HTML
-
Type B natriuretic peptide/brain natriuretic peptide (BNP) is synthesized in ventricular muscle cells. It was first isolated from porcine brain in 1988[1]. The gene encoding human BNP is located at the end of the short arm of chromosome 1 and consists of three exons and two introns[2]. The mRNA of BNP first translates a peptide chain that is composed of 134 amino acids, known as pre-proBNP[3]. Then, the signal peptide of pre-proBNP is cut off by a protease resulting in the BNP precursor (proBNP) that is composed of 108 amino acids. When ventricular volume load or pressure load increases, that is, when the ventricular muscle is stretched or the ventricular wall pressure increases, the stored proBNP is released immediately and rapidly broken down into amino-terminal proBNP (NT-proBNP), including 76 amino acids, and BNP, including 32 amino acids[4]. BNP has the following biological actions: diuresis, vasodilatation, reduction of systemic and regional (renaland cardiac) sympathetic tone, myocardial relaxation, antihypertrophic and antifibrotic effects, and cytoprotection of cardiomyocytes[5-6].
NT-proBNP, proBNP, and BNP coexist simultaneously in blood circulation. NT-proBNP is an inactive amino acid fragment, and its specific biological function is still not known. The apparent concentration of NT-proBNP is close to that of BNP in normal human plasma, but stored NT-proBNP and BNP are secreted rapidly in case of heart failure. The half-life of NT-proBNP is about 60 min, which is longer than the half-life of about 20 min for BNP[7], due to which the plasma concentration of NT-proBNP is several times higher than that of BNP. In contrast, it was recently shown that the complete fragment of BNP could not be detected in the blood sample of patients with heart failure having high BNP32 levels[8]. Thus, the detection of BNP is possible only by a complex method using a mixture of BNP chains with different lengths and cross-reaction with other members of the natriuretic peptide family[9].
BNP and NT-proBNP have been introduced into clinical practice owing to their stability in biological specimens and their utility in the management of suspected or established heart failure (diagnosis, prognosis, and guided medical treatment). In other words, NT-proBNP is the best biomarker of cardiac impairment as it is relatively stable in plasma and can be easily detected[10]. However, the limit of detection of the current NT-proBNP fluorescence lateral flow assay (LFA) is 220 pg/mL[11]. Hence, it is necessary to develop a highly sensitive detection method for NT-proBNP because of its low apparent concentration in early cardiac impairment. In the present study, based on a near-infrared point-of-care diagnostic (POCT) device, we developed a highly sensitive lateral flow detection method for NT-proBNP. This method is more rapid than that based on molecular motors[12], and the measured results also showed a good linear correlation with the results measured by the Roche E411 kit.
-
NT-proBNP capture antibody, labeled antibody, and recombinant NT-proBNP protein (as the standard) were purchased from HyTest Ltd. (Turku, Finland). Polyclonal goat anti-chicken IgY antibody and chicken IgY antibody were purchased from Biocare Biotech (Zhuhai, Guangdong, China). Sixty-two NT-proBNP plasma samples were collected from Beijing Hospital, and the near-infrared fluorescent dye Dylight-800 was purchased from Thermoelectric Company (Thermo Scientific, Illinois, USA). The buffer reagents, including sucrose, sodium azide, Tween-20, N, N-dimethylformamide (DMF), and isopropyl alcohol were purchased from Sigma-Aldrich (Darmstadt, Hessia, Germany), and phosphate buffer (PB) and borate were procured from Invitrogen China (Shanghai, China). The near-infrared POCT device Smart Scanner-1 was developed by Beijing Run Bio Biotechnology Development Co. Ltd.
-
The monoclonal antibody was conjugated with Dylight-800 according to the instructions of the DyLight Amine-Reactive Dye kit. Amine-reactive dyes contain N-hydroxysuccinimide (NHS) esters, which are the simplest and most commonly used reactive group for labeling proteins. NHS esters react with primary amines on the protein, forming a stable, covalent amide bond and releasing the NHS group. Briefly, 1 mg Dylight-800 was dissolved in 100 µL DMF. The capture and label antibodies were diluted to 1 mg/mL in 0.05 mol/L sodium borate buffer (pH 8.5), and then 7 µL Dylight-800 was transferred to the reaction tube containing 1 mg of the labeled antibody. The reaction solution was mixed thoroughly and then incubated for 1 h at room temperature. The nonreacted reagent was separated from the protein by dialysis. The labeled protein was stored at 4 ℃ in the dark. Chicken IgY antibody was also labeled in a similar manner.
-
The capture antibody and goat anti-chicken IgY antibody were diluted in a buffer containing 50 mmol/L PB (pH 7.0), 2% sucrose, 2% isopropyl alcohol, and 0.1 g/L sodium azide. The capture antibody and goat anti-chicken IgY were deposited onto a nitrocellulose membrane (Merck Millipore, Darmstadt Germany) at a concentration of 1.2 µL/cm using a Shanghai Kinbio HM3025. The striped membranes were dried at 42 ℃ for 45 min and stored in an aluminum foil bag with a desiccant until use. The conjugated antibodies (detection antibody and IgY) were diluted to 1:2000 using 10 mmol/L phosphate-buffered saline (PBS), 0.5% Tween-20, 1% polyvinylpyrrolidone, 2% sucrose, 0.01% sodium azide, and 10 mmol/L Tris-Cl (pH 7.4). Then, 2.15 µL of this solution was applied on a 1-mm conjugation pad and then dried at 37 ℃ for 2 h. The striped membrane, the conjugate release pad, and the absorbent pad were assembled together on a backing card with a 2-mm overlap between each component. The nitrocellulose membrane was cut into 3.5-mm-wide strips.
-
Human plasma (50 µL) contained in EDTA tubes was pipetted onto the sample pad. This was followed by pipetting out 50 µL of wash buffer (0.01 mol/L PBS, 1% Tween-20, 2% sucrose, 10 mmol/L Tris-Cl, and 0.01% sodium azide) onto the sample pad. After 15 min, the fluorescence signal was automatically scanned by a near-infrared POCT device (RunBio, Beijing, China), and the ratio of the fluorescence intensity of the test line to that of the control line was directly outputted. The corresponding concentration of NT-proBNP can be directly displayed if the work curve has been plotted.
-
The standard sample was diluted with NT-proBNP-free plasma, and 50 µL of each sample was added to the sample window. After 15 min at room temperature, the test card was inserted into the POCT device. The corresponding ratio of the fluorescence intensity of the test line to that of the control line was recorded for each standard concentration of NT-proBNP. The relationship between the ratio value and its corresponding concentration of NT-proBNP was plotted as the so-called work curve.
-
A total of 62 plasma samples were collected from patients with heart failure and from healthy subjects, and quantitative detection was performed using the Roche E411 NT-proBNP kit and by the method we developed in this study. Plasma samples (50 µL) were applied to the release pad of each test strip and allowed to run until completion. After 15 min, the card was inserted into the POCT device, and the concentration of NT-proBNP was automatically calculated. The sample concentration values obtained from the two detection methods were plotted in a log-log graph, and the linear correlation coefficient was calculated.
Reagents and Apparatus
Conjugation of Antibody with Near-infrared Fluorescent Dye
LFA Preparation
LFA
Work Curve
Comparison of Test Results of the Clinical Specimens
-
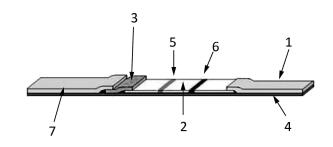
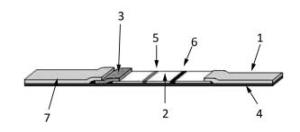
The LFA strip consists of a backing plate ④, an absorbent paper ①, a nitrocellulose membrane ②, a conjugation pad ③, and a sample pad ⑦ (Figure 1). When the sample is dropped onto the sample pad, it runs into the conjugation pad and then to the nitrocellulose membrane owing to the capillary effect. The target molecule in the sample binds to the labeled antibody fixed on the conjugation pad to form a complex. When the complex moves toward the detection line, it is intercepted by the capture molecule on the detection Line ⑤ to form a capture molecule-target-labeled molecule complex. The fluorescence-labeled IgY, which is the internal standard, moves toward the quality control line ⑥ and binds to the polyclonal goat anti-chicken IgY antibody. When an excitation light with a wavelength of 770 nm irradiates the quality control line and in turn the detection line, an excitation light with a wavelength of 794 nm, whose intensity is proportional to the number of fluorescent molecules, is emitted by DyLight-800 and detected by the POCT device.
-
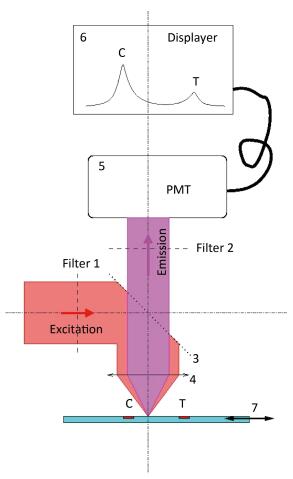
Figure 2 shows the mechanism of detection of the POCT device. A parallel excitation light with a wavelength of 770 nm is first outputted from filter 1 ①, which is then reflected completely by the light splitter ③ and finally focused on the LFA strip ⑦ by an objective lens ④. As a result, the irradiated strip emits another light, which is the so-called emission light. The parallel emission light is outputted from the objective lens ④, which then transmits it to the light splitter ③. The parallel emission light with a wavelength of 794 nm, which is emitted by Dylitht-800 in the sample and represents the measured signal, is outputted from filter 2 ② and finally detected by photomultiplier (PMT) ⑤. The two pulses that reflect the fluorescence intensities of the test line (T) and the control line (C), respectively, are directly displayed ⑥ in the POCT device. The ratio of the area covered by the T pulse to that covered by the C pulse can be automatically calculated. The ratio value, combined with the corresponding concentration of NT-proBNP, can be used to develop the work curves. After fixing the work curves in the POCT device, the unknown concentration of NT-proBNP can then be directly detected according to the linear mapping of the work curves as shown in Figure 3.
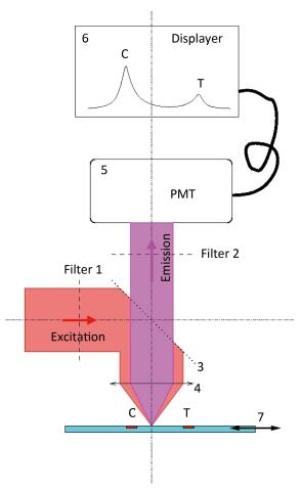
Figure 2. The mechanism of detection of the near-infrared POCT device. ① filter 1, ② filter 2, ③ light splitter, ④ objective lens, ⑤ PMT, ⑥ displayer, ⑦ LFA strip. An additional drive device is in charge of the one-dimensional movement of the LFA strip for scanning the two lines (T and C).

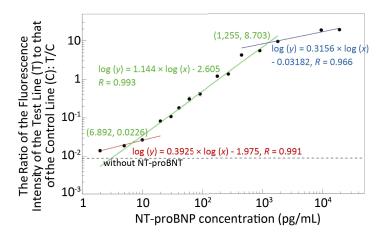
Figure 3. Work curves of NT-proBNP detection based on the near-infrared POCT device. The concentration range of NT-proBNP from 2 pg/mL to 20 ng/mL can be divided into three linear intervals. The low interval (red) is between 2 pg/mL and 6.892 pg/mL, the medium interval (green) is between 6.892 pg/mL and 1.255 ng/mL, and the high interval (blue) is between 1.255 ng/mL and 20 ng/mL. The results in each interval can be fitted linearly by a corresponding work curve in a log-log manner with correlation coefficients (R) of 0.991, 0.993, and 0.966, respectively.
-
DyLight-800 NHS ester is a near-infrared light fluorescent molecule with excitation and emission wavelengths of 770 and 794 nm, respectively. The antibody-to-fluorescent molecule ratio was about 1:6, as calculated by adding 1 mg of the antibody to 7 μL of the fluorescent molecule (10 mg/mL). After the condensation reaction, the near-infrared fluorescent molecule-labeled antibody remained stable for a long time at 4 ℃.
-
Fluorescence-labeled chicken IgY antibody was added to the NT-proBNP test strip antibody release pad (conjugate pad). The fluorescence intensity of the control line was relatively constant because IgY interacts only with the goat anti-chicken IgY antibody on the control line and not with the mouse anti-NT-proBNP monoclonal antibody. The fluorescence intensity of the detection line reflects only the amount of mAb-NT-proBNP complex detected. After diluting the standard series, the NT-proBNP rapid test strip was used for detecting the ratio of the fluorescence intensity of the detection line to that of the control line for each concentration of NT-proBNP. The working curve was obtained by plotting the ratio value of the fluorescence intensity and the NT-proBNP concentration. The results are shown in Figure 3 with a log-log plot. The concentration range of NT-proBNP from 2 pg/mL to 20 ng/mL can be divided into three linear intervals. The results in each interval can be fitted linearly by a corresponding work curve in a log-log manner with correlation coefficients (R) of 0.991, 0.9933, and 0.966, respectively. The work curves were then plotted into the POCT device, which, combined with the LFA strip, can now detect the NT-proBNP-free plasma whose concentration is unknown.
-
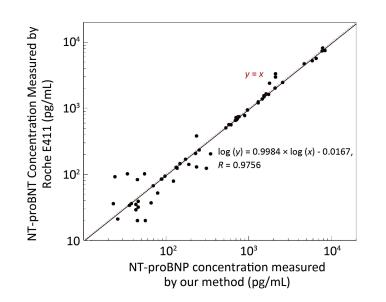
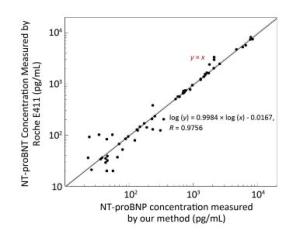
A total of 62 plasma samples were collected from Beijing Hospital and measured in parallel by our method and the Roche E411 kit. The results were compared using a log-log plot due to the large concentration range from 20 pg/mL to 10 ng/mL, as shown in Figure 4. The results showed a good linear correlation with a correlation coefficient (R) of 0.9756.
LFA Strip Preparation
Design of Near-infrared POCT Device
Conjugation of Antibody with Near-infrared Fluorescent Dye
Work Curve
Comparison of Test Results of the Clinical Specimens
-
Acute heart failure (AHF) is an important disease that seriously impacts the life expectancy. According to China's official cardiovascular disease report in 2016, an upward trend of AHF-related morbidity and mortality was observed between 2002 and 2014 in China. In particular, AHF-related mortality showed a more obvious increasing rate in rural areas, at 68.6% in a sample of 100, 000 individuals, whereas it was 55.32% in the urban areas in 2014. Timely diagnosis and symptomatic treatment in the early stages of onset are important to save the life of patients with AHF. Currently, rapid detection of emergency cases is difficult because the biochemical analyzers used by large hospitals are time-consuming and tedious to operate. Therefore, an urgent need exists for accurate, quantitative bedside diagnostic reagents and portable devices.
Currently, NT-proBNP levels are detected by methods such as electrochemical luminescence assay, enzyme-linked immunosorbent assay, and LFA[13]. Of these, the LFA is the most commonly used POCT test method. However, as the currently commercialized LFA methods use high-energy ultraviolet irradiation for excitation, the tomographic backplane, the membrane, and the matrix also emit fluorescence, thereby causing a high background noise, which in turn reduces the sensitivity of detection[14-18]. In contrast, our method uses low-energy near-infrared light (770 nm) for excitation, and thus the sample matrix and the tomographic materials do not emit fluorescence whose wavelength is higher than 770 nm[19]. Therefore, the near-infrared fluorescent dye markers ensure that the detection has higher sensitivity due to lower background noise. In addition, the PMT detector supplies a very large detection span from 2 pg/mL to 25 ng/mL as shown in Figure 3. Therefore, our method appears to have great potential for clinical applications.
In a future follow-up study, we intend to use a larger clinical sample size and conduct a greater number of alignment experiments to verify the feasibility of this method for the rapid detection of AHF, especially in a lower concentration interval as shown in Figure 4.
the Workstation of Academician ZENG Yi project 2014IC027







 Quick Links
Quick Links
 DownLoad:
DownLoad: