HTML
-
Postoperative cognitive dysfunction (POCD) is one of the most common postoperative complications[1], and is associated with poor short-term and long-term outcomes[2]. POCD diminishes the quality of the patient's life and adds costs to hospitalization and out-of-hospital care[3]. POCD has received considerable attention over the past 15 years[1]. Postoperative cognitive dysfunction syndromes include postoperative delirium (POD) and postoperative cognitive dysfunction (POCD). POD is an acute, transient and fluctuating decline in cognitive functioning in the early postoperative period, whereas POCD is a chronic impairment with more-subtle deterioration in memory, attention and speed of information processing following anaesthesia and surgery. The precise pathogenesis of POCD is not known and may involve perioperative as well as patient-related factors. However, there is still a lack of effective treatments for POCD, and many studies aim to find new and novel drugs to treat and/or prevent POCD.
Recent animal studies have strongly suggested the role of neuroinflammation in the development of cognitive dysfunction after surgery under volatile anesthetics[3-5]. These previous studies have shown that anesthetics/surgery increase the expression of inflammatory cytokines in the brain[3, 6]. It has been postulated that neuroinflammation response to the oxidative stress may lead to the synapse dysfunction, which can result in cognitive dysfunction[7].
Ginseng is the root of Panax ginseng C. A. Meyer (Araliaceae family) and has been used as a tonic remedy in traditional Chinese medicine for a long time[8-9]. Currently, ginseng is one of the most commonly used herbal medicines in the world[8-10]. Ginsenosides are thought to be the main active components of ginseng with multiple pharmacological activities including anti-inflammation, anti-aging, anti-tumor, anti-oxidation, and anti-fatigue[11-16]. Modern science has identified more than 50 kinds of ginsenosides. Ginsenoside Rb1 has been frequently used to reduce inflammatory process in various diseases[17-21]. However, the relationship between ginsenoside Rb1 and postoperative cognitive dysfunction is unknown.
We therefore assessed whether ginsenoside Rb1 could attenuate the isoflurane/surgery-induced cognitive impairment in mice and the related mechanism. Since the postsynaptic density 95 (PSD-95)[22]and synaptophysin (SVP)[23]are markers of synapse, we compared the levels of hippocampus PSD-95 and SVP as parts of the mechanistic investigation. Elevation of inflammatory cytokine tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) levels has been reported to be associated with the neuroinflammation and contribute to the cognitive impairment induced by surgery under anesthesia[3, 7, 24-25]. Oxidative stress is defined as an imbalance between production of free radicals and reactive metabolites, reactive oxygen species (ROS) could activate inflammatory pathways leading to over express of inflammatory cytokines[26]. Taken together, we assessed the effects of the ginsenoside Rb1 on isoflurane/surgery induced neuroinflammation (the levels of TNF-α and IL-6), oxidative stress (the levels of ROS) and synapse function (the levels of PSD-95 and SVP) in the hippocampus of mice, as well as the cognitive function (Barnes Maze Test) in the mice.
-
All animal procedures were approved by the Ethical Committee of Beijing Friendship Hospital, Capital Medical University (Beijing, China), and were performed in accordance with the Guidelines for Animal Experimentation of the International Association in Research and Teaching. Efforts were made to minimize the number of animals used. To avoid selection bias, we assigned animals randomly to different experimental groups by a computer-generated randomization list.
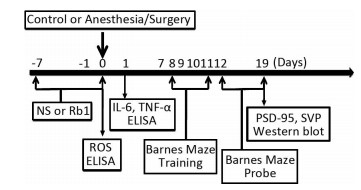
Wide-Type C57BL/6J mice (5-months-old, female) were housed in a controlled environment (20-22 ℃, 12 h of light/dark on a reversed light cycle) for seven days prior to the studies. The mice were randomly assigned to the isoflurane/surgery group or the control condition group with ginsenoside Rb1 or normal saline (the vehicle of ginsenoside Rb1) pretreatment. A simple laparotomy was performed under isoflurane anesthesia using the methods described in previous studies[27]. Specifically, anesthesia was induced and maintained with 1.4% isoflurane in 100% oxygen in a transparent acrylic chamber. Fifteen minutes after the induction, the mouse was moved out of the chamber, and anesthesia was maintained via a cone device. One 16-gauge needle was inserted into the cone near the nose of the mouse to monitor the concentration of anesthesia. A longitudinal midline incision was made from the xiphoid to the 0.5 centimeter proximal pubic symphysis on the skin, abdominal muscles and peritoneum. Then, the incision was sutured layer by layer with 5-0 Vicryl thread. At the end of the procedure, 2.5% lidocaine and 2.5% prilocaine cream was applied to the incision wound, and then every eight hours for two days to treat the pain associated with the incision. The temperature of the anesthetizing chamber was controlled (DC Temperature Control System; FHC, Bowdoinham, Maine) to maintain the rectal temperature of the mice at 37 ± 0.5 ℃ during the isoflurane/surgery. After recovering from the anesthesia, each mouse was returned to a home cage with food and water available ad libitum. The mice in the control group were placed in their home cages with room air for two hours, which was consistent with the condition of non-surgery patients. Ginsenoside Rb1 was at > 98% purity and obtained from the National Institutes for Food and Drug Control (Beijing, China) in the form of white powder-like crystals, and has a molecular weight of 1109.29 and general formula C54H92O23. Ginsenoside Rb1 or normal saline was administered to the mice via intraperitoneal injection from 7 days before isoflurane/surgery to the operation day (Figure 1). The dose of Ginsenoside Rb1 (60 mg/kg, daily) was selected according to the previous studies[21].

Figure 1. Diagram of the experimental Design. dhe mice received Barnes Maze training from day 8 to day 11 after operation. Then, the mice received Barnes Maze probe test on day 12 and 19, respectively. Ginsenoside Rb1 treatment (60 mg/kg) started 7 days before isoflurane/surgery. Western blot analysis was performed after the behavior tests on day 19. ROS measurement was performed immediately after the isoflurane/surgery on day 0. ELISA test of IL-6 and TNF-α was performed 24 h after operation.
-
We performed the Barnes maze test using the methods described in other studies[5, 25]with modifications. Six days after being exposed to various experimental conditions, animals were subjected to Barnes maze to test their spatial learning and memory (Figure 1). Barnes maze was a circular platform with 20 equally spaced holes. One of the holes was connected to a dark chamber that was called escape box. The test started by placing animals in the middle of the Barnes maze. Aversive noise (85 dB) and bright light (200 W) shed on the platform were used to encourage rats to find this box. Animals were trained in a spatial acquisition phase that took 4 days (from day 8 to day 11 after isoflurane/surgery) with 3 min per trial, 2 trials per day and 15 min between each trial. Their reference memory was tested on day 12 and day 19. Each animal had one trial on each of these two days. No test was performed during the period from day 12 to day 19. The latency to find the escape box during each trial was recorded as escape latency with the assistance of ANY-Maze video tracking system and the data were analyzed. The increase in escape latency of the Barnes Maze suggests cognitive impairment of the mice[5, 25]. Between each test, the Barnes maze was cleaned with 75% alcohol solution to avoid olfactory cues.
-
At the end of last behavior test on day 19, each of mice was killed by decapitation. The brain tissues (hippocampus) of the mice were harvested and subjected to Western blot analysis. Different group of mice brain tissues (hippocampus) were used for ROS measurement immediately after surgery or IL-6 and TNF-α test 24 h after surgery. The harvested hippocampus tissues were homogenized on ice using immunoprecipitation buffer (10 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 2 mmol/L EDTA, 0.5% Nonidet P-40) plus protease inhibitors (1 mg/mL aprotinin, 1 mg/mL leupeptin, 1 mg/mL pep-statin A). The lysates were collected, centrifuged at 12, 000 rpm for 15 min, and quantified for total proteins by bicinchoninic acid protein assay kit (Pierce, Iselin, NJ) as described in our prior studies[15].
-
The postsynaptic density protein 95 (PSD-95) antibody (95 kD, 1:1, 000, Cell Signaling, Danvers, MA) and synaptophysin (SVP) antibody (38 kDa, 1:1, 000, Cell Signaling, Abcam, MA) was used in the Western blot analysis to detect PSD-95 and SVP, respectively. The quantification of Western blot was performed as described in our previous studies[15]. Specifically, signal intensity was analyzed using image analysis program Quantity One (Bio-Rad, Hercules, CA). The Western blots were then quantified in two steps. First, we used β-actin levels to normalize protein levels (e.g., determining the ratio of PSD-95 to β-actin amount) and control for loading differences in the total protein amount. Second, we presented the changes in protein levels from the mice undergoing isoflurane/surgery as a percentage of those in the mice from control group. 100% changes of protein levels refer to control levels for the purpose of comparison with experimental conditions.
-
The mouse TNF-α Immunoassay kit (R & D Systems, Minne-apolis, MN, Catalog number, MTA00B) and IL-6 Immunoassay kit (R & D Systems, Catalog number, M6000B) was used to determine the levels of TNF-α and IL-6 in the hippocampus tissues of the mice. Briefly, a monoclonal antibody specific for mouse TNF-α or IL-6 has been coated onto microplates. We added 50 μL of standard or samples, and then added 50 mL of assay diluent RD1-14 to the center of each well. Wells were incubated for two hours at room temperature, and washed five times. Then 100 mL of mouse TNF-α or IL-6 conjugate was added to each well and incubated for another two hours and repeated the washing. At last, wells were incubated in 100 mL of substrate solution for 30 min and stopped with stop solution. Determination of the optical density of each well was set at 450 nm, and corrected at 570 nm.
-
An OxiSelect In Vitro ROS/RNS Assay Kit (Cell Biolabs, San Diego, CA) was used to measure the amount of ROS in the hippocampus of the mice, according to the protocols provided by the company and the methods described in other studies[27].
-
Data were expressed as mean ± standard error of mean (SEM). The number of samples varied from 6 (biochemistry studies) to 10 (behavioral studies). A two-way ANOVA was used to determine the interaction between group and treatment on escape latency and the levels of PSD-95, SVP, ROS, IL-6, and TNF-α, followed by Bonferroni test for post-hoc comparisons. PSD-95, SVP, ROS, IL-6, and TNF-α levels were presented as a percentage of those of the control group. P values less than 0.05 were considered statistically significant. Prism 6 software (Graph Pad Software, Inc, La Jolla, CA) was used to analyze the data.
Mice Anesthesia/surgery and Treatment
Barnes Maze Test
Brain Tissue Lysis, Protein Amount Quantification
Western Blot Analysis
Enzyme-linked Immunosorbent Assay (ELISA) Determination of TNF-α and IL-6
ROS Measurement
Statistics Analysis
-
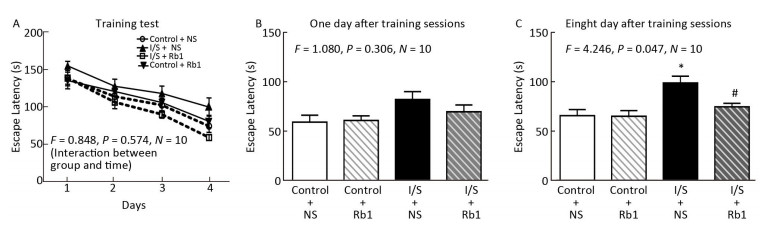
All mice survived until the end of the study. Firstly, we found that isoflurane/surgery did not induce cognitive impairment during Barnes Maze Training sessions (escape latency as compared to control condition) in mice pretreated with normal saline (Figure 2A, F = 0.848, P= 0.574, two-way ANOVA), the isoflurane/surgery did not induce cognitive impairment during Barnes Maze Training sessions in the mice pretreated with ginsenoside Rb1. Secondly, we found that the isoflurane/surgery did not induce cognitive impairment one day after Barnes Maze Training sessions in mice pretreated with normal saline, the isoflurane/surgery did not induce cognitive impairment one day after Barnes Maze Training sessions in the mice pretreated with ginsenoside Rb1 (Figure 2B, F= 1.080, P = 0.306, two-way ANOVA). Thirdly, We found that the isoflurane/surgery induced cognitive impairment eight day after Barnes Maze Training sessions in mice pretreated with normal saline, the ginsenoside Rb1 attenuated the isoflurane/surgery induced the escape latency prolongation as compared to isoflurane/surgery plus NS eight day after Barnes Maze Training sessions in the mice (Figure 2C, F = 4.246, P = 0.047, two-way ANOVA). These data suggest that ginsenoside Rb1 may mitigate the cognitive impairment induced by isoflurane/surgery in the mice.

Figure 2. Ginsenoside Rb1 attenuated isoflurane/surgery-induced cognitive impairment. (A) Two-way ANOVA with repeated measurement showed no significant interaction (F = 0.848, P = 0.574) of treatment (isoflurane/surgery versus control condition) and time (days) on escape latency of Barnes maze training test in the mice. The isoflurane/surgery plus normal saline (NS) did not significantly increase the escape latency in Barnes Maze training test as compared to control condition plus NS in the mice. The isoflurane/surgery plus ginsenoside Rb1 did not significantly increase the escape latency in Barnes Maze training test as compared to control condition plus ginsenoside Rb1 in the mice. (B) Two-way ANOVA showed no significant interaction (F = 1.080, P = 0.306) of group and treatment on escape latency of Barnes maze 1 day after the training sessions in the mice. (C) Two-way ANOVA showed significant interaction (F = 4.246, P = 0.047) of group and treatment on escape latency of Barnes maze 8 days after the training sessions in the mice. Isoflurane/surgery plus NS increased the escape latency as compared to control condition. Ginsenoside Rb1 attenuated the isoflurane/surgery induced the escape latency prolongation as compared to isoflurane/surgery plus NS. *P < 0.05 compared with control condition plus NS. #P < 0.05 compared with isoflurane/surgery plus NS. N = 10 in each group. Rb1, ginsenoside Rb1; NS, normal saline; I/S, isoflurane/surgery.
-
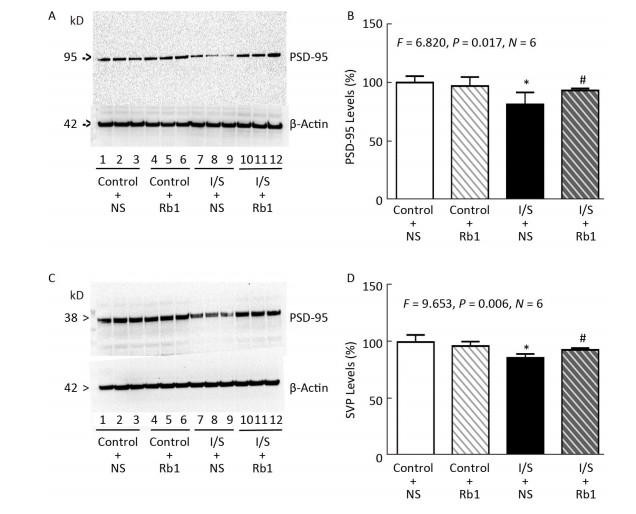
Next, we compared the effects of isoflurane/surgery on the levels of synaptic markers in hippocampus of the mice. We performed quantitative Western blot and found that ginsenoside Rb1 (lanes 10-12 in Figure 3A and striped black bar in Figure 3B) mitigated the isoflurane/surgery-induced reduction in hippocampus PSD-95 levels (lanes 7 to 9 in Figure 3A and black bar in Figure 3B) in the mice (F = 6.820, P = 0.017, two-way ANOVA). The isoflurane/surgery plus normal saline (lanes 4 to 6 in Figure 3A and black bar in Figure 3B) decreased the hippocampus PSD-95 levels as compared to the control condition (lanes 1 to 3 in Figure 3A and white bar in Figure 3B). However, there was no significant difference in the hippocampus PSD-95 levels between control condition plus normal saline (lanes 1 to 3 in Figure 3A and white bar in Figure 3B) and control condition plus ginsenoside Rb1 (lanes 4 to 6 in Figure 3A and striped bar in Figure 3B).
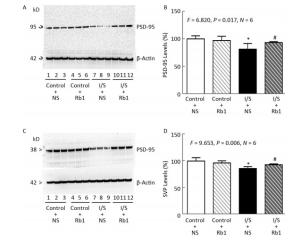
Figure 3. Ginsenoside Rb1 mitigated the reductions in PSD-95 and SVP levels induced by isoflurane/surgery in the hippocampus of the mice. (A) Isoflurane/surgery (lanes 7 to 9) decreased PSD-95 levels as compared to control condition plus normal saline (lanes 1 to 3) in hippocampus of the mice. Ginsenoside Rb1 plus control condition (lanes 4 to 6) did not significantly alter PSD-95 levels as compared to control condition plus normal saline (lanes 1 to 3). However, Ginsenoside Rb1 plus isoflurane/surgery (lanes 10 to 12) led to lesser reductions of PSD-95 levels as compared to isoflurane/surgery plus normal saline (lanes 7 to 9). (B) Quantification of Western blot showed that Ginsenoside Rb1 (striped and black bar) inhibited the reduction in PSD-95 levels induced by the isoflurane/surgery (black bar). (C) Isoflurane/surgery (lanes 7 to 9) decreased SVP levels as compared to control condition (lanes 1 to 3) in hippocampus of the mice. Ginsenoside Rb1 plus control condition (lanes 4 to 6) did not significantly change SVP levels as compared to control condition (lanes 1 to 3). However, Ginsenoside Rb1 plus isoflurane/surgery (lanes 10 to 12) led to lesser reductions of SVP levels as compared to isoflurane/surgery (lanes 7 to 9). (D) Quantification of Western blot showed that Ginsenoside Rb1 (striped and black bar) inhibited the reduction in SVP levels induced by isoflurane/surgery (black bar). *P < 0.05 compared with control condition plus NS. #P < 0.05 compared with isoflurane/surgery plus NS. N = 6 in each group. Rb1, ginsenoside Rb1; NS, normal saline; I/S, isoflurane/surgery.
Similarly, we found that ginsenoside Rb1 was able to mitigate the isoflurane/surgery-induced reduction SVP levels in mice hippocampus (Figure 3C and Figure 3D). There was no significant difference in hippocampus β-Actin levels in mice among various treatments (Figure 3). Taken together, these data suggest that ginsenoside Rb1 can mitigated the isoflurane/surgery-induced synapse dysfunction in the mice.
-
Given the findings that ginsenoside Rb1 attenuated isoflurane/surgery induced cognitive dysfunction and synapse dysfunction in the hippocampus of the mice, we asked whether the effects were associated with neuroinflammation and oxidative stress mechanism.
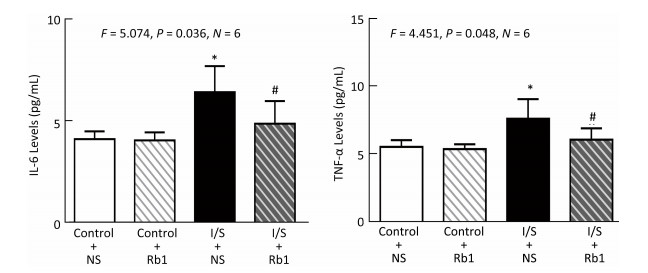
Firstly, we measured the IL-6 levels in the hippocampus of the mice, the isoflurane/surgery (black bar in Figure 4A) increased the IL-6 levels as compared to control condition (white bar in Figure 4A), the treatment of control condition plus ginsenoside Rb1 (striped bar in Figure 4A) did not significantly alter the IL-6 levels in the hippocampus of the mice as compared to control condition (white bar in Figure 4A). Two-way ANOVA showed significant interaction of treatment and group and ginsenoside Rb1 (striped and black bar in Figure 4A) specifically mitigate the elevation of the IL-6 levels induced by isoflurane/surgery in the hippocampus of the mice (black bar in Figure 4A) (F = 5.074, P = 0.036, two-way ANOVA).
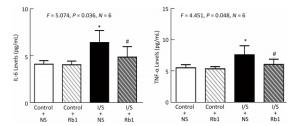
Figure 4. Ginsenoside Rb1 mitigated the elevation of IL-6 and TNF-α levels induced by isoflurane/surgery in the hippocampus of the mice. (A) ELISA showed that isoflurane/surgery plus normal saline (black bar) significantly increased IL-6 levels in hippocampus of the mice as compared to control condition (white bar) at 24 h after isoflurane/surgery. Treatment with ginsenoside Rb1 alone (white and striped bar) did not significantly change IL-6 levels as compared to control condition (white bar). Ginsenoside Rb1 mitigated the elevationofIL-6 levels induced by the isoflurane/surgery. (B) ELISA showed that isoflurane/surgery plus normal saline (black bar) significantly increased TNF-α levels in hippocampus of the mice as compared to control condition (white bar) at 24 h after isoflurane/surgery. Treatment with ginsenoside Rb1 alone (white and striped bar) did not significantly change TNF-α levels as compared to control condition (white bar). Ginsenoside Rb1 mitigated the elevation of TNF-α levels induced by isoflurane/surgery. *P < 0.05 compared with control condition plus NS. #P < 0.05 compared with isoflurane/surgery plus NS. N = 6 in each group. Rb1, ginsenoside Rb1; NS, normal saline; I/S, isoflurane/surgery.
Nextly, we measured the TNF-α levels in the hippocampus of the mice, the isoflurane/surgery (black bar in Figure 4B) increased TNF-α levels as compared to control condition (white bar in Figure 4B), the treatment of control condition plus ginsenoside Rb1 (striped bar in Figure 4B) did not significantly alter the TNF-α levels of the mice as compared to control condition (white bar in Figure 4B). Two-way ANOVA showed significant interaction of treatment and group and ginsenoside Rb1 (striped and black bar in Figure 4B) specifically mitigate the elevation of the TNF-α levels induced by isoflurane/surgery in the hippocampus of the mice (black bar in Figure 4B) (F = 4.451, P= 0.048, two-way ANOVA).
Finally, we asked whether ginsenoside Rb1 attenuated neuroinflammation via inhabiting the oxidative stress. We performed ELISA studies to measure the ROS accumulation in the hippocampus of the mice immediately after isoflurane/surgery, the isoflurane/surgery (black bar in Figure 5) increased the ROS levels as compared to control condition (white bar in Figure 5), the treatment of control condition plus ginsenoside Rb1 (striped bar in Figure 5) did not significantly alter the ROS levels of the mice as compared to control condition (white bar in Figure 5). Two-way ANOVA showed significant interaction of treatment and group and ginsenoside Rb1 (striped and black bar in Figure 5) specifically mitigate the elevation of the ROS levels induced by isoflurane/surgery in the hippocampus of the mice (black bar in Figure 5) (F = 4.458, P = 0.048, two-way ANOVA).
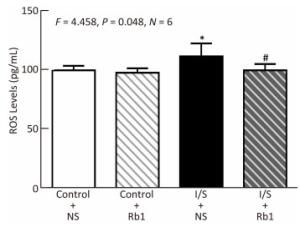
Figure 5. Ginsenoside Rb1 mitigated the elevation of ROS levels Induced by isoflurane/surgery in the hippocampus of the Mice. ELISA showed that isoflurane/surgery plus normal saline (black bar) significantly increased ROS levels in hippocampus of the mice as compared to control condition (white bar) immediately after isoflurane/ surgery. Treatment with ginsenoside Rb1 alone (white and striped bar) did not significantly change ROS levels as compared to control condition (white bar). Ginsenoside Rb1 mitigated the elevation of ROS levels induced by the isoflurane/surgery. *P < 0.05 compared with control condition plus NS. #P < 0.05 compared with isoflurane/surgery plus NS. N = 6 in each group. Rb1, ginsenoside Rb1; NS, normal saline; I/S, isoflurane/surgery.
Ginsenoside Rb1 Attenuated Isoflurane/surgery-induced Cognitive Impairment
Ginsenoside Rb1 Attenuated Isoflurane/surgery-induced Synapse Dysfunction
Ginsenoside Rb1 Attenuated Isoflurane/surgery-induced Neuroinflammation and Oxidative Stress
-
The neuropathogenesis of POCD remains largely to be determined. Neuroinflammation has been reported to be associated with POCD in humans and animal cognitive impairment[3-5, 24-25]. Ginsenoside Rb1 may protect against the negative effects of neuroinflammation and oxidative stress[21, 28]. Therefore, in the present study, we assessed the interaction between isoflurane/surgery and ginsenoside Rb1 on inflammatory cytokine activation, ROS accumulation, synapse function and Barnes Maze performance. We were able to demonstrate that ginsenoside Rb1 ameliorated the isoflurane/ surgery-induced cognitive impairment observed in mice. These findings suggested that ginsenoside Rb1 could be used to ameliorate the neurobehavioral deficits induced by isoflurane/surgery, pending further studies. We found that the ginsenoside Rb1 treatment attenuated the isoflurane/surgery-induced synapse dysfunction, which was consisted with the behavior changes. Isoflurane/surgery-induced IL-6 and TNF-α elevation, ROS accumulation as shown, at least partially, to be the up-stream mechanisms of the isoflurane/surgery-induced synapse dysfunction. We investigated that after surgery under anesthesia isoflurane, mice exhibited impaired memory that was temporally associated with inflammatory cytokine expression and ROS accumulation in the hippocampus. We found that ginsenoside Rb1 could attenuate the isoflurane/surgery-induced ROS accumulation and elevation of IL-6 and TNF-α levels in the hippocampus of the mice. Taken together, these data suggest that ginsenoside Rb1 could mitigate the isoflurane/surgery-induced synapse dysfunction by inhibiting the isoflurane/surgery-induced neuroinflammation and oxidative stress. In addition, these data further suggested that ROS accumulation and neuroinflammation could be the up-stream mechanisms of the isoflurane/surgery-induced synapse dysfunction and cognitive impairment.
Previously, it had been speculated that inflammatory cytokines could play a role in the development of cognitive decline following isoflurane/surgery[6]. IL-6 levels were increased between 4-12 h and remain elevated for up to 3 days postoperatively[29-31]. Cytokines originating from the periphery could exert effects within the central nervous system (CNS) through both direct and indirect means. Both IL-6[32]and TNF-α[33]had been shown to gain entry into the CNS through the relatively permeable blood-brain barrier in the periventricular regions. Other study showed that the increases in TNF-α levels occurred earlier than IL-6 levels after surgery[31]. These data suggested that surgery-induced increases in TNF-α levels could be precedent of surgery-induced increases in IL-6 levels. Terrando et al. had shown that TNF-α was upstream of IL-6 following a surgery under anesthesia in mice[31]. The future studies to test this hypothesis were warranted. Our results showed that the expression of IL-6 and TNF-α was increased in the hippocampus of mice at 24 h after isoflurane/ surgery, however, mice had cognitive impairment at 19 days after operation. These results suggest that neuroinflammation may induce secondary event for cognitive impairment to occur in a delayed phase. Note that isoflurane/surgery did not induce cognitive impairment on day 12 but induced cognitive impairment on day 19 with the longer escape latency of mice in the probe test of Barnes maze. The probe trial of Barnes maze on day 12 is used to assess spatial memory retention and the probe trial on day 19 is used to assess long-term memory retention. The explanation maybe that isoflurane/surgery more likely to induce long-term memory retention instead of spatial memory retention in our study system. The exact reason of such difference is unknown at the present time. The future studies to test this hypothesis are warranted. Strategies designed to mitigate the central neuroinflammatory component of surgery may represent a means of preventing postoperative cognitive dysfunction. Blocking the effects of inflammatory cytokines could reduce cognitive dysfunction. However, many of these blocking methods used in animals were antibodies against cytokines or transgenic mice lacking certain components for cytokine responses[3-4]and, therefore, had a low potential for clinical translation. Ginseng root had been used for several thousand years with few side effects prompting that ginsenoside Rb1 might have more potential value for clinical translation.
Substantial evidence had indicated that oxidative stress was a major contributor to the pathophysiology of a variety of neurodegenerative disorders, including Alzheimer's disease and acute CNS injuries[34-35]. Oxidative stress was served as the accumulation of ROS[36]. During inflammation, mast cells and leukocytes were recruited to the site of damage, which leaded to a 'respiratory burst' due to an increased uptake of oxygen, and thus, an increased release and accumulation of ROS[37-38]. On the other hand, inflammatory cytokines IL-6 and TNF-α had also been reported to play a role in oxidative stress-induced inflammation[39]. This sustained inflammatory/oxidative environment led to a vicious circle, which could damage healthy nerve cells and over a long period of time lead to synapse dysfunction[40-41]. Ginsenoside Rb1 also could significantly reduce the isoflurane/surgery induced elevation of ROS levels of the hippocampus of the mice in our study. Other results showed that ginsenoside Rb1 might have multiple mechanisms of action that by both reducing ROS generation and increasing the anti-inflammatory effects[18, 20, 28, 42-44].
There are several limitations in the current studies. First, we did not assess the effects of different concentrations of ginsenoside Rb1 on the isoflurane/surgery-induced cognitive dysfunction. It is possible that different concentrations of ginsenoside Rb1 may have different effects on the isoflurane/surgery-induced cognitive dysfunction, increases in ROS levels, IL-6 and TNF-α levels. Second, we did not determine whether ginsenoside Rb1 could attenuate the isoflurane/surgery-induced cognitive impairment through other behavioral methods, e.g., Morris Water Maze or Fear Condition System, to test a hypothesis that isoflurane/surgery may impairs certain different domains of cognitive function. Finally, we did not determine whether other antioxidant agents might also be able to attenuate isoflurane/surgery-induced neurotoxicity and neurobehavioral deficits. Nevertheless, the primary objective of the current study was to establish a system and to generate a concept. We will use this established system to systematically investigate the interaction of ginsenoside Rb1 (as well as other antioxidant agents, e.g., ginsenoside Rg1) and isoflurane (as well as other anesthetics, e.g., sevoflurane) on neurotoxicity and neurobehavioral deficits in the future.
In conclusion, we found that ginsenoside Rb1 attenuated the isoflurane/surgery-induced synapse dysfunction, elevation levels of ROS, IL-6 and TNF-α in the hippocampus of the mice. Moreover, ginsenoside Rb1 was able to ameliorate the isoflurane/surgery-induced cognitive impairment in the mice. Pending further studies, these results suggested that ginsenoside Rb1 might mitigate the isoflurane/surgery-induced synapse dysfunction via oxidative stress and neuroinflammation associated mechanisms, which may lead to the inhibition of isoflurane/surgery-induced cognitive impairment. Moreover, ginsenoside Rb1 may be useful to consider anti-inflammatory and anti-oxidation to reduce the risk of POCD in elderly patients in the future.







 Quick Links
Quick Links
 DownLoad:
DownLoad: