-
Modern technology has witnessed milestone achievements in the telecommunication industry. However, the widespread application of telecommunication technology is believed to heighten electromagnetic field (EMF) ‘pollution’ in our environment[1] and subject living organisms to various sources of electromagnetic emissions. These emissions include; microwaves, electromagnetic (radio) irradiations, mobile phone signals, and even wireless internet modems. All of these may exert subtle but significant biological effects. In the last two decades, there has been tremendous interest in the health effects of chronic long-term exposure to what has previously been assumed to be a biologically imperceptible level of electromagnetic radiation[2].
Recent studies have suggested several implications of extended exposure to EMFs. First, the generation of free reactive oxygen species (ROS) through the activation of nicotinamide adenine dinucleotide (NADH) oxidases in the plasma membrane has been described as a possible effect of magnetic fields in biological systems[3]. Some researchers have reported that ROS can be beneficial, but at high levels they may cause cellular activation which can result in both beneficial and negative effects. Similarly, the ability of magnetic fields to activate electron sharing processes and affect the H-bonding of several macromolecules has been previously described[3].
Excessive exposure to EMFs can also perturb the plasma membrane potential and alter calcium flux, a phenomenon that impacts calcium signaling and decreases protein kinase C (PKC) activity[4]. Various studies have suggested that long-term exposure to high levels of EMF stress can cause pancreatic cell apoptosis, mitochondrial dysfunctions, and DNA and liver stress. Increasing EMF exposure has also been associated with numerous health concerns[1]. The present study aimed to evaluate how moderate EMF emissions produced by WiFi signals might affect biochemical and hematological parameters, using rats as a model of human health.
All chemicals and reagents used in the study were of analytical grade and purchased from Sigma-Aldrich Inc. (St Loius, MO), BDH Chemical Inc. (Poole England), and Randox Inc. (United Kingdom)
The design and method of exposure to radiation were created according to the protocol described by Faraone et al., 2004[5].
Sixteen male Wistar rats (12-week old) were used in this study. Upon arrival, the animals were weighed and handled according to NIH guidelines and in accordance with the guidelines of Afe Babalola University (ABUAD) for the care and use of laboratory animals. Feed was weighed daily and provided in equal proportions to the four groups with ad libitum access to water. The animals were housed in steel mesh cages and placed approximately 10 cm away from an installed Wi-Fi device in a room with glass shields, which was built to serve the University community of ABUAD, Ekiti State, Nigeria.
The 16 male Wistar rats were randomly divided into four (4) treatment groups of four rats each (n = 4) as follows: Control: no exposure to EMF; Week 4: exposed to EMF for four (4) weeks; Week 6: exposed to EMF for six (6) weeks; and Week 8: exposed to EMF for eight (8) weeks.
At the end of each treatment period, the rats were anaesthetized (under mild diethyl ether), dissected, and blood samples were collected through cardiac puncture (these steps were performed at the 8-week mark for the control group). Tissues were subsequently collected and placed on ice. These tissues were homogenized in cold 0.1 mol/L Tris-HCl buffer (1∶10 w/v) using a Teflon homogenizer. The homogenates were centrifuged for 10 min at 3,000 g and the supernatants of the various tissues were stored for further assays.
The blood samples were collected in vials containing EDTA to measure hematological parameters such as mean cell volume (MCV), mean corpuscular hemoglobin concentration (MCHC), mean cell hemoglobin (MCH), red blood cell (RBC) count, white blood cell (WBC) count, packed cell volume (PCV), and hemoglobin (Hb) count.
Serum total protein was determined using the protocol devised by Peter et al.[6], alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were analyzed using a procedure by Reitman and Frankel (1957)[7], and lipid peroxidation was quantified using the method described by Ohkawa et al.[8].
The specific absorption rate (SAR) of the non-thermal exposure on the rats was calculated according to the method described by Durney, 1980[9].
Data were analysed using a one-way ANOVA (nonparametric test) (GraphPad software, San Diego, CA, USA) followed by Tukey’s test where appropriate. Differences were considered significant at P < 0.05.
Table 1 reveals that there were significant (P < 0.05) differences in the levels of MCH, PCV, and Hb between the group that had the highest exposure (week 8) and the control group. Furthermore, all the groups exposed to the EMF showed a significant (P < 0.05) difference in their MCV levels when compared to the control.
Exposure span MCV (fl) MCHC (%) MCH (pg) RBC (106/µL) WBC (103/µL) PCV (%) Hb (g/dL) control 84.50 ± 7.41 33.00 ± 0.00 30.23 ± 2.53 4.00 ± 0.00 2,400 ± 129.09 33.30 ± 3.20 11.00 ± 1.10 week 4 102.75 ± 1.03* 33.00 ± 0.00 34.00 ± 0.41 3.75 ± 0.30 2,425 ± 62.92 36.50 ± 1.85 12.25 ± 0.75 week 6 108.00 ± 1.00* 33.00 ± 0.00 35.75 ± 0.25 3.25 ± 0.30 2,525 ± 170.17 37.00 ± 1.41 12.50 ± 0.50 week 8 114.75 ± 2.50* 33.00 ± 0.00 39.00 ± 1.15* 3.75 ± 0.25 2,475 ± 110.87 41.25 ± 1.49* 13.75 ± 0.96* Note. The values are expressed as the mean ± SD (n = 4) in each group. *P < 0.05 when compared to the control group. MCV, mean cell volume; MCHC, mean corpuscular hemoglobin concentration; MCH, mean cell hemoglobin; RBC, red blood cell; WBC, white blood cell; PCV, packed cell volume; Hb, hemoglobin. Table 1. Effects of EMF exposure from indoor Wi-Fi devices on blood cells parameters of 8 weeks old Wistar rats for 30, 45, and 60 days
Figure 1 shows the observed changes in the levels of protein concentration (g/dL) across the EMF-exposed groups (week 4, 6, and 8) compared to the control. Data revealed that EMF-exposed groups had a significant (P < 0.05) increase in hepatic total protein compared to the control. Similarly, testicular protein levels in week 6 and 8 groups were significantly (P < 0.05) higher than in the week 4 group and the control, whereas heart total protein was significantly (P < 0.05) higher in the control group compared to EMF-exposed groups.
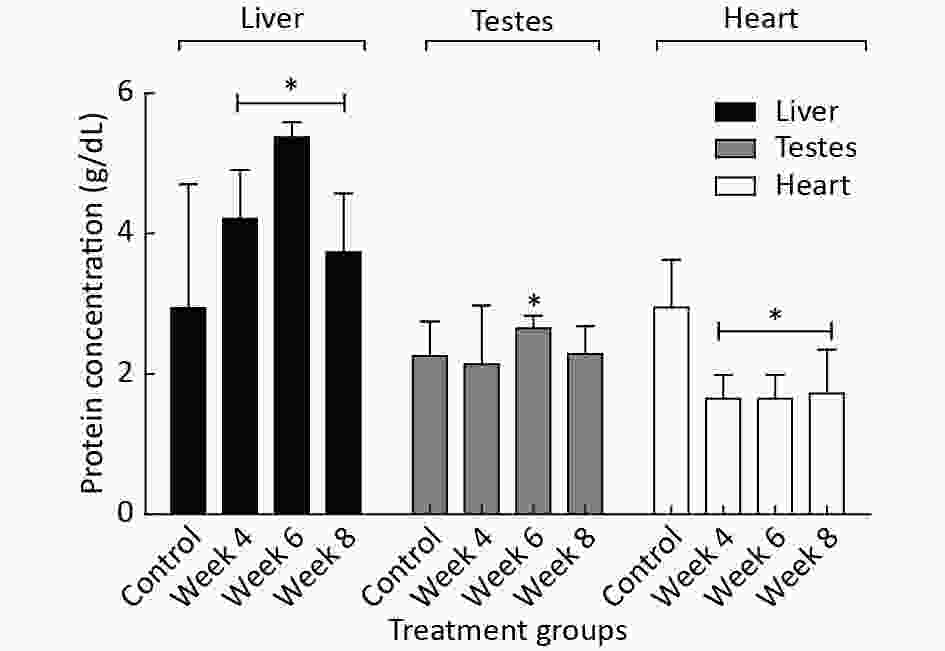
Figure 1. The effects of EMF radiation on the protein concentrations (g/dL) in different organs of EMF-exposed and control male rats. The results are expressed as the mean ± SD of four trials (n = 4). *Indicate the significant (P < 0.05) differences within the group compared to the control.
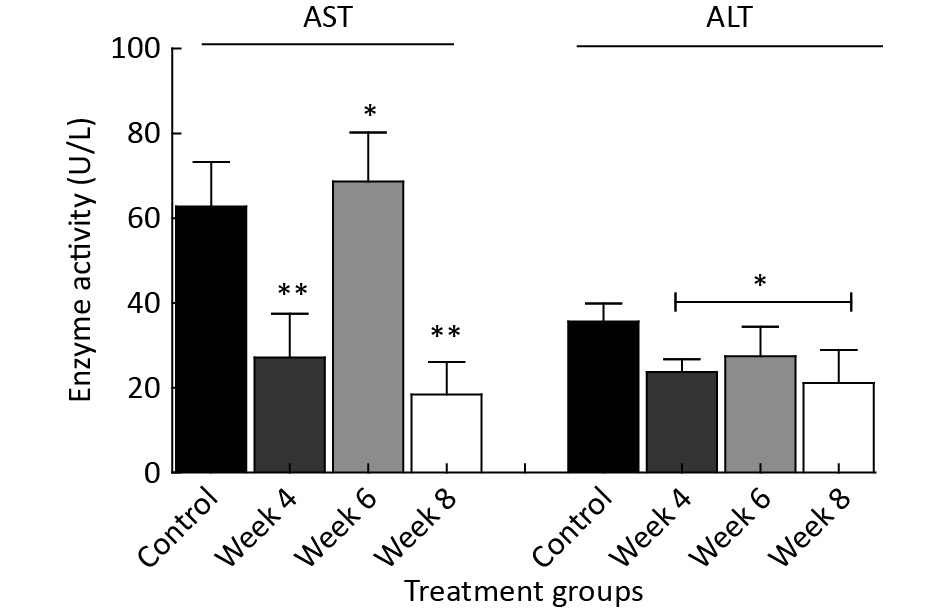
Figure 2 reveals several variations in serum enzymatic activities of the EMF-exposed groups when compared to the control group. The results show significantly (P < 0.05) reduced serum AST activities in the week 4 and 8 EMF-exposed rats, while a significant (P < 0.05) increase was noted in week 6 EMF-exposed rats when compared to the control. Similarly, a significant (P < 0.05) reduction was noted in serum ALT activities of the EMF-exposed rats (week 4 and 8), while a significant (P < 0.05) increase was observed in the Week 6 group when compared to the control.

Figure 2. The effects of EMF exposure on serum enzymatic activities over a period of 4 to 8 weeks. The results are expressed as the mean ± SD of four trials (n = 4). *Indicate the levels of significant (P < 0.05) differences in the compared to the control.
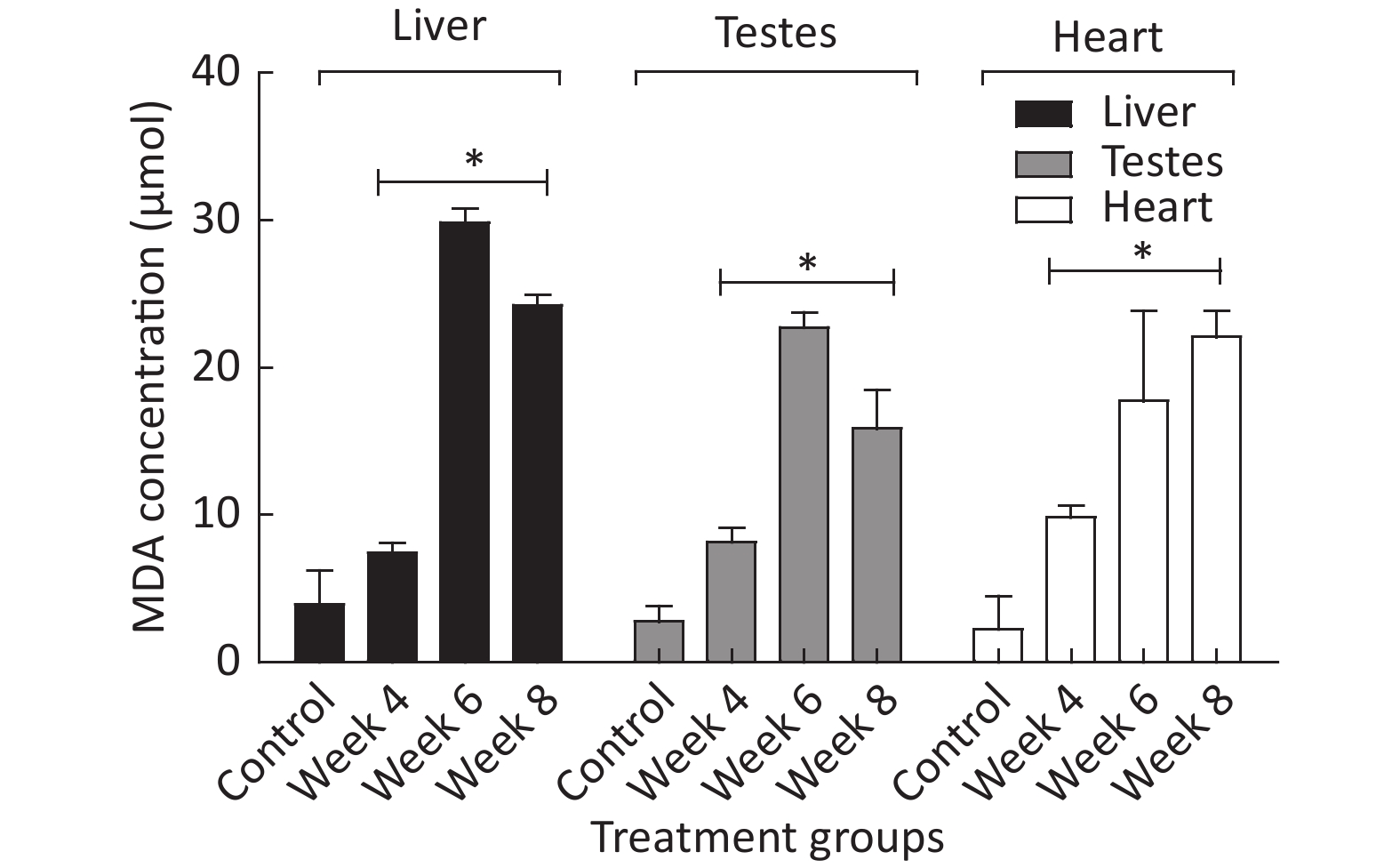
Figure 3 shows different MDA levels (µmol) produced in several tissues of EMF-exposed groups when compared to the control group. The results revealed a significant (P < 0.05) difference in MDA levels produced in hepatic, testicular, and heart tissue of EMF-exposed groups when compared to the control.
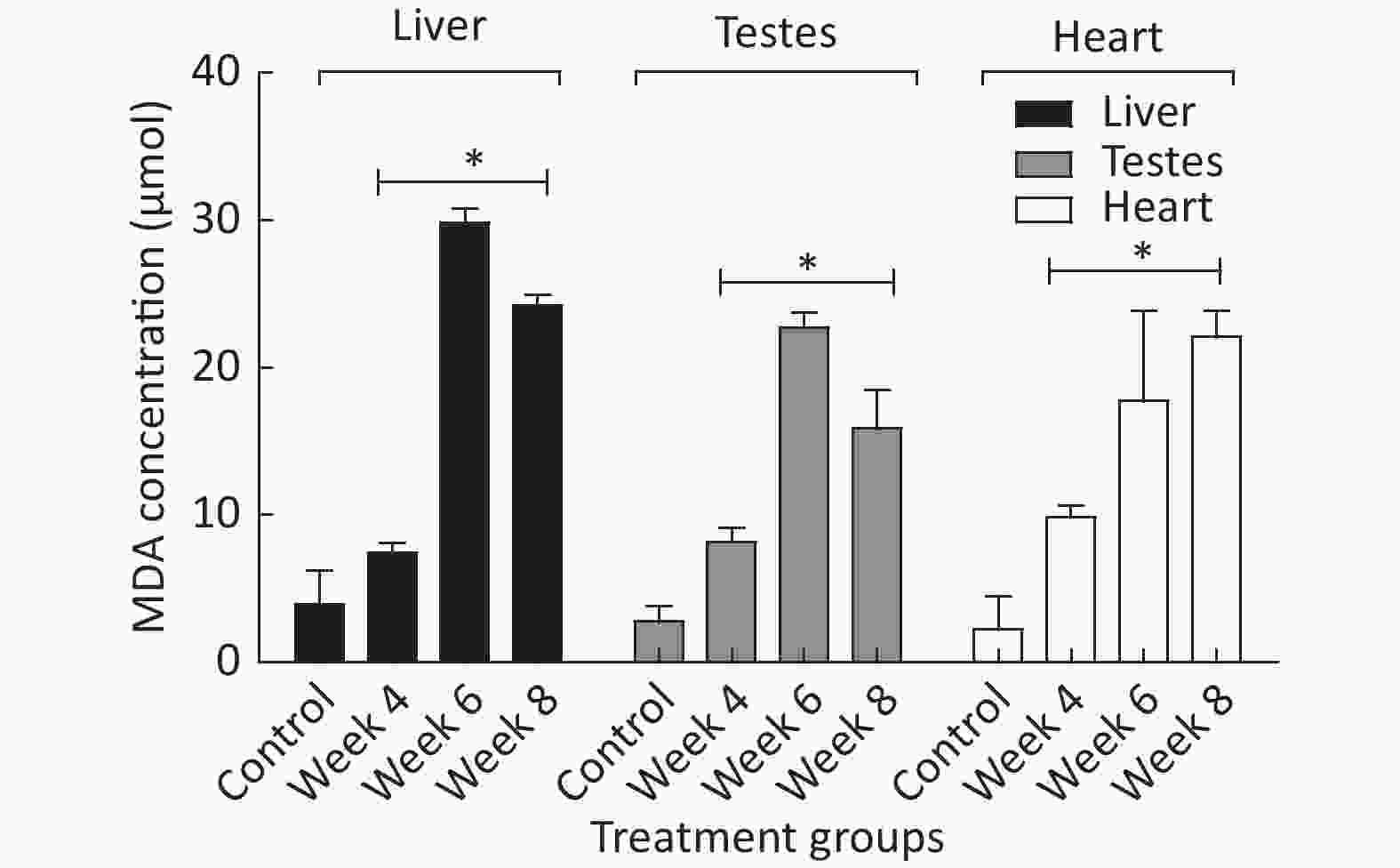
Figure 3. The MDA produced in µmol over a period of 4 to 8 weeks of EMF exposure. *Indicate a statistical difference (P < 0.05) in the EMF-exposed groups compared to the control. The results are expressed as the mean ± SD of four trials (n = 4).
Exposure to EMF radiation over a long period has been shown to pose a serious threat to human health. EMFs alter several metabolic processes in the human body and exert various biological and chemical effects on cells through a wide range of mechanisms such as the disruption of cellular structures and the excessive generation of ROS[3].
When exposure to EMFs was increased, a reduction in multiple hematological parameters was observed due to hemodynamic effects caused by the interaction between EMF radiation and the arterial system. The imbalance in blood parameters observed in the current study (Table 1) after extended EMF exposure might have been the direct result of EMF emissions on the walls of red blood cells, leading to iron/foliate deficiency[1].
In addition, our study discovered a significant alteration in the protein concentration (g/dL) among the exposed groups (Figure 1). The fluctuation observed in the enzyme activity from Week 4 (Figure 2) when compared to the control could be linked to an acute stress response of the enzyme localized tissues, which is connected with diverse signaling mechanisms. However, a marked increase was observed in the enzyme activity of the Week 6 EMF-exposed group, which suggests an adaptive immune response is a result of EMF exposure[10]. Contrarily, a decline in enzymatic activity was noted in the week 8 EMF-exposed group. The mechanism for this phenomenon has not yet been elucidated.
Malondialdehyde (MDA) has often been recognized as an oxidative stress/lipid peroxidation marker in biological systems[3]. In this study, there was a noticeable increase in the amount of MDA generated within several groups (Figure 3). This could have indirectly signaled oxidative stress and cellular damage that may be linked to the uncontrollable production of ROS within the groups that were exposed to various levels of EMF.
In the current study, alterations were noted in hematological and biochemical parameters of groups exposed to a 2.5 GHz EMF emission from an indoor Wi-Fi device over a period of 4-8 weeks. The precise mechanism underlying these effects was not identified in this study; however, the results demonstrate that long-term exposure to EMFs can induce tissue damage and alterations in biochemical parameters.
Acknowledgement The authors of this manuscript appreciate the contribution of the members of the ICT section of Afe Bablola University (ABUAD) for providing their full support during the study.
Exposure to a 2.5 GHz Non-ionizing Electromagnetic Field Alters Hematological Profiles, Biochemical Parameters, and Induces Oxidative Stress in Male Albino Rats
doi: 10.3967/bes2019.107
- Received Date: 2019-04-06
- Accepted Date: 2019-09-26
| Citation: | Afolabi Olakunle Bamikole, Obajuluwa Adejoke Olukayode, Tiwa Obajuluwa, Okiki Pius, Oloyede Omotade Ibidun, Fadaka Oluwaseun Adewale, Ojo Oluwafemi Adeleke. Exposure to a 2.5 GHz Non-ionizing Electromagnetic Field Alters Hematological Profiles, Biochemical Parameters, and Induces Oxidative Stress in Male Albino Rats[J]. Biomedical and Environmental Sciences, 2019, 32(11): 860-863. doi: 10.3967/bes2019.107 |








 Quick Links
Quick Links
 DownLoad:
DownLoad: