HTML
-
The connection between the environment and health is an important issue that afflicts the global health system. Climate change and ambient pollution not only cause serious economic losses but also considerably threaten human health. World Heart Day, 2017, reported that 17.7 million people per year have died due to cardiovascular diseases (CVDs); this figure is more than 31% of global deaths, thus, CVDs are now the leading cause of death in the world. In recent years, the occurrence of high-temperature heat waves caused by intensifying climate change have been frequent. Also, ozone concentrations have risen due to ambient air pollution. Therefore, studying the interactive effects of heat waves and ozone on atherosclerosis (AS) mice is particularly important.
A heat wave is a type of sustained hot weather. Epidemiological studies have shown that the risk of CVD increased significantly during heat wave exposure, especially for elderly patients[1-3]. In addition, high temperature is conducive to ozone production, as temperature is positively correlated with near-surface ozone concentration[4]. Ozone, as another cardiovascular risk factor, is an atmospheric secondary pollutant with robust oxidizing properties. Studies have suggested that morbidity and mortality due to CVDs increase dramatically with acute or long-term ozone exposure, the mechanism of which is associated with systemic inflammatory injury and cardiac autonomic dysfunction[5-7]. Thus far, extreme weather and ozone pollution events have remained increasingly prominent, whereas research on the interactive effects of heat waves and ozone exposure on CVDs has not been reported; currently, their mechanisms are unknown.
CVD events have been reported to peak mainly during the summer season[8]. Therefore, we simulated an actual heat wave and ozone pollution process that occurred in July 2017 in Shijiazhuang, China as our model. We selected AS model mice which received a combined exposure of high heat and ozone concentrations by the Shanghai-METAS. The interactive effects after exposure were explored from myocardial anti-oxidation capability, inflammatory response, and endothelial and coagulation system function on ApoE−/− mice.
-
The Shanghai-METAS was provided by Zhejiang Qiushi Artificial Environment Co., Ltd. This system can simulate a real environment where meteorological elements (temperature, humidity, and pressure) and harmful gases (ozone) are individually or synergistically controlled. Temperature can range from -10 ℃ to 70 ℃ (with deviations of ± 1.0 ℃) and humidity can range from 30% to 90% RH (with deviations of ± 3% RH). The ozone accuracy is approximately 300 ppb. This animal exposure system itself did not result in additional environment variations and injury to animals. Moreover, the system also ensured fresh air (oxygen) injection during exposure.
A TH212 special thermal detector from Beijing Hong'ou Technology Co., Ltd., was used to measure temperature. A BP-2006 intelligent non-invasive blood pressure monitor (Beijing Soft Dragon Biotechnology Co., Ltd.), Tissue-Tearor Homogenizer (Biospec Products, America), Infinite M200 Pro NanoQuant Microplate Reader (Tecan Products, Switzerland), and electronic balance were used in this experiment.
In addition, 7% chloral hydrate solution and assay kits were used in this study. The assay kits included NO (microplate method), CK (colorimetric method), SOD (WST-1 method), D-LDH, HSP-60, TNF-α, IL-6, sICAM-1, HIF-1α, IFN-γ, MDA, ET-1, D2D, and PAI-1 ELISA kits, These kits were provided by Elabscience Biotechnology Co., Ltd. Blood lipids (including T-CHO, TG, LDL-C, HDL-C) biochemical test kits were purchased from Nanjing Jiancheng Biological Co., Ltd.
-
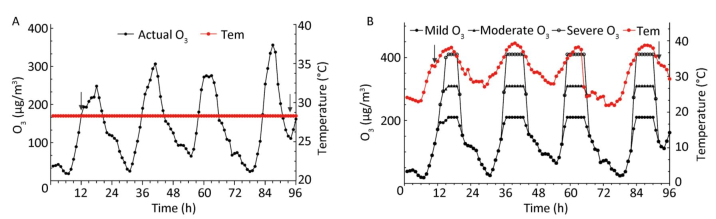
We collected per hour meteorological data (temperature, humidity, and wind speed) and air quality data from June to August, 2017 in Shijiazhuang. We used the heat wave standard based on Huang[9] and China Meteorological Administration regulations. Ozone was evaluated as the hourly average concentration in accordance with Ambient Air Quality Standards (GB3095-2012). The criteria for mild, moderate, and severe ozone pollution was set to 200 µg/m3, 300 µg/m3, and 400 µg/m3, correspondingly, to reflect the effect of different degrees of ozone exposure on mice. In addition, considering the safety of animals, we only simulated an actual heat wave and ozone pollution process for July 2017 in Shijiazhuang. The experiment ran from 1 a.m. on 7 July 2017 to 3 a.m. on 11 July 2017, thereby sustaining for more than 9 days. The temperature of the control group was set to 28.3 ℃ (the average summer temperature in July 2017 in Shijiazhuang). Figure 1 illustrates the simulation curve in which animals were exposed over 4 days with the 3 different ozone levels.
-
A total of 64 8-week-old specific-pathogen-free male ApoE−/− mice with an average weight of 18.0 ± 2.0 g were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. To develop the atherosclerotic model, ApoE−/− mice were fed with a high-fat diet (10% lard, 10% cholesterol, 2% bile salt, and 78% basal feed) for eight weeks. A high-fat diet has been confirmed to induce AS plate and thickening in the aorta and coronary artery wall[10]. Feed was purchased from Beijing Ke'Ao Xieli Feed Co., Ltd. Experimental mice were maintained in an animal house in standard cages at 28.3 ℃ (the average temperature in July), 40%-60% RH, and a 12:12 h light-dark cycle. All mice were provided with sufficient water and chew and trained several times daily to minimize the additional effects of capture during the experiment.
The 64 ApoE−/− mice were divided into eight blocks (n = 8) matched by weight. One mouse from each block was assigned to the control, and the others were assigned OMi, OMo, OS, Hw, H+OMi, H+OMo, or H+OS, as displayed in Table 1. The animal protocols used in this study were approved by the Animal Use and Ethic Committee of the Institute of Arid Meteorology, China Meteorological Administration (Grant NO. 2017_1).
Group Constant Temperature Heat Wave Ozone exposure — Control Hw Mild OMi H+OMi Moderate OMo H+OMo Severe OS H+OS Note. Hw indicates heat wave exposure; OMi, OMo, and OS correspond to mild, moderate and severe ozone exposure; and H+OMi, H+OMo, and H+OS refer to heat wave exposure plus mild, moderate and severe ozone exposure, respectively. n = 8/group. Table 1. Animal Grouping Protocol (n = 8)
-
All mice were kept in an adaptation period for 7 days in accordance with heat wave and ozone simulation curves before the exposure. Ozone-alone exposure and heat wave groups were randomly placed into the control cabin when the exposure system was stable. Simultaneously, the heat wave plus ozone groups were placed in the exposure cabin which was exposed to high heat and different levels of ozone, as depicted in Figure 1.
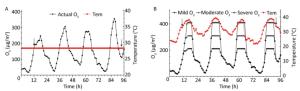
Figure 1. Experimental simulation curves of ozone and heat wave exposure (A and B). Tem indicates temperature, and the arrows represent sampling time points when basic physiological function indexes of ApoE−/− mice were measured.
Experimental mice were first removed, and the body weight, rectal temperature, heart rate, and blood pressure were measured when the exposure reached 72 h and the cabin temperature reached 28.3 ℃.
-
Experimental mice were anesthetized with 7% chloral hydrate (0.3 mL/100 g) and sacrificed through decollation. Blood samples (0.8-1.0 mL each) were collected immediately into centrifuge tubes. Samples were then centrifuged at 3, 000 rpm for 10 min at 4 ℃ to collect the plasma which was isolated and stored at -20 ℃ for future analysis. Removed hearts after the cardiac apex weighing 10 mg were homogenized in ice-cold 0.9% saline with Tissue-Tearor Homogenizer and centrifuged at 3, 000 rpm for 10 min at 4 ℃. The supernatant was collected and immediately kept at -20 ℃ until analysis.
The frozen plasma and heart homogenates were melted at 37 ℃ before detection. The levels of D-LDH, sICAM-1, TNF-α, ET-1, D2D, and PAI-1 in plasma and the contents of HSP60, HIF-1α, IL-6, CRP, and MDA in heart homogenates were detected using ELISA kits and a microplate reader. The activity of plasma CK and NO was determined by applying colorimetry and microplate methods, respectively, and the activity of SOD in the heart was determined through the WST-1 method.
-
Data were expressed as the mean ± standard deviation. All statistical analyses were performed with SPSS 22.0 IBM statistical software. The differences between paired groups were determined using a paired t-test. One-way analysis of variance and least significant difference test were used when three or more groups were compared. P < 0.05 was presented as statistical significance.
Equipment and Materials
Establishment of Experimental Simulation Curves
Animals and Grouping
Exposure Scheme
Sample Collections and Biomarker Estimations
Statistical Analysis
-
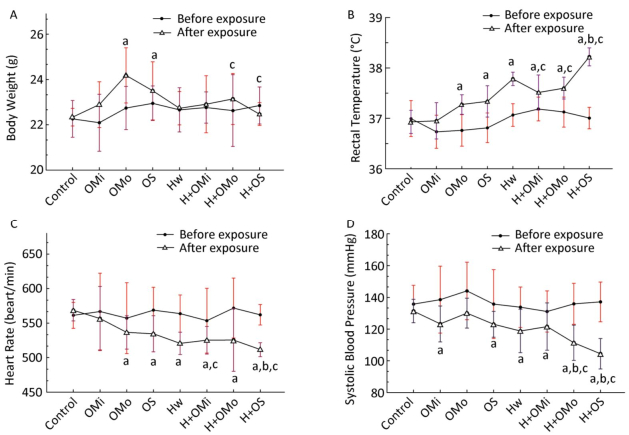
The basic physiological function indicators of AS mice were monitored during the entire exposure process, as demonstrated in Figure 2.

Figure 2. Basic physiological function indicator changes in all groups. (A) Body weight; (B) Rectal temperature; (C) Heart rate; (D) Systolic blood pressure. Hw indicates heat wave; OMi, OMo, and OS refer to mild, moderate and severe ozone exposure groups, respectively. Data were expressed as mean ± SD, n = 8/group. (aP < 0.05 compared with the control group; bP < 0.05 compared with the heat wave group; cP < 0.05 compared with the corresponding ozone-only exposure group).
The body weight of groups that experienced moderate and severe combined exposure decreased slower than the corresponding ozone group (P < 0.05). Compared with growth before exposure, heat and ozone exposure were shown to delay the growth of mice or even cause a shrinking of mice. The body temperature of mice was higher in each group than in the control group. The body temperature significantly increased in combined exposure groups (P < 0.05) compared with the heat wave or ozone-alone group, especially during heat exposure combined with severe ozone concentrations. Changes in heart rate and blood pressure are displayed in Figure 2. Compared with the control group, the heart rate of all mice significantly decreased with the increase in ozone concentration. That is, the heart rate was considerably lower in combined exposure groups than in the corresponding ozone-only group (P < 0.05). Similarly, compared with the heat wave and ozone-only groups, a declining trend in heart rate was detected in the severe combined exposure group (P < 0.05). Blood pressure was significantly lower in all groups than in the control group, whilst the differences in the moderate ozone exposure group were statistically insignificant (P > 0.05). Blood pressure presented a significant downward trend (P < 0.05) at moderate and severe combined exposure groups, especially compared with the corresponding ozone and heat wave exposure groups. Also, results indicated that ozone exposure combined with high heat exposure can retard the growth of mice, increase rectal temperature, and decrease heart rate and blood pressure.
-
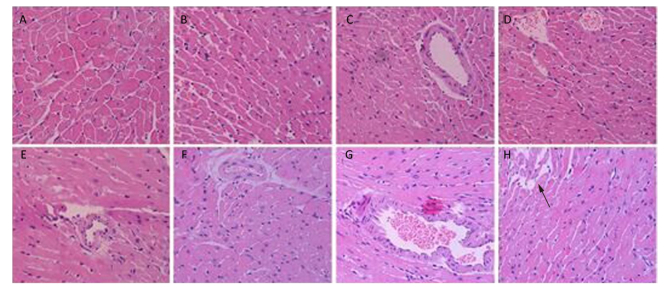
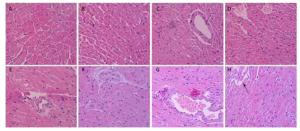
Figure 3 shows the pathological cardiovascular changes from heat wave and ozone exposure. The myocardial structure of the control group was essentially intact (Figure 3A). With an increase in ozone exposure, in the OMi and OMo groups, the smooth muscle cells were disordered, fat cells accumulated, and typical plaque formed (Figure 3D). Compared with the control group, the pathologic damage was more serious after ozone exposure. Under the simulation of heat waves, numerous fat cells accumulated in the intima and severe coronary stenosis or stoppage occurred (Figure 3E). With combined exposure of ozone and heat, the intima was severely damaged and massive plaques appeared (Figure 3F, 3G). In addition, smooth muscle cells proliferated and severe myocardial infarction lesions formed (Figure 3H).
-
The levels of CK and D-LDH in plasma were analyzed to evaluate the effects of heat waves and ozone exposure on heart injury, as presented in Figure 4.
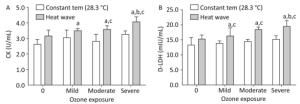
Figure 4. Heart injury factor level in plasma. (A) CK; (B) D-LDH. (aP < 0.05 compared with the control group; bP < 0.05 compared with the heat wave group; cP < 0.05 compared with the corresponding ozone-only exposure group).
For all groups except for the heat wave and ozone-only groups, the concentration of CK, was statistically elevated than in the control group (P < 0.05). Compared with the high heat and corresponding ozone-only groups, the levels of CK were enhanced in groups that experienced combined exposures; considerable changes were only apparent when ozone exceeded moderate exposure. Ozone exposure and the D-LDH of each group increased simultaneously, thus indicating a strong heart injury. The D-LDH levels of the three combined exposure groups were significantly higher than in the control and corresponding ozone groups (P < 0.05). The severe combined exposure group had the highest D-LDH levels among all exposure groups. In terms of the effects of heat waves plus ozone combined exposure on heart injury factors, the release of CK and D-LDH exhibit a dose-response relationship (P < 0.05).
-
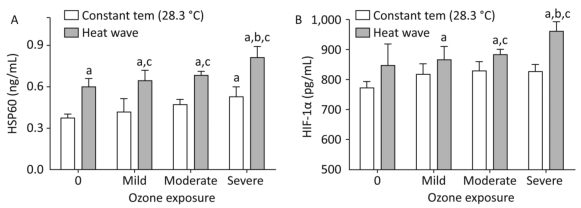
The effects of high heat and ozone exposure on the levels of HSP60 and HIF-1α in heart tissue are exhibited in Figure 5.

Figure 5. Heat stress factor level in heart tissue. (A) HSP60; (B) HIF-1α. (aP < 0.05 compared with the control group; bP < 0.05 compared with the heat wave group; cP < 0.05 compared with the corresponding ozone-only exposure group).
With statistical significance (P < 0.05), the combined exposure groups had elevated concentrations of HSP60 which indicated that this change was caused by increasing ozone exposure. In addition, the increase in HSP60 levels were more significant in the severe combined exposure group than in the heat wave or ozone-only group (P < 0.05). The HSP60 levels were higher in HIF-1α than in the control group; no significant difference (P > 0.05) was found between the heat wave and ozone exposure groups. Levels of HIF-1α simultaneously increased with the rising ozone exposure. This significant increase was discovered in the severe combined exposure group (P < 0.05). Therefore, it was concluded that heat waves can accelerate the changes in HSP60 and HIF-1α with association ozone exposure. The interactions between heat waves and ozone were significant, especially in the case of severe ozone exposure.
-
The release of mediators related to systemic inflammatory response were measured in plasma and heart tissue, and the results are displayed in Figure 6. Compared with the control group, levels of IL-6 declined slightly in the mild ozone group and increased in the heat wave and other ozone groups, although the differences were statistically insignificant (P > 0.05). In the combined exposure group, IL-6 levels increased with increased ozone exposure. Furthermore, compared with the heat wave and ozone-only groups, IL-6 only significantly increased in the severe combined exposure group (P < 0.05). Except for the mild ozone group, the concentrations of CRP in the heart tissues were significantly higher than in the control group (P < 0.05). In moderate and severe combined groups, CRP concentrations were significantly higher than in the heat wave and corresponding ozone groups (P < 0.05) by 1.44 and 1.26 times, respectively.

Figure 6. Inflammatory mediator levels in heart tissue and plasma. (A) IL-6; (B) CRP; (C) sCMA-1; and (D) TNF-α. (aP < 0.05 compared with the control group; bP < 0.05 compared with the heat wave group; cP < 0.05 compared with the corresponding ozone-alone exposure group).
Compared with the control group, the levels of sCMA-1 in all groups increased, whilst significant changes were found only in the three combined exposure groups (P < 0.05). Furthermore, additional SCMA-1 was produced in plasma when the ozone exposure reached a moderate or high level. The result indicated that the release of sCMA-1 in plasma has a dose-response relationship. In this study, the degree of O3 exposure positively correlated with the release of sCMA-1. However, the release of TNF-α showed a different trend, in which an inflection point was observed at a moderate ozone exposure. Released TNF-α was small when the ozone exposure was elevated higher than moderate. A significant difference was also found between the moderate combined group and the heat wave or corresponding ozone group (P < 0.05).
-
The SOD activity and MDA concentrations in heart tissues reflect the effects of heat and ozone exposure on oxidative injury AS mice, as depicted in Figure 7.
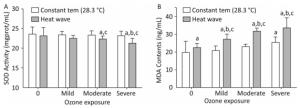
Figure 7. Oxidative stress factor level in the heart. (A) SOD activity; (B) MDA content. (aP < 0.05 compared with control group; bP < 0.05 compared with heat wave group; cP < 0.05 compared with the corresponding ozone-alone exposure group).
SOD is an antioxidant enzyme. Compared with the control group, reduced SOD activity was detected in ozone and heat wave exposure groups, while only the moderate and severe combined exposure groups demonstrated statistical significance (P < 0.05). The SOD activities in these two groups were inhibited by 64% and 68%, respectively. The control group had the highest SOD level. However, the level of MDA in all groups increased. A dose-response relationship was observed in the three combined exposure groups; when ozone exposure increased, MDA gradually increased as well.
Furthermore, significant MDA increases were more apparent in the combined exposure group compared with the heat wave and ozone-only groups (P < 0.05). The influences of heat and ozone exposure together on SOD and MDA were more pronounced than exposure to heat or ozone alone. A significant interaction was also observed between heat waves and ozone.
-
The plasma concentrations of NO and ET-1 were determined to detect changes in endothelial function of AS mice exposed to ozone during the heat wave simulation, as displayed in Figure 8.
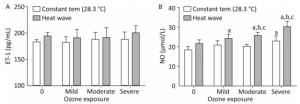
Figure 8. Vasomotor factor levels in the heart. (A) ET-1; (B) NO. (aP < 0.05 compared with the control group; bP < 0.05 compared with the heat wave groups; cP < 0.05 compared with the corresponding ozone-only exposure groups).
ET-1 is a vasoconstriction factor. In both the heat wave and ozone groups, the ET-1 levels were slightly higher than in the control group (P > 0.05). Moreover, compared with the heat wave and ozone exposure groups, the ET-1 level in the combined exposure group increased although not significantly (P > 0.05). NO is an important vascular expansion factor. Compared with the control group, NO levels were elevated in all exposure groups, particularly in the combined exposure groups. Compared with the heat wave or ozone-only groups, the moderate and severe combined exposure groups exhibited statistically significant elevated NO levels, indicating that the NO production was induced by the increase in ozone exposure. The results indicate that heat wave and ozone exposure do not significantly affect ET-1. The only significant change in NO was due to heat wave and ozone exposure.
-
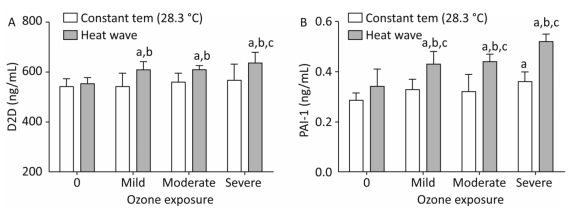
Figure 9 shows D2D and PAI-1 levels which were measured to estimate the effect of heat and ozone exposure on a coagulation system function in mice plasma.
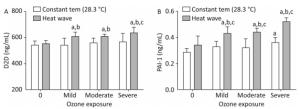
Figure 9. Thrombotic factor levels in plasma. (A) D2D; (B) PAI-1. (aP < 0.05 compared with the control group; bP < 0.05 compared with the heat wave group; cP < 0.05 compared with the corresponding ozone-only exposure group).
The D2D levels in groups with combined exposures were significantly elevated compared with those of the heat wave and control groups (P < 0.05). In addition, the D2D level was higher in the severe combined exposure group than in the ozone-alone group. However, the PAI-1 levels in the combined exposure groups were significantly different from their corresponding ozone-only exposure groups (P < 0.05). Compared with heat wave group, PAI-1 levels were found to have a positive relationship with ozone exposure. This relationship confounded with ozone exposure in the combined exposure groups (P < 0.05). A significant relationship was not observed in D2D by detecting the levels of D2D and PAI-1, while an interaction only existed in PAI-1.
-
Blood lipid levels were analyzed to assess the risks of CVDs in AS mice caused by ozone exposure during a heat wave simulation as summarized in Table 2.
Groups T-CHO/(mmol/L) TG/(mmol/L) HDL-C/(mmol/L) LDL-C/(mmol/L) Control 3.73 ± 0.37 1.93 ± 0.29 0.43 ± 0.06 1.89 ± 0.34 Hw 4.61 ± 0.47 3.19 ± 0.53* 0.38 ± 0.03 2.69 ± 0.09* OMi 4.23 ± 0.22 2.23 ± 0.13 0.41 ± 0.08 1.97 ± 0.18 OMo 4.10 ± 0.56 2.56 ± 0.39* 0.39 ± 0.06 2.23 ± 0.25 OS 5.01 ± 0.39* 2.89 ± 0.37* 0.36 ± 0.02 2.84 ± 0.27* H+OMi 5.13 ± 0.31*Δ 3.08 ± 0.43*Δ 0.29 ± 0.04*#Δ 3.10 ± 0.13*Δ H+OMo 5.54 ± 0.54*#Δ 3.37 ± 0.17*#Δ 0.25 ± 0.03*#Δ 3.44 ± 0.31*#Δ H+OS 5.59 ± 0.72*#Δ 4.14 ± 0.38*#Δ 0.21 ± 0.05*#Δ 3.85 ± 0.45*#Δ Note. Hw represents heat wave; OMi, OMo, and OS correspond to mild, moderate and severe ozone. Data were expressed as mean ± SE, n = 8/group. (*P < 0.05 compared with the control group; #P < 0.05 compared with the heat wave group; ΔP < 0.05 compared with the corresponding ozone-only exposure group). Table 2. Analysis of the CVD Risk Factors in all ApoE−/− Mice (n = 8, Mean ± SE)
Compared with the control group, T-CHO, TG, and LDL-C levels in all groups were elevated while HDL-C levels decreased. A significant change was found in the three combined exposure groups (P < 0.05). In addition, the HDL-C levels were significantly lower in the combined exposure groups than in their corresponding ozone-only exposure groups or heat wave groups (P < 0.05). However, a distinct increase in T-CHO, TG, and LDL-C levels was found when comparing the heat wave and ozone-only exposure group. The result indicated that the levels of T-CHO, TG, LDL-C, and HDL-C in plasma have an negative dose-response relationship with the increase in ozone exposure; significant interactions were found for such factors.
Basic Physiological Functions
Cardiovascular Pathological Changes
Heart Injury Factors
Heat Stress Factors
Inflammatory Mediators
Oxidative Stress Factors
Vasomotor Factors
Thrombotic Factors
Cardiovascular Risk Factors
-
Studies have determined that ozone generated by human activities can increase the morbidity and mortality of CVD[11]. In addition, excessive greenhouse gases have resulted in global warming and increased extreme weather events (such as heat waves). These changes are suspected to result in increases in CVD related deaths[12]. With serious increases in environmental pollution problems that affect ozone and heat waves and subsequent CVDs, this problem must be urgently solved.
Our previous research suggested that a high-temperature heat wave simulation can slowly increase the body weight of ApoE−/− mice[12]. However, in this study, we found that heat waves combined with ozone exposure can retard the growth of body weight and even result in weight loss. This finding may be attributed to the inflammation of the heart, thereby leading to body discomfort and appetite loss. Body adjustment firstly started with body temperature. An experimental study showed that the rectal temperature of ApoE−/− mice considerably increased with heat exposure compared with that of healthy mice[13]. Similarly, our results indicate that heat waves and ozone exposure can induce a marked increase in rectal temperature. Zanobetti et al. found a positive correlation between blood pressure and ozone in heart disease patients[14]. However, blood pressure and heart rate substantially decreased when hypertensive rats were exposed to ozone or ozone and PM2.5 combined[15-16]. This study obtained similar results. With combined ozone and heat wave exposure, a notable decrease in blood pressure and heart rate might be due to the dysfunction of the autonomic nervous system[17].
LDH is part of the cytoplasmic enzyme and is a marker that reflects cardiovascular cytotoxicity. Clinical studies have shown that serum CK activity sharply increased from 2 h to 12 h after myocardium injury and stayed elevated for 2-3 days[18]. Studies have also suggested that a high temperature can cause damage to the heart function of rats; this damage was related to changes in arterial blood pressure and myocardial ischemia or hypoxia[19]. The results of the present study show the existence of a dose-response relationship between the LDH and CK after heat wave plus ozone exposure; LDH and CK levels are positively correlated with an increase in ozone exposure.
HSP60, a heat stress protein, can promote inflammation and release various cytokines, resulting in atherosclerotic plaque instability and even thrombosis[20]. Lee et al. demonstrated that HIF-1α was detected during acute myocardial ischemia and early necrotic myocardium[21]. A toxicology experiment found that high-temperature heat wave simulations can increase the levels of HSP60 and HIF-1α in the myocardium, thereby exacerbating the risk of coronary heart disease[12]. In our experiment, we also showed that the expression levels of HSP60 and HIF-1α increase under heat wave and ozone exposure, especially severe exposure. Notably, an interactive effect is present between the two types of exposure.
Studies have suggested that inflammation caused by heat wave or ozone exposure may play a significant role in CVD onset[22-23]. Similarly, our results indicated that a heat wave combined with ozone exposure can induce severe inflammation in ApoE−/− mice, marked by increased expressions of sICAM-1 and secretions of IL-6, CRP, and TNF-α. TNF-α, an inflammatory factor, is elevated in AS mice and induces the increase in sICAM-1 expression[12]. Ozone and PM2.5 exposure lean to an increase TNF-α and IL-6 levels, respectively[16]. CRP and plasma fibrin production by the liver can be enhanced by IL-6, leading to vascular wall destruction[22]. In addition, many studies have found that the extent of coronary inflammatory injury are associated with the sICAM-1, IL-6, and CRP levels; high levels of sICAM-1, IL-6, and CRP lead to a severe degree of stenosis[16, 22-23]. In this study, combined heat and ozone exposure had a significant effect on inflammatory factors. We also found that heat with ozone exposure combined may cause more serious heart inflammation than each factor individually.
Our previous experimental research confirmed that heat waves can reduce the SOD activity of heart tissue and accelerate the deposition of cholesterol over the blood vessel wall, thus forming AS[12]. Few studies have also shown that the cardiac function reduction caused by ozone exposure may be associated with decreased SOD activities[24]. MDA is known to be an important indicator of oxidative stress. MDA levels are closely related to speed and degree of lipid peroxidation[16]. The results from this study demonstrated that heat plus ozone exposure can cause a reduction in SOD activity and MDA levels. In particular, ApoE−/− mice may produce additional free radicals and lipid peroxides when exposed to heat waves and ozone, thereby resulting in a severe oxidative/antioxidant system imbalance. This imbalance may be an important mechanism for forming coronary AS.
The past study also showed that an increased ET-1 concentration in plasma is caused by diesel exhaust and particulate exposure, but the mechanism remains unclear[25]. Bdelkader et al. revealed that a high level of ET-1 not only prolonged vasoconstriction but also lead to pro-oxidative and pro-coagulant effects[26]. Furthermore, our previous research demonstrated that heat waves can lead to a considerable increase in NO in ApoE−/− mice, while slightly affecting ET-1[16]. However, this study shows that combined heat wave and ozone exposure can induce the observed increase in ET-1 and NO levels. Only a substantial increase in NO levels was observed with an increase in ozone exposure. Therefore, the ApoE−/− mice vasomotor balance was inclined to expand when exposed to heat waves and ozone.
Numerous studies have found that D2D is a sensitive thrombotic factor that can elevate the expression levels of the inflammatory protein-1 and IL-6. These proteins promote inflammatory reactions and lead to acute coronary syndromes (ACS)[27]. PAI-1 is an independent factor that maintains the balance of the coagulation and fibrinolysis system and is typically used to predict ACS and myocardial infarction severity[28]. In this study, we determined that heat wave plus ozone exposure may cause higher levels of PAI-1 and D2D than each factor individually. However, no change in D2D levels was found with the increase in ozone exposure.
Studies have confirmed that the increase in LDL, TG and TC and the decrease in HDL as risk factors for CVD[29-30]. The expression level of PAI-1 is enhanced by TC elevation[29], which is positively correlated with LDL[30].
Therefore, we can speculate that a high level of LDL-C or TC and a high level of PAI-1 is likely to result in AS. Our results indicated that the combined heat wave with ozone exposure groups can produce more apparent changes in T-CHO, TG, LDL-C, and HDL-C than the control groups and each factor alone. This finding indicates that heat waves may enhance the risk of CVD induced by ozone exposure, accelerate coronary thrombosis, and promote the onset of diseases such as myocardial infarction.
-
Short-term exposure to heat waves and ozone can adversely affect heart health. Moreover, heat waves may enhance the toxic effect of ozone exposure, especially when each factor is severe. In addition, an interactive toxic mechanism for heat waves plus ozone that provokes serious heart inflammation, reduces heart antioxidant capacity, destroys vascular endothelium function, and accelerates dyslipidemia and thrombosis can be realized.
-
ZHANG Shu Yu conceived this study and designed the experiments. NIU Jing Ping and ZHOU Ji were responsible for the meteorological data collection and distribution. SONG Quan Quan and LIANG Ting Ting participated in the experiment and analyzed experimental data. SONG Quan Quan, NIN Jing Ping, ZHANG Shu Yu, and ZHOU Ji wrote this manuscript. FENG Shan Shan and SONG Quan Quan edited and revised this manuscript. All the authors read and approved the final manuscript.
-
The authors declare no conflicts of interest.






 Quick Links
Quick Links
 DownLoad:
DownLoad: