HTML
-
Oxidative stress plays a key role in the pathophysiology of neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease, and multiple sclerosis (MS)[1, 2]. Antioxidant therapy is employed for the prevention and treatment of these disorders[3, 4]. In MS, an autoimmune disorder of the central nervous system described as a demyelinating disease, oxidative stress and inflammation are the distinguishing effectors that cause damage to the myelin sheaths[3, 5]. Myelin is produced in the brain by oligodendrocytes and comprises mainly cholesterol and phospholipids along with some specific proteins[6]. Under oxidative stress, cholesterol may undergo autoxidation to produce cholesterol oxides (oxysterols), especially those that are oxidized at C7[7]. Among these lipid peroxidation products, 7 beta-hydroxycholesterol (7β-OHC) and 7-ketocholesterol (7KC) are present at significantly higher levels in the cerebrospinal fluid of patients with MS[8] and AD. Oxysterols, mainly those oxidized at C7, have been associated with cytotoxic and pro-inflammatory activities, especially at elevated concentrations[9]. A growing interest in elucidating the exact mechanism underlying oxysterol-mediated cytotoxicity has emerged. Several studies have revealed the involvement of 7KC, 7β-OHC, 24(S)- hydroxycholesterol (24SOHC), 25-hydroxycholesterol (25-OHC), and 27-hydroxycholesterol (27-OHC) in numerous neurodegenerative diseases[10]. Similar to 7KC, 7β-OHC is a potent inducer of oxiapoptophagy, a complex cell death process associated with oxidative stress and mitochondrial dysfunctions that lead to apoptosis and autophagy[11-13]. Oxiapoptophagy could constitute a risk factor contributing to demyelination. It is, therefore, imperative to identify natural or synthetic molecules that can attenuate 7β-OHC-induced side-effects. As observed with 7KC, 7β-OHC is difficult to metabolize at the cellular level except in liver cells, wherein it is converted to bile acid. Thus, it is important to know the signaling pathways associated with oxysterol to prevent its deleterious effects, and to identify natural or synthetic molecules or combination of molecules such as oils and plant extracts that may prevent or reduce its toxicity. Treatment regimens that included antioxidants together with anti-inflammatory agents were more efficient than other treatments[14]. In addition, an increase in the consumption of phenolic-rich food was shown to be associated with reduced risks of oxidative stress-induced diseases[15, 16]. Further, flavonoids limit demyelination in MS and are thought to play a role against neuroinflammation and related disorders[17].
Carpobrotus edulis (C. edulis) is an edible plant used as a food and therapeutic agent in traditional medicine. This plant is used by traditional healers to treat tuberculosis, diabetes mellitus, sores, high blood pressure, intestinal worm-related infections, and constipation. C. edulis is thought to contain some bioactive secondary metabolites that act against opportunistic infections[18]. In addition, the plant extract serves as a natural source of antioxidants and contains potential phytochemicals that are effective against protein glycation and colon cancer[19]. The ethanol-water extract (EWe) of C. edulis was demonstrated to contain high levels of polyphenols and flavonoids[19]. In vitro and in situ studies have shown that the flavonoids and some metabolites of C. edulis could traverse the blood-brain barrier (BBB), owing to their lipophilic nature[20-22]. Thus, the EWe of C. edulis may be an efficient therapeutic owing to its high content of flavonoids, and has attracted the attention of researchers aiming to develop dietary therapeutic strategies in order to combat nervous system damages induced by oxidative stress.
In our previous study, we compared the effects of EWe and an aqueous extract of C. edulis leaves and found that EWe contained higher levels of polyphenols and flavonoids and exhibited better antioxidant activity than the aqueous extract[19]. Thus, in the present study, we evaluated i) the capacity of 7β-OHC to trigger cellular modifications associated with aging, age-related diseases, and neurodegenerative diseases, such as oxidative stress and cell death, in response to mitochondrial dysfunction. We also analyzed the ability of EWe to attenuate 7β-OHC-induced side-effects. Murine oligodendrocytes (158N) were cultured in the absence or presence of 7β-OHC [20 μg/mL (50 μmol/L), 24 h] to determine the cytoprotective and antioxidant effects of EWe.
-
Murine oligodendrocytes (158N)[23] were seeded at 104 cells/well in a 12-well plate and cultured in Dulbecco's modified Eagle's medium (DMEM) (Lonza, Amboise, France) supplemented with 5% (v/v) heat-inactivated fetal bovine serum (Dutscher, Brumath, France) and 1% antibiotics (penicillin, streptomycin) (Dutscher) in a humidified atmosphere containing 5% CO2. The cells were incubated in the presence or absence of EWe at different final concentrations for 2, 4, and 6 h at 37 ℃. After the pretreatment with EWe, 158N cells were incubated without or with 7β-OHC (20 µg/mL). The concentration of 7β-OHC was chosen in the range that induced cell death. Parallel experiments were carried out using ethanol as a vehicle.
-
The protective effect exerted by EWe against 7β-OHC cytotoxicity was assessed using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded in a 96-well plate at a density of 0.5 × 105 cells/wells in the presence of different concentrations of EWe (20-200 µg/mL) for 2 h. After treatment with 7β-OHC (20 µg/mL) and incubation for 6 h, cell viability was determined using the colorimetric MTT assay. A total of 200 µL of 5 mg/mL MTT solution was added to each of a 96-well plate. After 3 h of incubation at 37 ℃, the medium was removed and 100 µL of dimethyl sulfoxide (DMSO) was added to each well. The plates were shaken, and the percentage of viable cells was measured based on the reduction of MTT dye into formazan crystal at 570 nm wavelength.
-
Lipid peroxidation was indirectly determined by measuring the production of malondialdehyde (MDA) from the treated cells following the method of Yoshioka et al.[24]. Briefly, to precipitate the proteins, 158N cell lysate was mixed with trichloroacetic acid (20%). Thiobarbituric acid (0.67%) was added to the mixture and the reaction was carried out for 30 min at 95 ℃. After cooling to 25 ℃, 4 mL of n-butanol was added to the mixture and the absorbance was measured at 530 nm wavelength. Total MDA concentration was expressed in nanomole per milligram of protein.
The level of conjugated diene (CD), another indicator of lipid peroxidation, was measured as described by Esterbauer et al.[25]. For this assay, lipids were extracted from the cells using a chloroform-methanol (2:1) mixture. The extracted lipids were re-dissolved in hexane, and CD level was calculated using the value of molar extinction coefficient [2.52 × 104 mol/(L·cm)]. The results were expressed as micromole of hydroperoxide per milligram protein.
-
CP levels were measured using a method based on the reaction between 2, 4-dinitrophenylhydrazine (DNPH) and the PC groups to form protein hydrazones[26]. The cell lysate was incubated with DNPH [10 mmol/L in 2.5 N hydrochloric acid (HCl)] and treated with 20% trichloroacetic acid. The tube was centrifuged at 1, 800 ×g for 5 min and the precipitate obtained was washed with trichloroacetic acid (10%) as well as with a mixture of ethanol-ethyl acetate (1:1; v/v). The precipitate was dissolved in a 6 mol/L guanidine hydrochloride solution and its absorbance was measured at 370 nm. CP levels were determined using the molar extinction coefficient of DNPH [22, 000 mol/(L·cm)].
-
Catalase activity was measured as per the method of Awasthi et al.[27] with hydrogen peroxide (H2O2) as the substrate. The assay mixture contained 100 µL of Tris-HCl buffer (pH 7.4; 1 mol/L), 100 µL of H2O2 (400 µmol/L), and 20 µL of diluted cell fraction in a total volume of 0.22 mL. The reaction was spectrophotometrically (240 nm) analyzed at 37 ℃ every 15 s for 90 s. The results were calculated using the extinction coefficient of H2O2 [0.043 nmol/(L·cm)] and expressed as unit per milligram (U/mg) protein. Protein levels were determined using Bradford method.
GPx activity was measured as described by Flohe and Günzler[28]. The enzyme activity was expressed as U/mg protein.
-
SPSS version 22 (Statistical Package for Social Science, SPSS Inc., Chicago, IL) was used to conduct statistical analysis. Analyses were carried out with the Mann-Whitney U-test. Data were considered statistically different at a value of P ≤ 0.05.
Cell Culture
Evaluation of the Effects of C. edulis Extract on Cell Growth and Mitochondrial Activity with the Colorimetric MTT Assay
Determination of Lipid Peroxidation Product Levels
Analysis of Carbonylated Protein (CP) Level
Determination of Antioxidant Enzyme Activities: Catalase and Glutathione Peroxidase (GPx)
Statistical Analysis
-
We have previously demonstrated higher levels of polyphenols and flavonoids and higher antioxidant activity in the EWe from C. edulis leaves than in its aqueous extract[19]. In the present study, we investigated whether EWe from C. edulis leaves could attenuate 7β-OHC-induced side-effects on 158N murine oligodendrocytes.
-
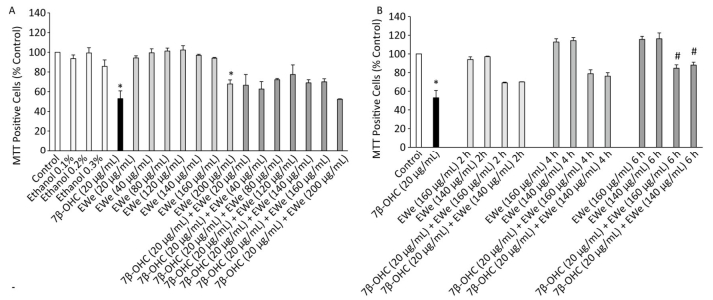
We used the MTT assay to quantify succinate dehydrogenase activity, which is reflective of mitochondrial dysfunctions and/or cell growth inhibition (or loss of cell adhesion). No significant difference was observed between untreated cells and vehicle controls. EWe showed no cytotoxicity at 20-160 µg/mL concentrations; at 200 µg/mL concentration, however, Ewe affected cell viability (Figure 1A). The treatment with 7β-OHC (20 µg/mL) for 24 h resulted in a significant reduction in cell viability as compared with that of untreated cells. However, the pretreatment of the cells with EWe (140 and 160 µg/mL) for 6 h resulted in a significant improvement in viability (P < 0.05) (Figure 1B). These data show that EWe is able to counteract the 7β-OHC-induced mitochondrial dysfunction, cell growth inhibition, and loss of cell adhesion.

Figure 1. Effect of Carpobrotus edulis (C. edulis) EWe and 7β-OHC on 158N cell viability. (A) Cells were incubated in the absence or presence of 7β-OHC for 24 h with or without pretreatment with EWe of C. edulis (20-200 µg/mL; 2 h). (B) Cells were incubated in the absence or presence of 7β-OHC for 24 h with or without pretreatment with EWe of C. edulis (140 and 160 µg/mL) for the indicated times. Data shown are mean ± SD from two independent experiments conducted in triplicates. Significance of the difference between vehicle- (ethanol) and EWe- or 7β-OHC-treated cells is indicated with * (Mann- Whitney test, P < 0.05). Significance of the difference between 7β-OHC and 7β-OHC + EWe groups is indicated with # (Mann-Whitney test, P < 0.05).
-
EWe treatment resulted in a significant increase in catalase and GPX activities of the cells treated with 7β-OHC (P < 0.05) as compared with that of the untreated or vehicle-treated cells. The pretreatment of the cells with 160 µg/mL of EWe for 6 h resulted in a minor but not significant decrease in the antioxidant enzyme activities (Figure 2).
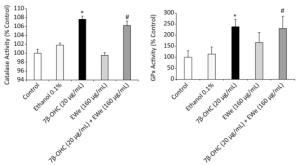
Figure 2. Effect of Carpobrotus edulis EWe and 7β-OHC on catalase and glutathione peroxidase activities. Cells were incubated in the absence or presence of 7β-OHC for 24 h with or without pretreatment with EWe of C. edulis (160 µg/mL; 6 h). Data shown are mean ± SD from two independent experiments conducted in triplicates. Significance of the difference between vehicle- (ethanol) and 7β-OHC-treated cells is indicated with *. Significance of the difference between 7β-OHC and 7β-OHC + EWe groups is indicated with # (Mann-Whitney test; P < 0.05).
-
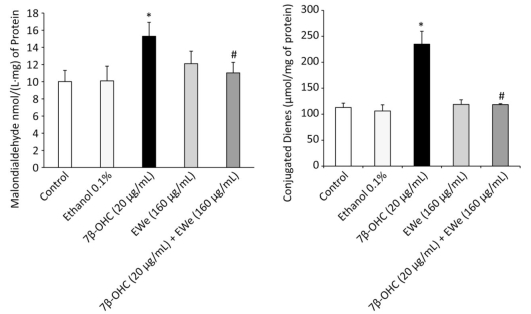
The levels of MDA and CD, the indicators of lipid peroxidation, were remarkably higher in the cells treated with 7β-OHC (20 µg/mL) alone than in the untreated or vehicle-treated cells. These data support that 7β-OHC is a potent inducer of oxidative stress. In comparison with 7β-OHC-treated cells, the cells pretreated with C. edulis extract (160 µg/mL, 6 h) showed a significant reduction in MDA and CD levels (Figure 3).
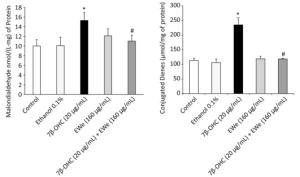
Figure 3. Effect of Carpobrotus edulis EWe and 7β-OHC on lipid peroxidation products. Cells were incubated in the absence or presence of 7β-OHC for 24 h with or without pretreatment with EWe of C. edulis (160 µg/mL; 6 h). Data shown are mean ± SD from two independent experiments conducted in triplicates. Significance of the difference between vehicle- (ethanol) and 7β-OHC-treated cells is indicated with *. Significance of the difference between 7β-OHC and 7β-OHC + EWe groups is indicated with # (Mann-Whitney test; P < 0.05).
-
In comparison to untreated or vehicle-treated cells, the cells treated with 7β-OHC (20 µg/mL) showed significantly higher levels of CPs (Figure 4), indicating that 7β-OHC is a potent oxidative stress inducer. A significant reduction in the levels of CPs was observed in the cells pretreated with 160 µg/mL of C. edulis extract as compared with that in 7β-OHC-treated cells (P < 0.05).

Figure 4. Effect of Carpobrotus edulis EWe and 7β-OHC on carbonylated protein levels. Cells were incubated in the absence or presence of 7β-OHC for 24 h with or without pretreatment with EWe of C. edulis (160 µg/mL; 6 h). Data shown are mean ± SD from two independent experiments conducted in triplicates. Significance of the difference between vehicle- (ethanol) and 7β-OHC-treated cells is indicated by *. Significance of the difference between 7β-OHC and 7β-OHC + EWe groups is indicated with # (Mann-Whitney test; P < 0.05).
Effects of C. edulis Extract on the Growth and Mitochondrial Activity of 7β-OHC-treated 158N Cells
Effects of C. edulis Extract on the Antioxidant Enzyme Activities (catalase and GPx) of 7β-OHC-treated Cells
Effects of C. edulis Extract on Lipid Peroxidation Product Levels in 7β-OHC-treated Cells
Effects of C. edulis Extract on the Levels of CPs in 7β-OHC-treated Cells
-
The present study demonstrated that 7β-OHC induced oxidative stress-mediated death of 158N murine oligodendrocytes and that EWe of C. edulis attenuated the 7β-OHC-induced side-effects. The levels of 7KC and 7β-OHC have been shown to increase in lesions and plasma samples from patients with atherosclerosis and AD. These oxysterols are thought to contribute to the pathogenesis of these diseases[29, 30]. In other studies, the 158N cells were cultured in the presence of 7β-OHC, wherein the lipotoxicity of 7β-OHC induced cell death through oxidative stress[11-13]. The EWe of C. edulis is known to prevent 158N cells from 7β-OHC-mediated alterations resulting from mitochondrial dysfunction, the major event implicated in neurodegeneration. In our study, the cytotoxic effects were evident following treatment with 7β-OHC. We also observed induction of cell death (mitochondrial dysfunctions), enhancement in antioxidant enzyme activity (GPx and catalase), and overproduction of oxidized derivatives of lipids (CD and MDA) and proteins (CPs).
The increase in the antioxidant enzyme activities may be considered as a cellular reaction to ROS overproduction. In concordance with this hypothesis, 7KC was shown to induce an increase in the mRNA level of manganese superoxide dismutase in human macrophages[31]. Further, a marked overproduction of superoxide anions (O2.-) and H2O2 was observed in the 158N cells treated with 7β-OHC or 7KC[12]. The overproduction of O2.- may result from the upregulation of NADPH oxidase isoforms, as shown in 7KC-treated human aortic smooth muscle cells[32] as well as from the mitochondrial dysfunction observed in 158N cells (MTT assay result). The mitochondrial alteration observed in the present study is consistent with previous study results obtained in the presence of 7β-OHC or 7KC. In 158N and microglial BV-2 cells, the percentage of metabolically active cells was low and a considerable decrease in the transmembrane mitochondrial potential was reported[12, 33, 34]. Furthermore, mitochondrial alterations are associated with cell death and organelle dysfunctions. In fact, 7KC triggers morphological, topographical, and functional peroxisomal alterations associated with mitochondrial changes at concentrations that may or may not induce cell death[35, 36]. In addition, an increase in acidic vesicle formation was reported that may correspond to the formation of autophagic vesicles or may serve as an adaptive response to the alterations in lysosomes resulting from the accumulation of 7KC in these organelles, subsequently leading to lysosomal membrane damage[35]. These cytoplasmic vacuoles associated with autophagosomes and autophagolysosomes were also reflected by transmission electron microcopy of cells with condensed and/or fragmented nuclei and following the conversion of microtubule-associated protein light chain 3 (LC3-I) to LC3-Ⅱ, a specific marker of autophagy[12, 35]. Oxidative stress may probably be a key event in this mode of cell death, as antioxidant molecules such as α-tocopherol could prevent this phenomenon[11]. Thus, lipid membrane peroxidation as a marker of oxidative stress could be related to membrane permeation and cell death.
Changes in catalase and GPx activities correlate with the toxicity of oxysterols, and may cause damage to macromolecules and accumulation of MDA, CD, and CPs in 158N cells treated with 7β-OHC. Thus, it would be interesting to determine whether these enzymes linked to the redox status could constitute pharmacological targets. In this direction, the identification of new compounds that attenuate the production of these oxysterols to prevent their toxic effects is desirable. At present, a few molecules have been shown to attenuate the toxic effects of 7KC and/or 7β-OHC in oligodendrocytes, microglial, and neuronal cells, such as α-tocopherol[12], polyphenols (quercetin, resveratrol, indicaxanthin)[37, 38], docosahexaenoic acid (DHA)[12, 39, 40], oleic acid and elaidic acid (oleic acid trans-isomer)[40], and dimethyl fumarate (the active substance in BG-12 (Tecfidera) used for the treatment of the relapsing form of MS[13]), AG126 (tyrosine kinase inhibitor)[41], calmidazolium, and W7 (calmodulin agonists)[42]. Among these compounds, only DHA has been shown to attenuate the 7β-OHC-induced oxiapoptophagy in 158N cells[12] and human neuronal cells (SK-N-BE cells)[39]. Thus, studies have been directed to investigate natural products used in traditional medicine for their ability to prevent multiple human diseases. Despite reported biological and pharmacological activities of C. edulis, there are no published reports on the effects of this plant extract on 158N murine oligodendrocytes. Pretreatment with EWe for 6 h resulted in the reduction in 7β-OHC-induced cytotoxicity. This extract could counteract the 7β-OHC-induced mitochondrial dysfunction, cell growth inhibition, and loss of cell adhesion. It also restored the activities of catalase and GPx to normal and diminished lipid and protein oxidation derivative levels in pretreated cells. These data are consistent with those of a study that evaluated the protective effects of sea urchin egg oil against 7β-OHC-induced cytotoxicity[34]. The high polyphenol and flavonoid contents present in the EWe extract may be responsible for this protective effect. As mentioned in our previous study, the contents of total polyphenols and flavonoids reached 151.9 mg (GAE) and 38.8 mg (quercetin)/mg of dry extract, respectively, in the EWe of C. edulis[19]. Some flavonoids are capable of penetrating the BBB in in vitro and in situ models[20-22] and may protect the neurons against stress-induced injury through the suppression of axonal demyelination and neuroinflammation. Seven major phenolic compounds were successfully identified in EWe, including sinapic acid, ferulic acid, luteolin-7-O-glucoside, hyperoside, isoquercitrin, ellagic acid, and isorhamnetin 3-orutinoside[19]. The potential effect of ellagic acid against free radicals and neural damage was demonstrated in vivo in a 6-hydroxydopamine-induced Parkinson's disease model[43]. Furthermore, the neuroprotective and anti-inflammatory potentials of the phytocompound luteolin-7-O-glucoside have been evaluated by many researchers[44-46]. Its significant anti-inflammatory activity comprising ROS scavenging and inhibition of the recruitment and activation of peripheral blood leukocytes in patients with MS[47, 48]. Moreover, luteolin was able to inhibit human mast cell activation induced by myelin basic protein and their interaction with activated Jurkat cells, which have been recently implicated in MS pathogenesis[49]. Furthermore, luteolin treatment exhibited additive effects by modulating cell proliferation and pro-inflammatory cytokine (interleukin-1β and tumor necrosis factor-α) production[47]. Zhu et al. reported that ferulic acid increased the proliferation of Schwann cells and expression of myelin-associated glycoprotein (MAG) and myelin basic protein (MBP) through mitogen-activated protein kinase 1 (MEK1)/extracellular signal-regulated kinase (ERK)-1/2 signaling, and that ferulic acid may also accelerate remyelination in injured peripheral nerves[50]. Potential protective effects against amyloid β-induced neurotoxicity in cultured murine hippocampal neurons were associated with isoquercitrin[51]. As these compounds are present in the extract, it is possible that some of them could synergistically act to exert cytoprotective effects, whereas several other compounds present in this extract may be involved in imparting these beneficial effects. Thus, different compounds present in the extract may simultaneously activate or repress several signaling pathways and contribute to cytoprotection.
The consumption of dietary flavonoids present in foods has been correlated with several beneficial effects against cardiovascular risk factors. These molecules may exhibit the potential to prevent cardiovascular and neurodegenerative diseases such as vascular dementia[52]. Studies have been directed for the identification of polyphenols with cardioprotective properties. Many reports suggest that the intake of polyphenol-rich foods is beneficial, owing to their influence on several cardiovascular risk factors and that the mechanisms involved in the cardioprotective effects of polyphenols are numerous and may include antioxidant, antiapoptotic, and metabolic functions[53]. These studies confirm the antioxidant and cytoprotective effects of EWe of C. edulis, and highlight its potential application for the prevention of cardiovascular risk factors and neurodegenerative and age-related diseases, as oxidative stress and mitochondrial dysfunctions are considered the key events in these degenerative diseases.
The combination of these different molecules in the same extract is of great importance and could be used to design functional foods to prevent pathologies. The extract could be encapsulated to prevent the metabolism and the conjugation of key ingredients. Thus, it may prevent the elimination of flavonoids and polyphenols from the extract. In fact, encapsulation of α-tocopherols into a chitosan hydrogel was carried out to protect its antioxidant capacity. This complex has shown excellent resistance to oxidative stress and improved the survival of cardiomyocytes[54]. However, whether the protective effects of C. edulis EWe vary with cell lines and among neuronal, glial, and microglial cells is yet unknown. Thus, the application of this extract may vary based on the type and stage of pathology. A better understanding of the protective effects of C. edulis EWe on various cellular models may contribute to optimization of its use.
Altogether, our data demonstrate that 7β-OHC induces a disequilibrium in the redox status of 158N cells and contributes to cell death. Further, C. edulis EWe prevents 7β-OHC-induced toxicity. As the levels of 7β-OHC and other oxysterols such as 7KC and 24S-OHC may be simultaneously increased in various neurodegenerative diseases, a better knowledge of their biological activities and associated metabolic pathways as well as identification of molecules (or mixtures of molecules) capable of counteracting their side-effects may evoke physiopathological and pharmacological interests[55].
-
The authors declare no conflict of interest. This study was supported by The Ministry of Higher Education, Scientific Research and Information and Communication Technologies, Tunisia.







 Quick Links
Quick Links
 DownLoad:
DownLoad: