fructus Lycii 0.2 mL/10 (g∙d) (n = 10) vs. 0.1 mL/10 (g∙d) (n = 10) vs. Placebo (Equivalent saline, n = 10) 1 week The time of exhaustive swimming Liu 2019 Kunming mice, 6 week, male Extract of Fructus Lycii 0.3 mg/(g∙d) (n = 10) vs. 0.6 mg/(g∙d) (n = 10) vs. 0.9 mg/(g∙d) (n = 10) vs. Placebo (n = 10) 2 weeks The time of exhaustive swimming, muscle glycogen and liver glycogen, BUN and lactic acid, SOD, MDA Yang et al 2018 Kunming mice, weighing 18–22 g, male, SPF Extract of Fructus Lycii 0.5 mg/(g∙d) (n = 10) vs. 1 mg/g/d (n = 10) vs. 1.5 mg/(g∙d) (n = 10) vs. Placebo (n = 10) 30 days The time of exhaustive swimming, muscle glycogen and liver glycogen, BUN and lactic acid Ma 2019 Rats, SPF Fruit of Fructus Lycii 0.5g/(kg∙d) (n = 10) vs. 3g/(kg∙d) (n = 15) vs. Placebo (5 mL/kg saline, n = 15) 6 weeks The time of exhaustive swimming Niu et al. 1994 Kunming mice, weighing 18–24 g, half-male and half-female, SPF Decoction of Fructus Lycii 5g/(kg∙d) (n = 10) vs. 2.5/(kg∙d) (n = 10) vs. Placebo (Equivalent running water, n = 10) 2 weeks The time of exhaustive swimming, blood glucose, and lactic acid Wu et al. 2008 Kunming mice, 3 week, weighing 18–22 g,female Raw juice of Fructus Lycii 0.2 mL/10 (g∙d) (n = 10) vs. 0.25 mL/10 (g∙d) (n = 10) vs. Placebo (Equivalent saline, n = 10) 3 weeks The time of exhaustive swimming, BUN and lactic acid Wang et al. 2017 Kunming mice, weighing 60–80 g, female Extract of Fructus Lycii 0.2 mL/10 (g∙d) (n = 10) vs. Placebo (n = 10) 3 weeks The time of exhaustive swimming Note. MDA, malondialdehyde; ROS, reactive oxygen species; SOD, superoxide dismutase; CAT, catalase; GSH-Px, glutathione peroxidase; BUN, blood urea nitrogen. A total 15 RCTs studies included, the information in detail were showed in the Supplementary Table S5. The exhaustive time, which the main parameter of anti-exercise-fatigue ability, were assessed in 14 articles. blood lactate (included in 6 articles) and BUN (included in 5 articles) were the main product of exercise metabolism and important marker for the evaluation of exercise fatigue. As the important energy source, muscle glycogen was included in 2 articles and liver glycogen in 3 articles. SOD (in 3 articles) and MAD (in 4 articles) also were the important outcomes included in our study. Based on the placebo group, the experimental group received decoction of Fructus Lycii (8 articles), extract of Fructus Lycii (4 articles), raw juice of Fructus Lycii (2 articles) and fruit of Fructus Lycii (1 article). Treatment duration ranged from 10 to 42 days. -
[1] Morales-Alamo D, Losa-Reyna J, Torres-Peralta R, et al. What limits performance during whole-body incremental exercise to exhaustion in humans? J Physiol, 2015; 593, 4631-48. [2] Wang PX, Wang DH, Hu JM, et al. Natural bioactive peptides to beat exercise-induced fatigue: A review. Food Biosci, 2021; 43, 101298. doi: 10.1016/j.fbio.2021.101298 [3] Nakai N, Kawano F, Ohira Y. Control of muscle protein synthesis in response to exercise and amino acids. J Phys Fit Sports Med, 2012; 1, 297−305. doi: 10.7600/jpfsm.1.297 [4] Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr, 2011, 93; 884S-90S. [5] Feng SY, Wu SJ, Chang YC, et al. Stimulation of GLUT4 glucose uptake by anthocyanin-rich extract from black rice ( Oryza sativa L.) via PI3K/Akt and AMPK/p38 MAPK signaling in C2C12 cells. Metabolites, 2022; 12, 856. doi: 10.3390/metabo12090856 [6] Zhou YP, Cao FL, Wu Q, et al. Dietary supplementation of octacosanol improves exercise-induced fatigue and its molecular mechanism. J Agric Food Chem, 2021; 69, 7603−18. doi: 10.1021/acs.jafc.1c01764 [7] Huang WY, Pan JH, Jeong I, et al. Antifatigue and anti-inflammatory effects of Cervus elaphus L. , Angelica gigas Nakai, and Astragalus membranaceus bunge complex extracts in physically fatigued mice. J Med Food, 2022; 25, 1126-32. [8] Neelam K, Dey S, Sim R, et al. Fructus lycii: a natural dietary supplement for amelioration of retinal diseases. Nutrients, 2021; 13, 246. doi: 10.3390/nu13010246 [9] Yang J, Wei YQ, Ding JB, et al. Research and application of Lycii fructus in medicinal field. Chin Herb Med, 2018; 10, 339−52. doi: 10.1016/j.chmed.2018.08.006 [10] Wen XH, Lin JR, Xu GQ, et al. Preventing exercise-induced low blood testosterone by supplementation of Chinese Wolfberry Juice. Chin J Sports Med, 2016; 35, 344−8. (In Chinese [11] Luo TT, Lu Y, Yan SK, et al. Network pharmacology in research of Chinese medicine formula: methodology, application and prospective. Chin J Integr Med, 2020; 26, 72−80. doi: 10.1007/s11655-019-3064-0 [12] Xu X, Zhang WX, Huang C, et al. A novel chemometric method for the prediction of human oral bioavailability. Int J Mol Sci, 2012; 13, 6964−82. doi: 10.3390/ijms13066964 [13] Tao WY, Xu X, Wang X, et al. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J Ethnopharmacol, 2013; 145, 1−10. doi: 10.1016/j.jep.2012.09.051 [14] Dhanya R, Arun KB, Syama HP, et al. Rutin and quercetin enhance glucose uptake in L6 myotubes under oxidative stress induced by tertiary butyl hydrogen peroxide. Food Chem, 2014; 158: 546−54. doi: 10.1016/j.foodchem.2014.02.151 [15] Zupin L, Psilodimitrakopoulos S, Celsi F, et al. Upside-down preference in the Forskolin-induced in vitro differentiation of 50B11 sensory neurons: a morphological investigation by label-free non-linear microscopy. Int J Mol Sci, 2023; 24, 8354. doi: 10.3390/ijms24098354 [16] Xu M, Wang XH, Li YN, et al. Arachidonic acid metabolism controls macrophage alternative activation through regulating oxidative phosphorylation in PPARγ dependent manner. Front Immunol, 2021; 12, 618501. doi: 10.3389/fimmu.2021.618501 [17] Gerwyn M, Maes M. Mechanisms explaining muscle fatigue and muscle pain in patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): a review of recent findings. Curr Rheumatol Rep, 2017; 19, 1. doi: 10.1007/s11926-017-0628-x [18] Ren YH, Li Y, Lv JN, et al. Parthenolide regulates oxidative stress-induced mitophagy and suppresses apoptosis through p53 signaling pathway in C2C12 myoblasts. J Cell Biochem, 2019; 120, 15695−708. doi: 10.1002/jcb.28839 [19] López-Soldado I, Guinovart JJ, Duran J. Increased liver glycogen levels enhance exercise capacity in mice. J Biol Chem, 2021; 297, 100976. doi: 10.1016/j.jbc.2021.100976 [20] Peternelj TT, Coombes JS. Antioxidant supplementation during exercise training. Sports Med, 2011; 41, 1043−69. doi: 10.2165/11594400-000000000-00000 [21] Zhang XF, Chen J, Yang JL, et al. UPLC-MS/MS analysis for antioxidant components of Lycii Fructus based on spectrum-effect relationship. Talanta, 2018; 180, 389−95. doi: 10.1016/j.talanta.2017.12.078 [22] Li Y, Yao JY, Han CY, et al. Quercetin, inflammation and immunity. Nutrients, 2016; 8, 167. doi: 10.3390/nu8030167 [23] Manjeet KR, Ghosh B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-α production in murine macrophages. Int J Immunopharmacol, 1999; 21, 435−43. doi: 10.1016/S0192-0561(99)00024-7 [24] D’Andrea G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia, 2015; 106, 256-71. [25] Chen XL, Liang DH, Huang ZQ, et al. Anti-fatigue effect of quercetin on enhancing muscle function and antioxidant capacity. J Food Biochem, 2021; 45, e13968. [26] Hargreaves M. Exercise, muscle, and CHO metabolism. Scand J Med Sci Sports, 2015; 25, 29−33. doi: 10.1111/sms.12607 [27] Seiler SE, Koves TR, Gooding JR, et al. Carnitine acetyltransferase mitigates metabolic inertia and muscle fatigue during exercise. Cell Metab, 2015; 22, 65−76. doi: 10.1016/j.cmet.2015.06.003 [28] Wagatsuma A, Sakuma K. Mitochondria as a potential regulator of myogenesis. Sci World J, 2013; 2013, 593267. [29] Gan ZJ, Fu TT, Kelly DP, et al. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell Res, 2018; 28, 969−80. doi: 10.1038/s41422-018-0078-7 [30] Davis JM, Murphy EA, Carmichael MD, et al. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol, 2009; 296, R1071−7. doi: 10.1152/ajpregu.90925.2008 [31] Bouche C, Serdy S, Kahn CR, et al. The cellular fate of glucose and its relevance in type 2 diabetes. Endocr Rev, 2004; 25, 807−30. doi: 10.1210/er.2003-0026 [32] Tsao JP, Bernard JR, Hsu HC, et al. Short-term oral quercetin supplementation improves post-exercise insulin sensitivity, antioxidant capacity and enhances subsequent cycling time to exhaustion in healthy adults: a pilot study. Front Nutr, 2022; 9, 875319. doi: 10.3389/fnut.2022.875319 [33] Guo W, Liu S, Zheng XT, et al. Network pharmacology/metabolomics-based validation of AMPK and PI3K/AKT signaling pathway as a central role of Shengqi Fuzheng injection regulation of mitochondrial dysfunction in cancer-related fatigue. Oxid Med Cell Longev, 2021; 2021, 5556212. [34] Chen ZP, Stephens TJ, Murthy S, et al. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes, 2003; 52, 2205−12. doi: 10.2337/diabetes.52.9.2205 [35] Zhang XY, Wang LF, Peng LZ, et al. Dihydromyricetin protects HUVECs of oxidative damage induced by sodium nitroprusside through activating PI3K/Akt/FoxO3a signalling pathway. J Cell Mol Med, 2019; 23: 4829−38. doi: 10.1111/jcmm.14406 [36] Rezatabar S, Karimian A, Rameshknia V, et al. RAS/MAPK signaling functions in oxidative stress, DNA damage response and cancer progression. J Cell Physiol, 2019; 234: 14951−65. doi: 10.1002/jcp.28334 [37] Zhuang CL, Mao XY, Liu S, et al. Ginsenoside Rb1 improves postoperative fatigue syndrome by reducing skeletal muscle oxidative stress through activation of the PI3K/Akt/Nrf2 pathway in aged rats. Eur J Pharmacol, 2014; 740, 480−7. doi: 10.1016/j.ejphar.2014.06.040 [38] Li Q, Wang YZ, Cai GS, et al. Antifatigue activity of liquid cultured Tricholoma matsutake mycelium partially via regulation of antioxidant pathway in mouse. Biomed Res Int, 2015; 2015, 562345. [39] Suzuki K, Nakaji S, Yamada M, et al. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol, 2002; 8, 6−48. [40] Wan JJ, Qin Z, Wang PY, et al. Muscle fatigue: General understanding and treatment. Exp Mol Med, 2017; 49, e384. doi: 10.1038/emm.2017.194 [41] Simpson RJ, Florida-James GD, Whyte GP, et al. The effects of intensive, moderate and downhill treadmill running on human blood lymphocytes expressing the adhesion/activation molecules CD54 (ICAM-1), CD18 (β2 integrin) and CD53. Eur J Appl Physiol, 2006; 97, 109−21. doi: 10.1007/s00421-006-0146-4 [42] Ye J, Shen CH, Huang YY, et al. Anti-fatigue activity of sea cucumber peptides prepared from Stichopus japonicus in an endurance swimming rat model. J Sci Food Agric, 2017; 97, 4548−56. doi: 10.1002/jsfa.8322 -
 23182+Supplementary Materials.pdf
23182+Supplementary Materials.pdf

-




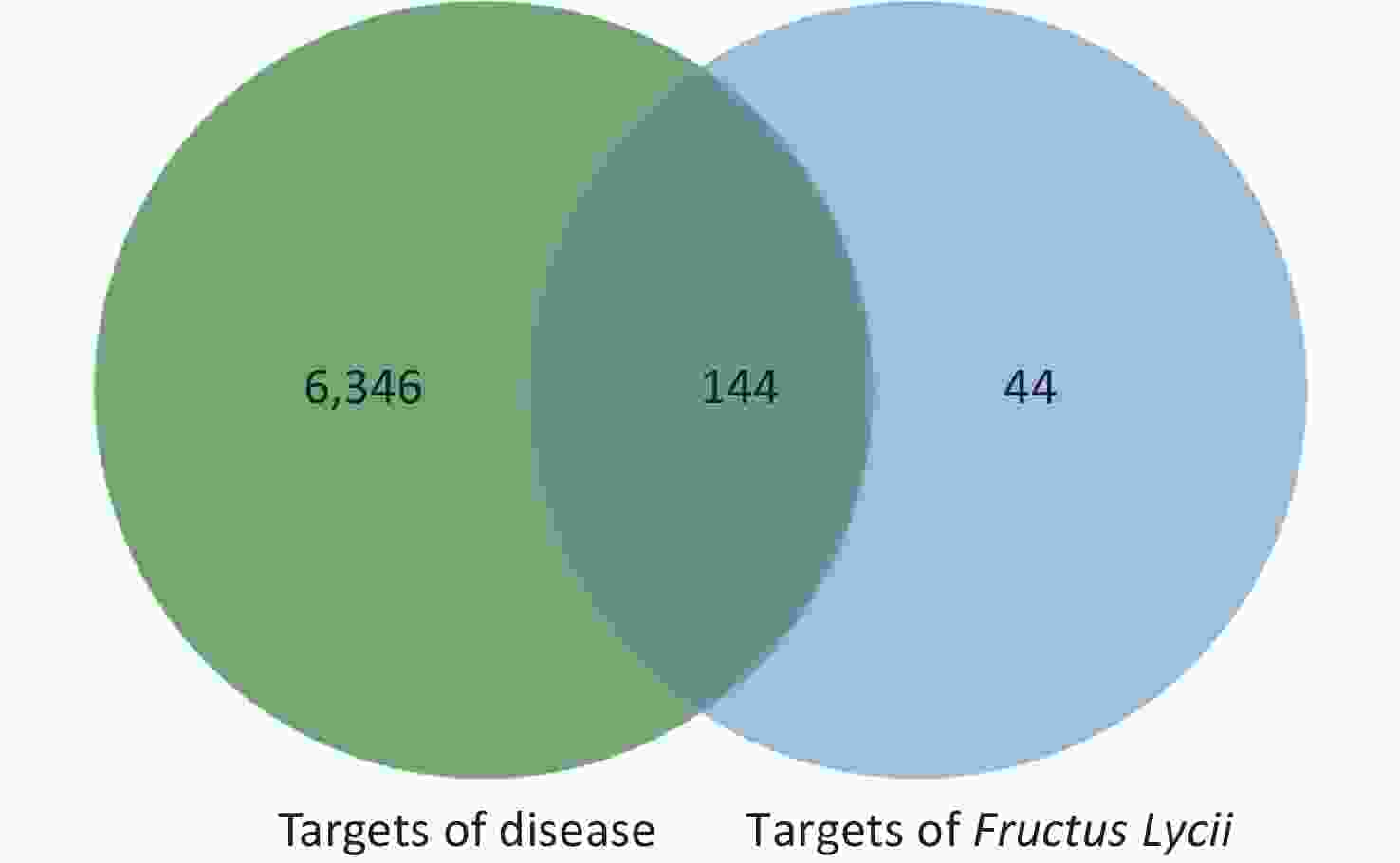
 下载:
下载:
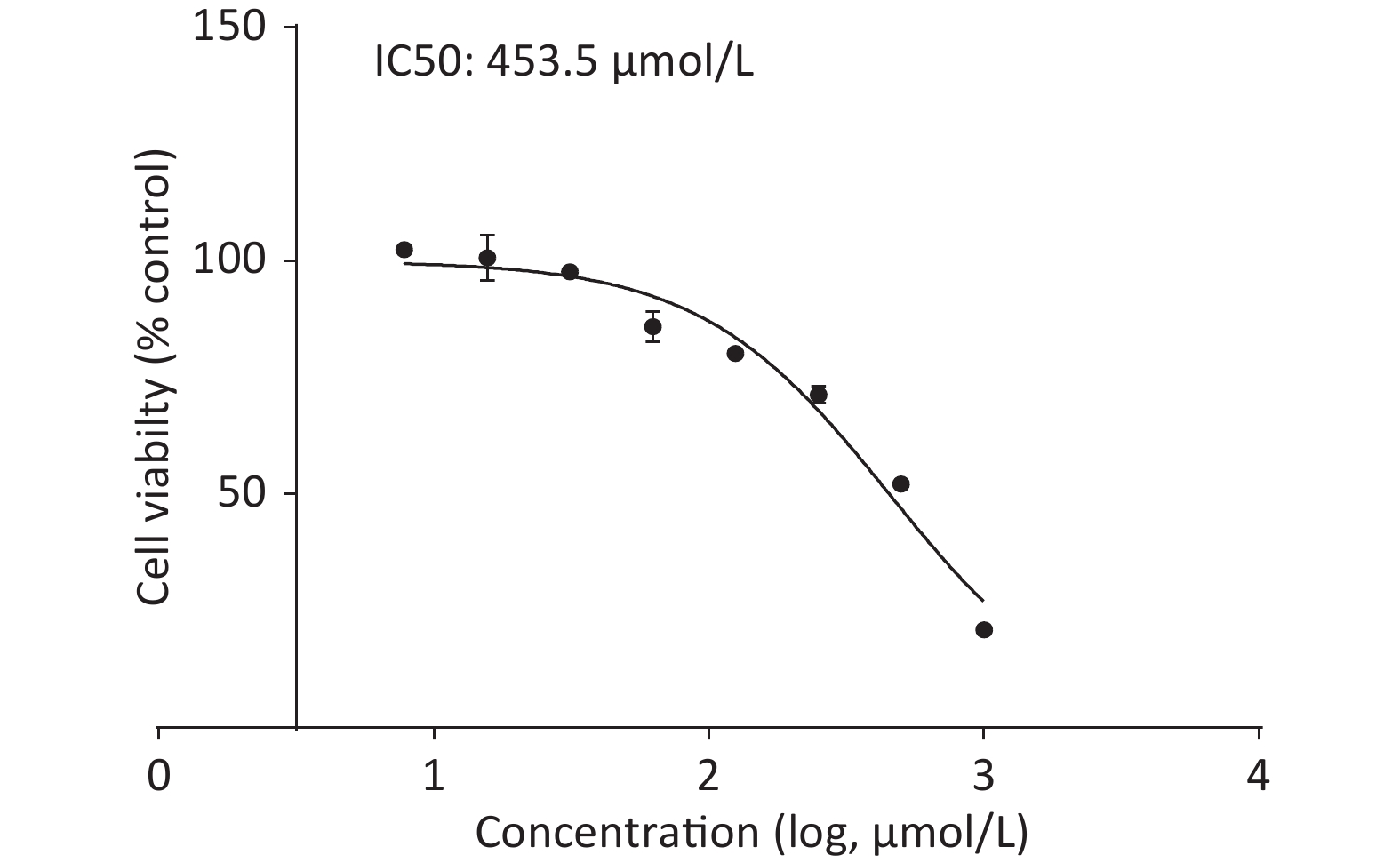




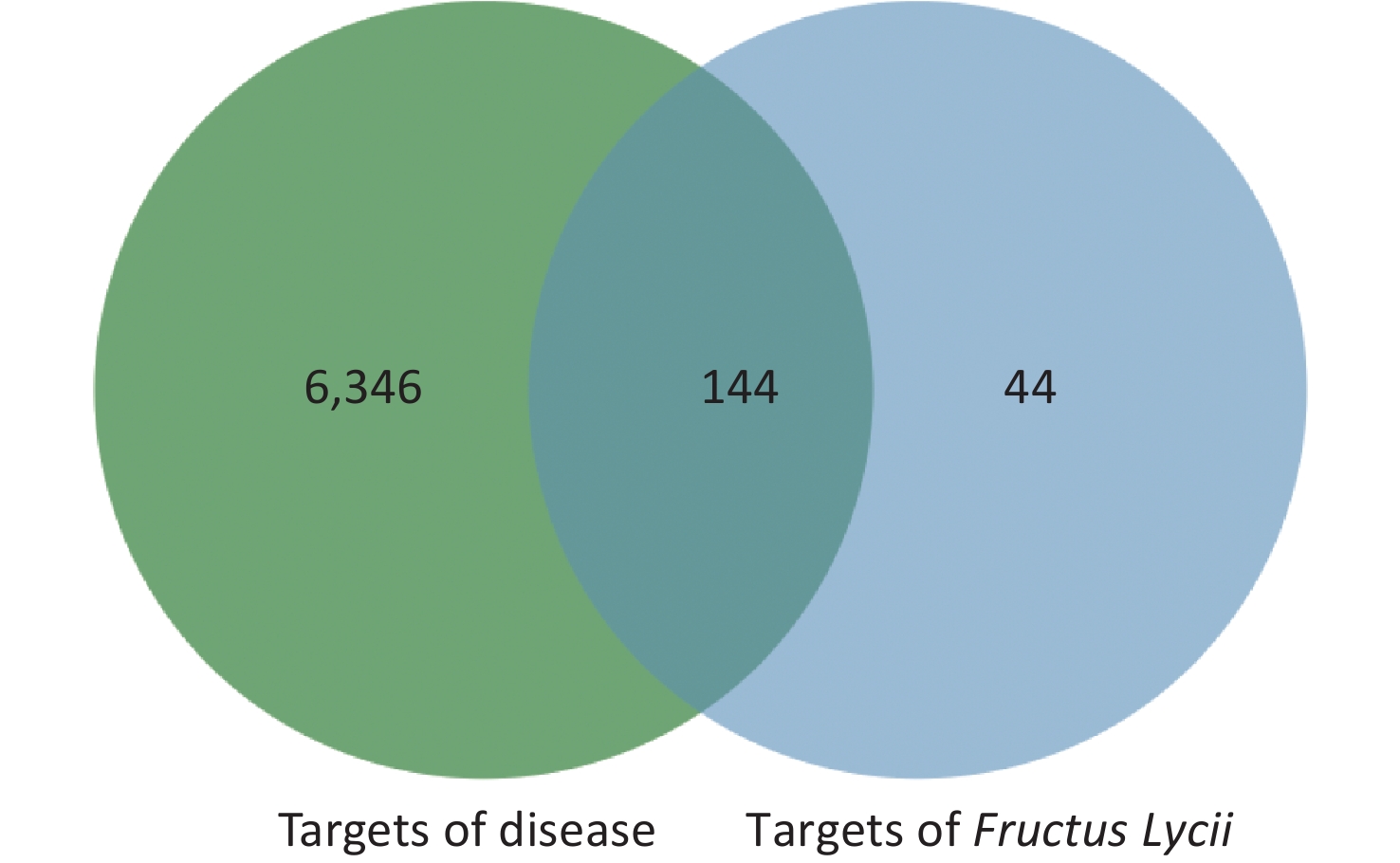

 Quick Links
Quick Links