-
Acinetobacter baumannii is an opportunistic pathogen which causes infections among hospital patients who are injured, immuno-compromised, or in the intensive care unit (ICU)[1]. These infections are becoming increasingly untreatable with current therapy because of the emergence of multi-drug resistant strains and the lack of suitable new antibiotics. Vaccination may be an alternative treatment for controlling this emerging pathogen, however, there is currently no vaccine available against A. baumannii. The research and development of an effective A. baumannii vaccine for clinical use is urgently needed.
Several A. baumannii vaccine candidates have been investigated in the past few years, including those based on formalin-inactivated whole cells (IWCs), outer membrane vesicles (OMVs), and recombinant antigens. IWC or OMV immunized animals often exhibit high antibody titers, resulting in effective protection against A. baumannii challenge. However, neither is an ideal vaccine candidate because of their complex compositions and potential safety concerns. Recombinant antigens (such as the biofilm-associated protein Bap, the outer membrane protein OmpA, the outer membrane protein OmpK, and Omp22) have been proved to be crucial antigens for A. baumannii vaccine design in animal models[2-5], though a recent study found recombinant single outer membrane proteins sometimes provided only weak protection against A. baumannii infection or poor cross-protection against certain strains[6]. Consequently, some researchers have suggested that multivalent subunit vaccines may be the most promising candidates.
Pathogen-free BALB/c female mice were obtained from the Laboratory Animal Center of Chongqing Medical University (Chongqing, China) and housed in a specific-pathogen-free laboratory, six to a cage and fed a commercial diet. Animal experiments were performed in accordance with the guidelines of the Chinese Council on Animal Care. The mice were 6-8 weeks old at the time of vaccination. A. baumannii ATCC19606 strain was obtained from China General Microbiological Culture Collection Center (CGMCC, China). A clinically isolated A. baumannii strain was obtained from the teaching hospital of North Sichuan Medical College and identified using a VITEK® 2-Compact automatic bacteria identification device and PCR. Prokaryotic expressing vector pColdI and Escherichia coli BL21 (DE3) strain were purchased from Takara Company.
Chromosomal DNA of A. baumannii ATCC19606 was isolated using a bacterial genomic DNA extraction kit and then used as a template for PCR. Primers used in this study are as follow: P1, 5′-GCGGATCCATGCAATTAAAGCAACTTG-3′; P2, 5′-GCGTCGACTTAGAAGTGGTATTTAACCA-3′; P3, 5′-TAACTAATGCACGCATGAAGTGGTATTTAACC-3′; P4, 5′-GGTTAAATACCACTTCATGCGTGCATTAGTTA-3′; P5, 5′-GCGGATCCATGCGTGCATTAGTTA-3′; P6, 5′-GCGTCGACTTATTGTTTAGCATAAATG-3′. Primers P1 and P2 were used for amplification of the gene encoding OmpK, and P5/P6 for the gene encoding Omp22. The fused gene encoding OmpK/Omp22 fusion protein was generated by using overlap extension, with P1 and P3 used for amplification of the ompK gene fragment, P4/P6 for omp22 gene fragment, and P1/P6 were used to amplify the fused gene with the mixture of ompK and omp22 gene fragments as the template. The PCR products were digested with restriction enzyme BamHI/SalI and cloned into the corresponding site of the prokaryotic expressing plasmid pColdI. This process produced a recombinant plasmid named pCold-K/22 expressing OmpK/Omp22 fusion protein, and two individual antigen-expressing plasmids named pCold-K and pCold-22. Protein expression and purification were conducted as described previously[7]. The purified protein was refolded using the urea gradient dialysis method according to the manufacturer's (Qiagen) guidelines. Protein concentration was determined using a Bradford Protein Assay kit.
BALB/c mice were randomly divided into four groups: OmpK, Omp22, OmpK/Omp22 immunized groups, and a control group. Mice in the treatment groups were immunized subcutaneously with 20 μg of the appropriate recombinant protein in 50 μL PBS formulated with an equal volume of Freund's Complete Adjuvant (Sigma), and boosted with the same dose of corresponding protein formulated with Freund's Incomplete Adjuvant (Sigma) at day 14 and 21. Mice in the control group were injected subcutaneously with 50 μL PBS plus 50 μL Freund's Complete Adjuvant on the first day and 50 μL PBS plus 50 μL Freund's Incomplete Adjuvant at day 14 and 21.
The A. baumannii clinically isolated strain was grown to late-logarithmic phase in LB broth at 37 ℃/150 rpm. Cells were harvested by centrifugation at 6, 000 ×g for 10 min, washed, and resuspended in PBS. Three weeks after receiving their third immunization, mice in each group were injected intraperitoneally with 2 × 108 CFU/per mouse of A. baumannii cells. Six mice were randomly selected from each group for the detection of bacterial load in the blood, and evaluation of pathological changes in the lung tissue. The remaining mice were observed daily to check survival rate.
After blood samples had been aseptically taken from the caudal vein of 6 mice in each group 12 h post-challenge, 10 μL of each blood sample were serially diluted by PBS and spread plated on Luria agar followed by incubation at 37 ℃ overnight. From the number of colony forming units (CFU) present the total bacterial CFU in 1 mL blood was calculated. Results were expressed as mean log10 CFU in 1 mL blood.
Three mice in each group were sacrificed 24 h post challenge, the lung tissue was aseptically collected and fixed in 10% buffered formalin, stained with hematoxylin-eosin and observed under microscope at ×100 magnification. Pathological changes were recorded by a veterinary pathologist with no prior knowledge of the treatment groups.
Statistical analysis was performed with SPSS software version 19.0 (SPSS Inc, Chicago, IL, USA). Data were analyzed using analysis of variance (ANOVA) followed by Dunnett's test. The data were considered significantly different at P < 0.05.
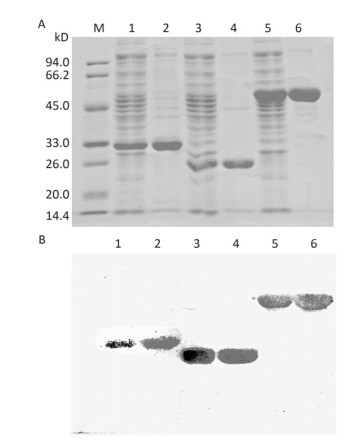
The genes encoding OmpK, Omp22, and OmpK/Omp22 fusion protein were acquired by PCR amplification, and the corresponding recombinant plasmids were successfully constructed and identified by DNA sequencing. The recombinant plasmids were separately transformed into E.coli BL21 strain and expressed by IPTG induction. Due to over-expression, the recombinant proteins formed inclusion bodies and accumulated in the insoluble fraction. After purification using Ni-affinity chromatography, highly-purified OmpK, Omp22, and OmpK/Omp22 fusion protein were obtained and confirmed by SDS-PAGE (Figure 1A) and Western-blot analysis (Figure 1B). Protein concentration detection showed that at least 5 mg of highly purified proteins could be obtained from 300 mL of bacterial culture.

Figure 1. SDS-PAGE and Western-blot analysis of purified recombinant proteins. (A) 12% SDS-PAGE; (B) Western-blot analysis of the recombinant proteins using anti-6×His antibody. Lane M: standard protein markers; Lane 1: whole bacterial lysate after induction of OmpK expression by IPTG; Lane 2: purified recombinant OmpK protein; Lane 3: whole bacterial lysate after induction of Omp22 expression by IPTG; Lane 4: purified recombinant Omp22 protein; Lane 5: whole bacterial lysate after induction of OmpK/Omp22 fusion protein expression by IPTG; Lane 6: purified recombinant OmpK/Omp22 fusion protein.
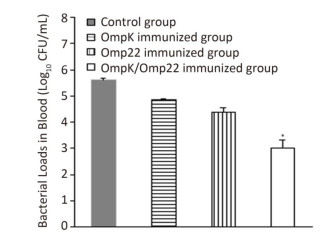
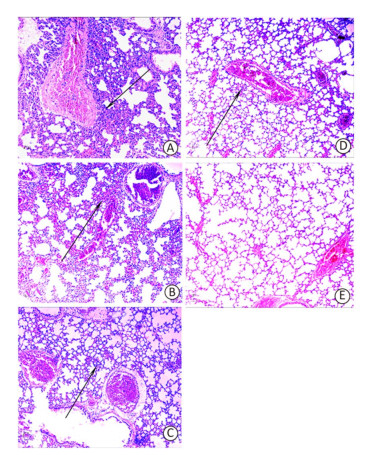
Although the majority of researchers believe that hospital-acquired and ventilator-associated pneumonia (VAP) are the most common infections caused by A. baumannii, bacteremia caused by A. baumannii has been identified increasingly in critical patients admitted to ICUs[8, 9]. Therefore, in this study, rather than use the pneumonia model to evaluate the protective efficacy of the recombinant proteins, we employed a mouse sepsis model. Post-treatment, the control mice (treated with only PBS and adjuvant) developed severe bacteremia, both the OmpK and Omp22 immunized mice showed moderate bacteremia, while only a small number of bacteria were detected in the blood of the OmpK/Omp22 fusion protein immunized mice (Figure 2). At 24 h post challenge, the most severe pathological changes were detected in the lung tissue from the control group, with widespread and severe interstitial pneumonia and intensive infiltration of inflammatory cells in the perivascular areas evident. Moderate interstitial pneumonia and a reduced number of inflammatory cells were found in the lung tissue from OmpK or Omp22 immunized mice. Only minor pathological changes were found in OmpK/Omp22 fusion protein immunized mice, with the lung tissue appearing almost normal and only very few inflammatory cells in the perivascular areas (Figure 3). The survival rate of mice immunized with OmpK/Omp22 (66.7%) was higher than either OmpK or Omp22 immunized mice (25% and 37.5%, respectively) to eight days post-challenge, while all the control mice had died by 72 hours post-challenge. From these results, we conclude that OmpK/Omp22 fused antigen provides a significantly greater protective effect than either of these antigens individually.
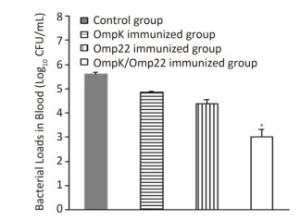
Figure 2. Bacterial load in mouse blood 12 h post-challenge (n = 6). Bars indicate mean log10 CFU ± standard deviation of six mice in each group. *The value is significantly different from the other group at P < 0.01.
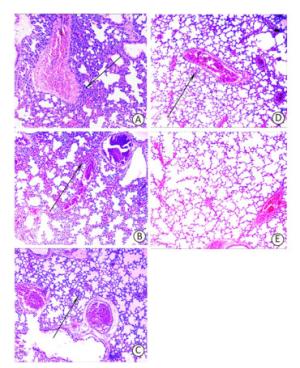
Figure 3. Mouse lung histopathology 24 h post-challenge. (A) Control, showing intensive inflammatory cell infiltration in the perivascular areas (arrow). (B) OmpK immunized, showing moderate inflammatory cell infiltration in the perivascular areas (arrow). (C) Omp22 immunized, showing moderate to minor inflammatory cell infiltration in the perivascular areas (arrow). (D) OmpK/Omp22 immunized, showing very minor inflammatory cell infiltration in the perivascular areas (arrow). (E) Unimmunized, uninfected mouse showing normal histological characteristics. (HE, ×100).
The response to fused antigen or antigen mixture is superior to that for the antigens individually. This is probably because they contain more antigenic epitopes which widen the active T and B cell repertoire against A. baumannii. It can also be concluded that fused antigens (or antigen mixture) elicit a synergistic effect while stimulating the host's immune system. Since fused antigens could be recognized and processed by the same antigen-presenting cells, they may produce a more potent synergistic effect than the corresponding antigen mixtures. Yet, as shown in this and several other studies, the protection provided by these fusion antigens and antigen mixture is still not entirely satisfactory[10]. Ideally, future vaccine design studies should involve a greater number of immunodominant antigens of A. baumanii, as it is likely that a multi-epitope subunit vaccine would be optimal.
In summary, the efficacy of a recombinant OmpK/Omp22 fusion protein and two individual antigens OmpK and Omp22 were evaluated and compared for protection against A. baumanii in BALB/c mice. OmpK/Omp22 induced a more potent protective immunity than either of the two antigens individually. These results provide novel clues for the future development of an effective vaccine against A. baumannii.
We wish to express our gratitude to HE Xing Rong, and LI Yang You for their assistance with experiments.
HTML
a project from Educational Commission of Sichuan Province of China 17ZA0166







 Quick Links
Quick Links
 DownLoad:
DownLoad: