-
Endocrine disruptors (EDCs), also known as Environmental Hormones (EHs), refer to substances in the environment that interfere with the endocrine systems of humans and animals. These substances can lead to genital disorders in humans and animals[1]. Nonylphenol (Nph) is a kind of relatively distributed substance of EDCs. Nph is the metabolite of nonylphenol polyethoxy and an important fine chemical, raw material, and intermedium[2], which is one of the most widely used raw materials in detergent, plastic, paint, and other chemical products. Nph is not only used in the production of surfactants but also used in that of antioxidants. The increasing pollution of freshwater systems by thousands of industrial and natural compounds around the world is one of the major environmental problems that mankind is facing[3]. In recent years, media from various countries have reported the feminization phenomenon of male fish, which is mostly caused by wastewater with EHs discharged into rivers. EHs will disrupt not only the human endocrine system but also the ecological balance.
The conventional detection methods of Nph are gas chromatography/mass spectrometry, high-performance liquid chromatography, and electrophoresis[4]. Although these methods have excellent sensitivity and automated operation, they also have disadvantages, such as high cost, inadequate pre-treatment of complex samples, and time-consuming. Compared with these methods, an electrochemical sensor is an analytical method with low cost and simple operation. In recent years, the electrochemical sensor has been a rapidly developing technology and gradually applied to the detection of EHs.
The latest electrochemical sensor technology reduces the detection limit, shortens the response time, has a low manufacturing cost, is portable, and can be used for continuous real-time detection[3]. Molecular imprinting is a technique for preparing polymers with the capability to accurately identify target molecules. Molecularly imprinted polymers (MIPs) have been widely used in the fields of analytical chemistry and separation science. Furthermore, MIPs are particularly appropriate for biological and environmental samples and the separation of specific target molecules in complex systems, such as plants[4,5]. When AuNPs are subjected to functionalization with the appropriate metals and organic or biomolecular functional groups, the properties of AuNPs, such as surface plasmon resonance, color, electron affinity and conductivity, are remarkably improved[6]. Here we report on a novel approach based on functionalized AuNPs and MIPs for the sensitive electrochemical analysis of Nph. As a template molecule in MIPs, Nph forms a cavity that specifically recognizes binding sites and functionalizes AuNPs with p-aminothiophenol (PATP) to construct an electrochemical sensor with high specificity and affinity.
Sensors established similarly have been used in other studies. Florea et al. reported that the detection of the anticancer drug gemcitabine by linear scanning voltammetry has a detection limit of 3 fmol/L on the basis of the electropolymerization of PATP-AuNPs on gold electrode[7]. Guo et al.[8] reported the detection of 1,3,5-trinitrotoluene with a detection limit of 0.04 fmol/L. All of the aforementioned examples show that the MIP/PATP-AuNPs sensor has good sensitivity and reproducibility and is an effective electrochemical method to detect many kinds of substances, including organic pollutants, explosives, and drugs in different actual samples. Thus, the MIP/PATP-AuNPs sensor has good application prospects.
The synthesis of PATP-AuNPs was based on that reported by Guo et al.[8]. In a two-necked flask equipped with a spherical condenser, 31.6 mg of tetrachloroauric acid trihydrate and 30 mL of methanol were mixed. The apparatus was placed on a magnetic stirrer with heater for 10 min in the dark. Subsequently, 12 mL of methanol/water (v/v) mixture solution with 19.84 mg of PATP was added dropwise. Furthermore, 30.4 mg of NaBH4 dissolved in 2.2 mL of water was slowly added dropwise. The reaction was appropriately accelerated for 10 min, stirring was stopped, and the solution was kept in the dark for 1 h. The suspension was filtered using a polymer membrane and rinsed sequentially with water and diethyl ether. The resulting black powder was dried and gently scraped off. The granules were dried and placed in a vial away from light.
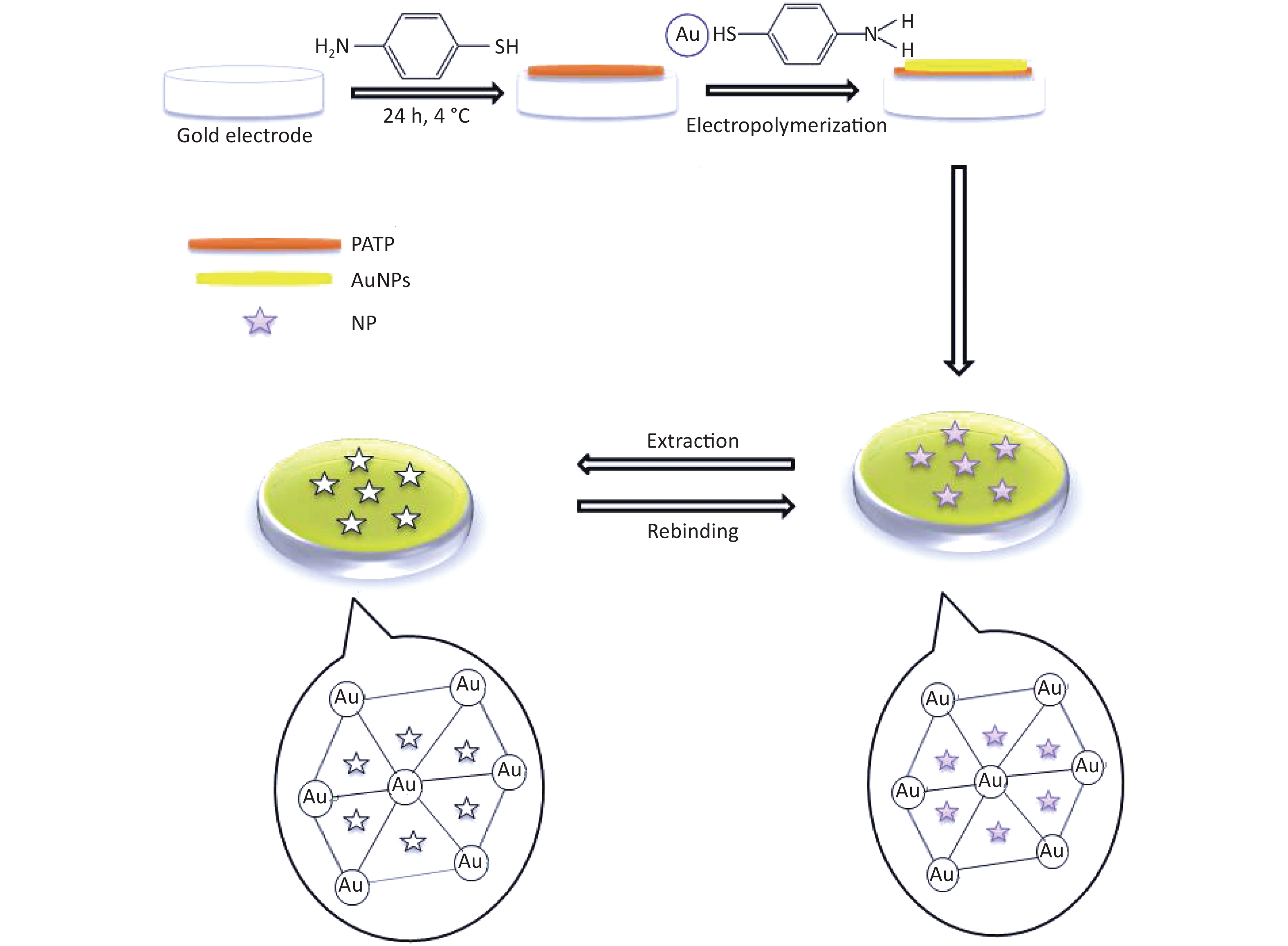
The preparation of the PATP-AuNPs hybrid modified gold electrode is shown in Figure 1. The gold electrode was lightly polished using Al2O3 powder and rinsed with distilled water. Then, the surface of the gold electrode was dried with nitrogen (gaseous) and immersed in an ethanol solution of PATP (1.6 × 10−4 mol/L) at 4 ℃ for 12 h to form a middle monolayer of PATP. After 12 h, the gold electrode was rinsed with ethanol and double-distilled water to remove nonspecifically adsorbed PATP and dried with nitrogen. PATP-AuNPs was deposited on the electrode through electropolymerization via cyclic voltammetry (CV). To obtain imprinted films, the prepared gold electrode was incubated for 20 min in a solution containing 3 mL of 0.1 g/L PATP-AuNPs, 3 mL of [Fe(CN)6]3−/4−, and 3 mL of Nph (0.1 g/L) solution by cycling from −0.20 to +0.60 V at a scan rate of 15 mV/s for 10 cycles (the redox probe was used to examine the electropolymerization of PATP-AuNPs on PATP-modified electrode). Then, the gold electrode was eluted with a mixed solution of acetone and phosphate buffer solution (1:1) for 30 min[8] to remove the blotting molecules.

Figure 1. Schematic illustration of the fabrication process of the molecularly imprinted polymer electrochemical sensor.
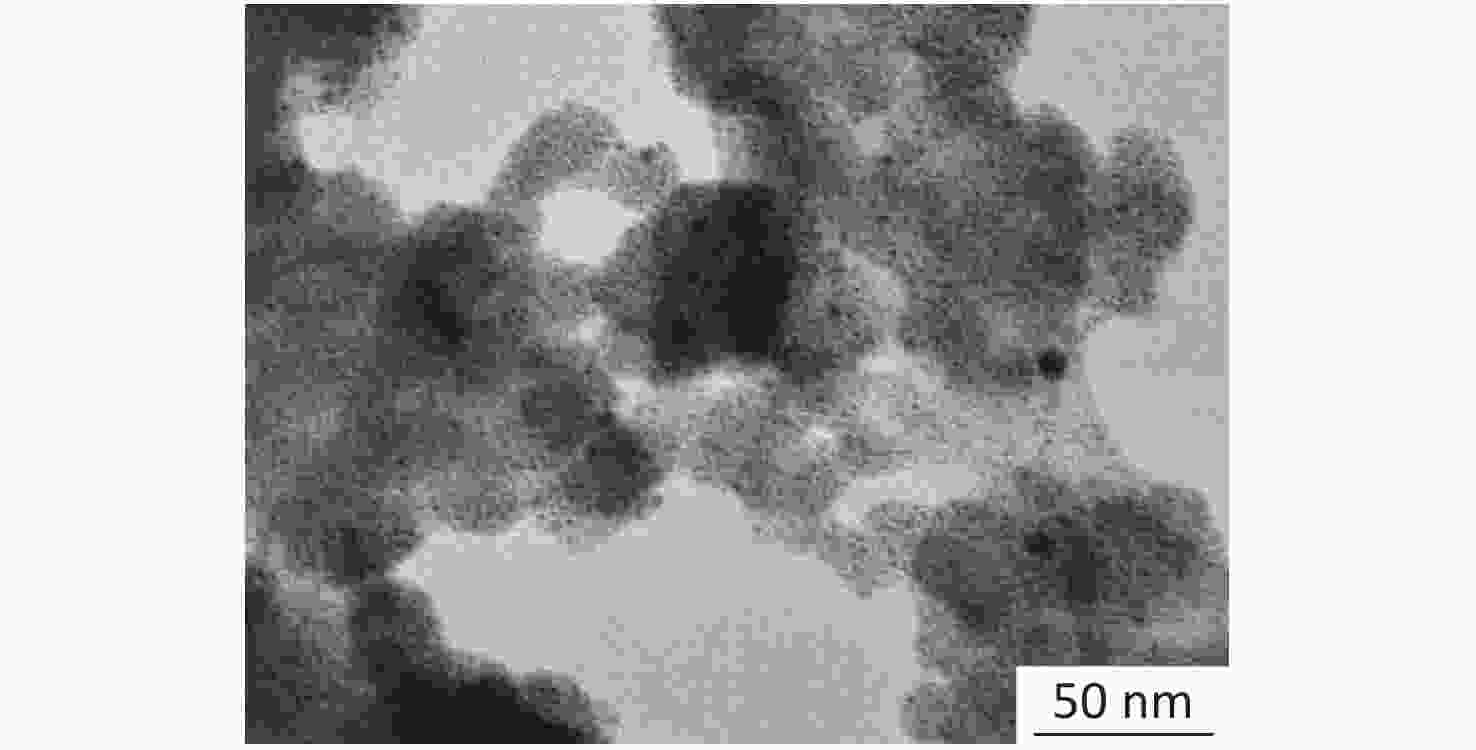
A clear redox peak is observed by CV scanning (Supplementary Figure S1 available in www.besjournal.com). With the increase in the number of cycles, the current decreases, indicating that the dense polymer film covering the surface of the gold electrode has grown gradually. This finding can be explained by the fact that the charge transfer of the redox probe on the gold electrode was hindered. The oxidation and reduction peaks deviated slightly during the cycling process. However, the curves were symmetrical and the peaks were identical, which indicate that the reaction is reversible. The [Fe(CN)6]3−/4− redox probe was used to determine the effect of its permeability on the formation of polymer films. The peak current of the oxidation/reduction of the redox probe decreases with each scan cycle because of the continuous formation of PATP-AuNPs/Nph composite films that obstruct the electron transfer from/to the redox probe [Fe(CN)6]3−/4− toward the surface of the gold electrode, indicating that the PATP-AuNPs composite film is formed on the electrode surface by electropolymerization. The transmission electron microscopy images (Supplementary Figure S2 available in www.besjournal.com) depict the MIP films with uniformly dispersed porous nanoparticles and nanostructure morphology.
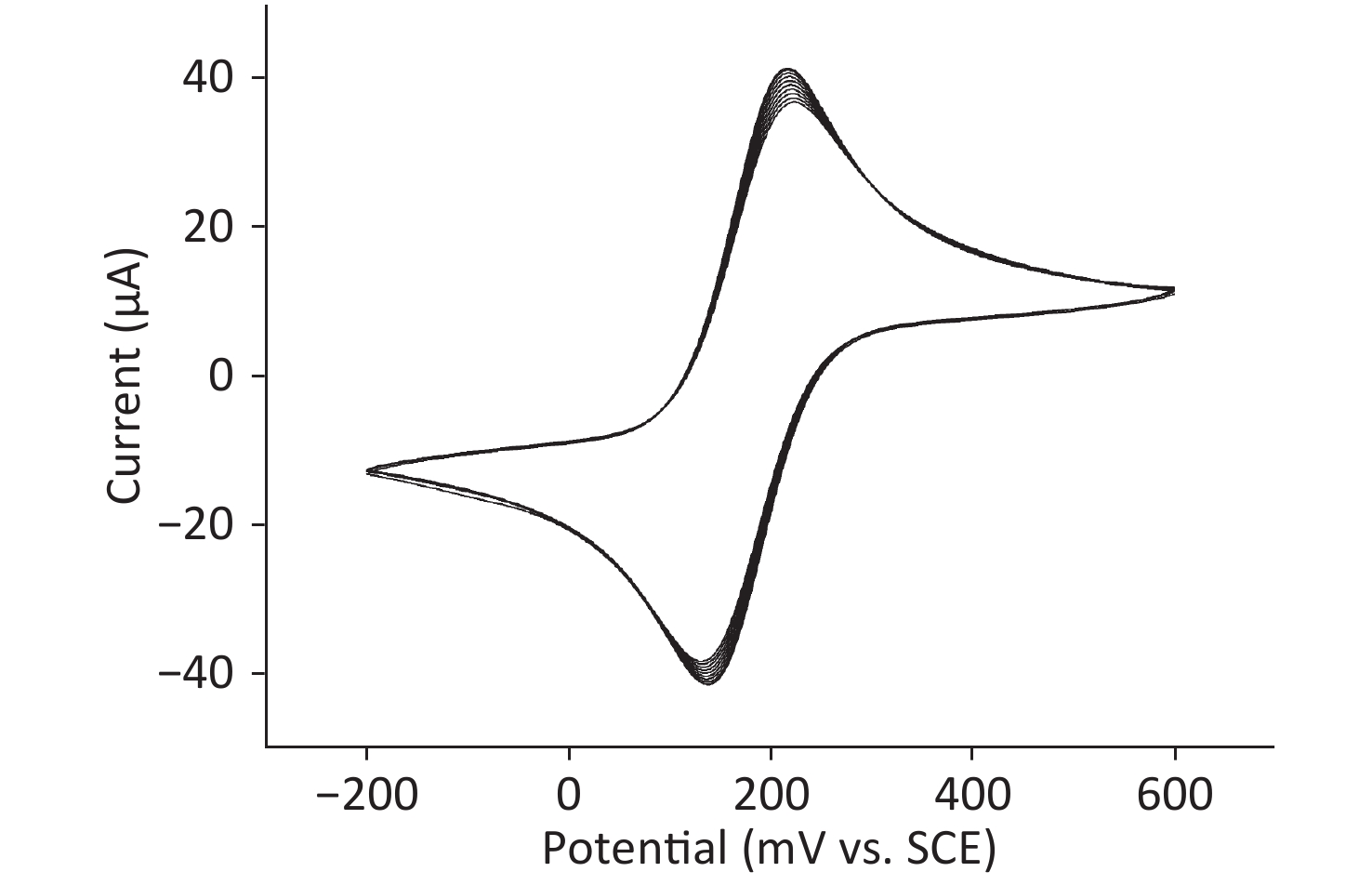
Figure S1. Cyclic voltammograms for the electropolymerization of MIPs/PATP-AuNPs in a solution of 0.1g/L [Fe(CN)6]3-/4- in PBS (pH 5.50) containing 0.1g/L PATP-AuNPs and 0.1 g/L NP solution. Potential range from −0.2 0V to +0.60 V vs. SCE, scan rate 100 mV/s
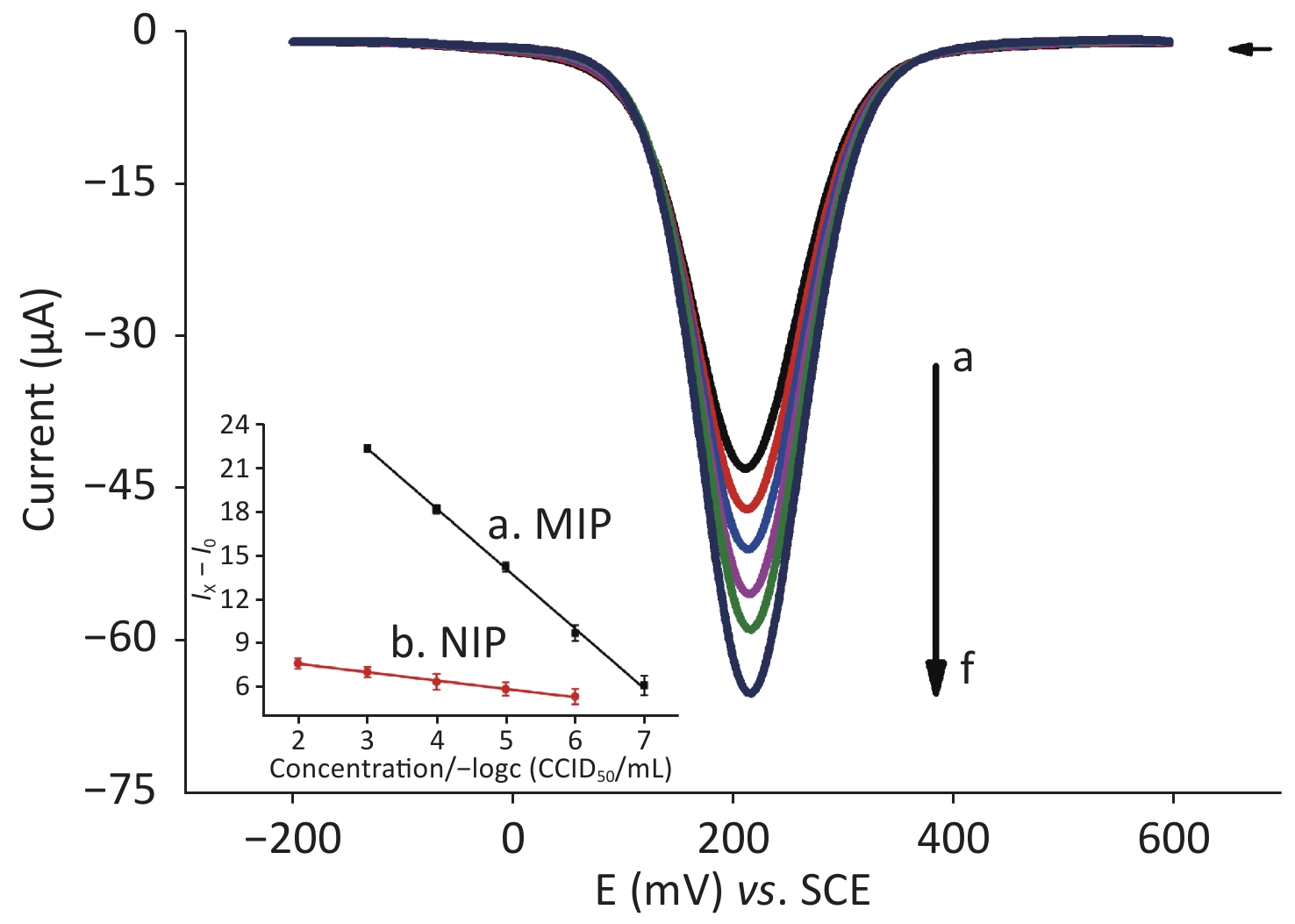
Square-wave voltammetry (SWV) is an excellent method for the detection of analytes. SWV was conducted in 3 mL of [Fe(CN)6]3−/4− solution and 3 mL of Nph solution with different concentrations (i.e. from 10−7 to 10−3 g/L) in the potential range of +0.20 to +0.60 V vs SCE at a scan rate of 100 mV/s. Figure 2 shows that, as the concentration of the Nph solution increases (i.e. from 1.0 × 10−7 g/L to 1.0 × 10−3 g/L), the absolute value of the peak current decreases gradually, which indicates that the MIP sensor has suitable binding sites for Nph. The degree of binding to the cavity changes with the increase in Nph concentration, which hinders the rate of electron transfer. This phenomenon can be explained by the combination of Nph (low electron acceptor) and PATP (electron donor) through π–π noncovalent binding. In the case of a high electron acceptor, such as TNT, an increase in peak current was observed[8]. In the case of Nph, the presence of the long alkyl chain could explain the increase in the resistance of the MIP film when its concentration increases.
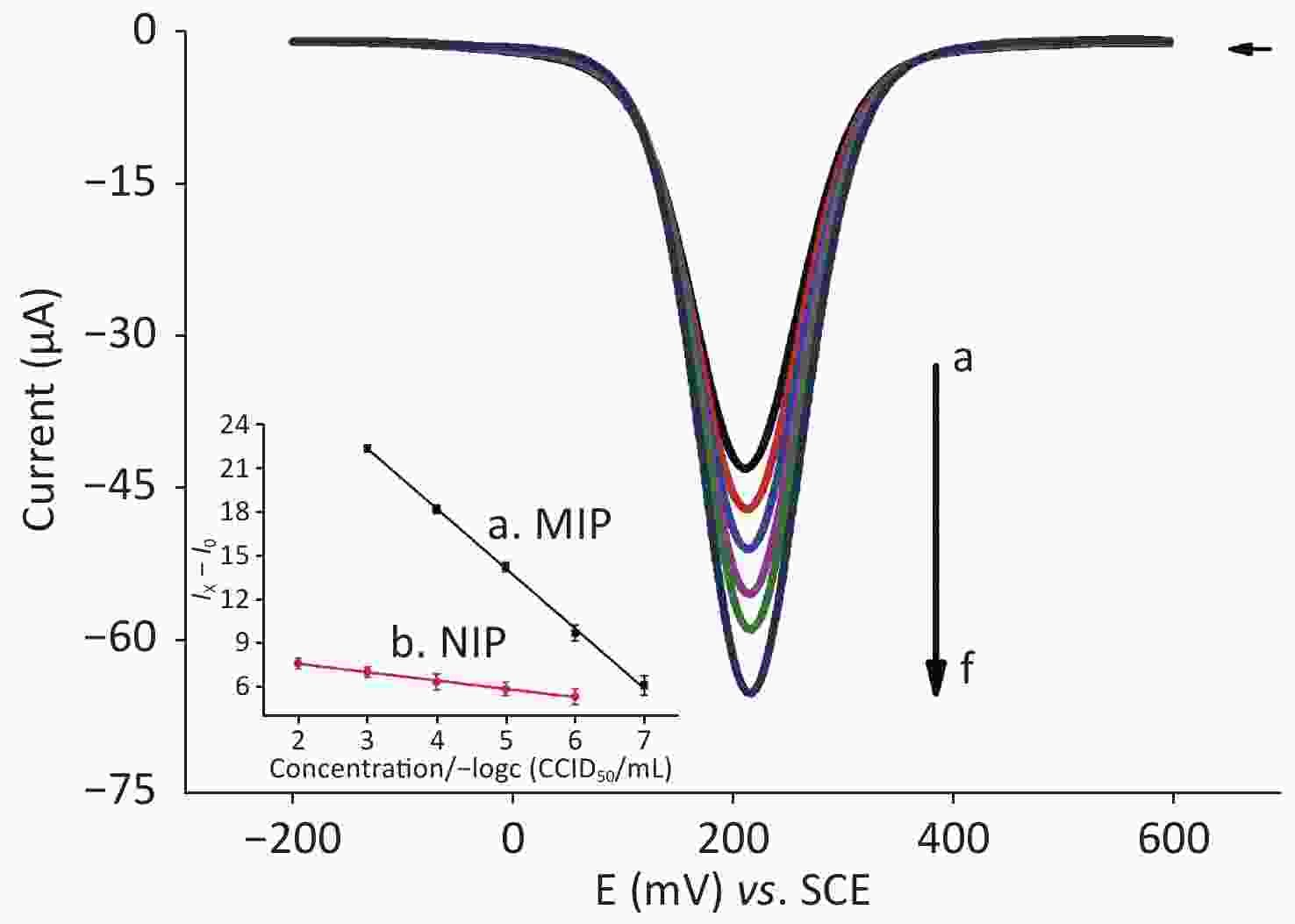
Figure 2. SWV scans of different concentrations of Nph for PATP-AuNPs/AuE in [Fe(CN)6]3−/4−. The potential range is from −0.20 to 0.60 V vs. SCE, and the scan rate is 100 mV/s. From a to f, the concentration of the solution containing Nph is 10−7, 10−6, 10−5, 10−4, and 10−3 g/L and blank control. As the concentration increases, the electrical signal decreases. Calibration curves of the analysis of various concentrations of Nph on the MIP-modified sensor (line a: y = −4.09378x + 34.71377, R2 = 0.99925) and NIP-modified sensor (line b: y = −0.57056x + 8.88532, R2 = 0.99735) (I0: the peak current of the blank sample; Ix: the peak current of each concentration gradient of Nph). (MIP: Molecularly imprinted polymers; NIP: Non-molecularly imprinted polymers).
Figure 2 shows that, for the MIP-modified sensor (line a), the difference between the measured peak current of each concentration gradient of Nph (Ix) and the peak current of the blank sample (I0) has a linear relationship with the negative logarithm of the concentration of Nph (−Logc), i.e. Ix − I0 = −4.09 (−Logc) + 34.71 (R2 = 0.999), yielding a linear concentration range from 10−3 to 10−7 g/L with a detection limit of 1.52 × 10−8 g/L (S/N = 3). Meanwhile, for the NIP-modified sensor (line b), we observed that the linear relationship between Ix − I0 and the negative logarithm of the Nph concentration of the NIP-modified sensor is not as good as that of the MIP-modified sensor and the slope is small (slope = −0.57), indicating that its sensitivity is low. The imprinting factor, as the ratio of both sensitivities, is equal to 8.6, which is high. Moreover, Nph with a concentration lower than 10−5 g/L cannot be detected by the NIP-modified sensor. Furthermore, the detection limit of the NIP-modified sensor is higher than that of the MIP-modified sensor. The advantages of the MIP-modified sensor have been demonstrated by the aforementioned findings.
The MIP sensor method established in this study exhibited a lower detection limit than many other sensors used for the determination of Nph (via either test, all units of the relevant values have been converted into g/L; Supplementary Table S1 available in www.besjournal.com).
Electrode Modified material Actual samples Range of linearity
(g/L)Limit of detection
(g/L)QGCE GNPs River water samples 2.20×10−6–2.20×10−3 6.61×10−6 GCE CTAB River water samples 2.20×10−5–5.51×10−3 2.20×10−6 GCE GR-DNA River water samples 1.10×10−5–8.81×10−4 2.20×10−6 GCE AuNPs/PILs Lake water samples 2.20×10−5–2.64×10−2 7.27×10−6 GCE MIL-101(Cr) @rGO Tap water samples 2.20×10−5–2.75×10−3 7.27×10−6 Gold electrode MIP/TiO2-NH2/
AuNPs/cystea mineTap water samples 2.09×10−4–1.06×10−1 7.05×10−5 GCE MIP/MWNT/NF water and soil samples 4.41×10−5–7.93×10−2 1.32×10−5 Gold electrode MIP/PATP-AuNPs Lake water samples and
tap water samples1×10−7–1×10−3 1.52×10−8 (this work) Table S1. Comparison of different methods for the detection of Nph
In the actual test, various analogs of Nph may affect the detection of its concentration. To further verify the selectivity of the sensor, in this experiment, bisphenol A (BPA) and octylphenol (Oph) were used as interference substances. We conducted separate electrochemical analysis of the three substances (i.e. Nph, BPA, and Oph) with the same concentration (10−3 g/L). The SWV current responses of the MIP/PATP-AuNPs and NIP/PATP-AuNPs electrodes to Nph and its analogs are shown in Figure 3. The results indicated that the response of the MIP/PATP-AuNPs electrode to Nph was higher than that toward its analogs. However, we observed no significant difference in the response of the NIP/PATP-AuNPs electrode to Nph, Oph, and BPA. Therefore, the MIP/PATP-AuNPs electrode-based sensor had excellent selectivity for Nph. This sensor was continuously measured for 5 days, and the deviation of the measured value was observed to be within 5%, indicating good stability.
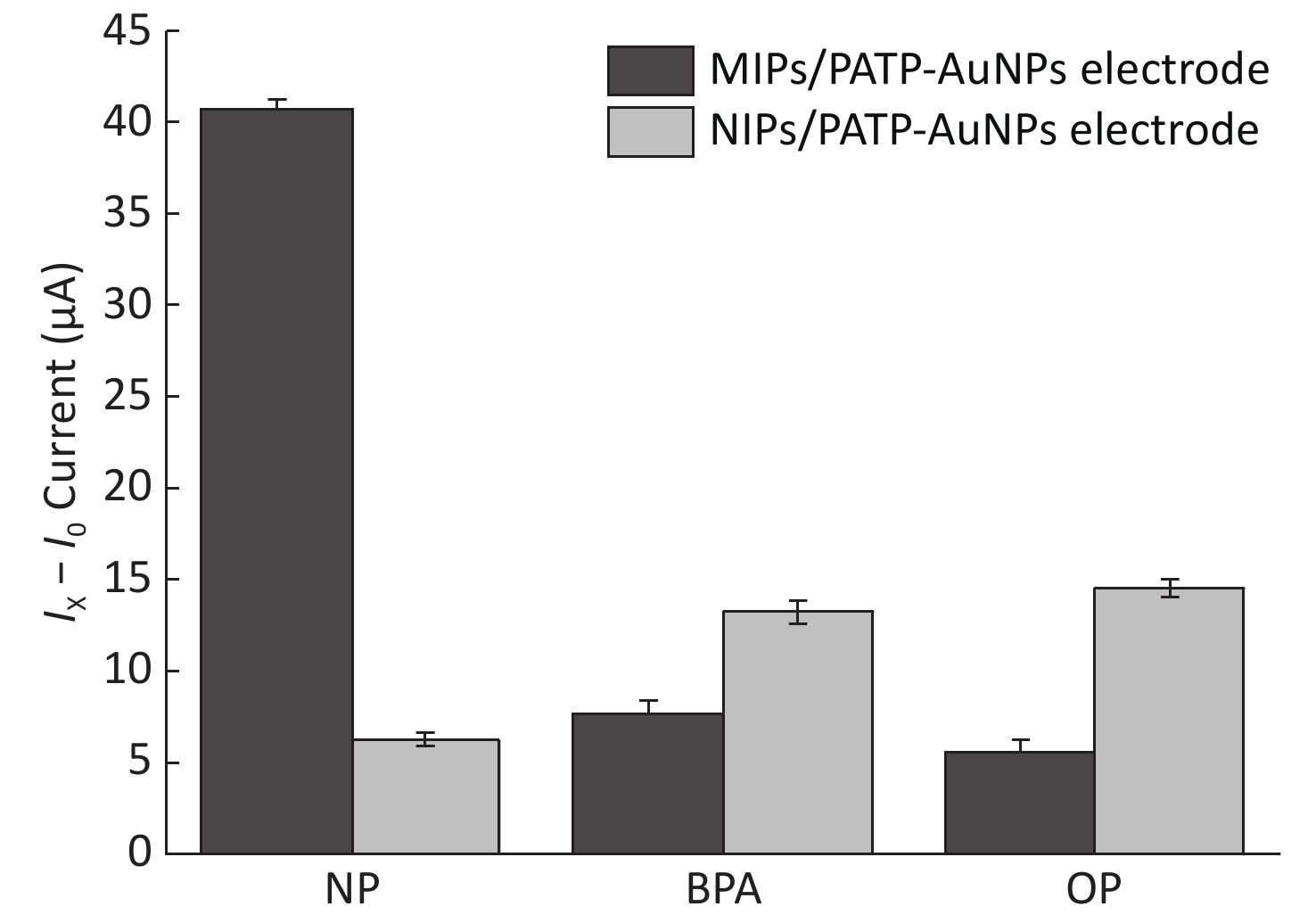
Figure 3. Histogram of the current responses of the MIP/PATP-AuNPs and NIP/PATP-AuNPs electrodes to the Nph solution and BPA and Oph interference substances. Nph: Nonylphenol;BPA: bisphenol A; Oph: octylphenol.
To verify the possibility of using this method to analyze Nph in actual samples, three actual samples were used to assess the analytical performance of the method. The three actual samples were water samples collected from Qinhu Lake (inner part of the lake), Huangjia Lake (outer part of the lake), and tap water. After the water samples were collected, they were filtered and diluted 100 times after standing for 24 h. The concentration of Nph for PATP-AuNPs/AuE in 3 mL of [Fe(CN)6]3−/4− and 3 mL of actual sample solution was determined using the standard addition method. The results were c = 5.69 × 10−5 g/L for Qinhu Lake and c = 4.47 × 10−5 g/L for Huangjia Lake.
To evaluate whether the MIP sensor is still functional under the interference of complex matrix in tap water, Nph is separately formulated into solutions with the concentrations of 10−4, 10−5, and 10−6 g/L using tap water. Then, the MIP sensor was used to detect these solutions by SWV. The sensor showed a good recovery rate for Nph ranging from 98.39% to 102.35%, which effectively proved the detection accuracy of the MIP sensor (Supplementary Table S2 available in www.besjournal.com). Moreover, the sensor can be used repeatedly many times, with good repeatability. The experiments showed that the sensor can be accurately used for up to 10 consecutive measurements. At the same time, we conducted intraday and interday stability tests. The relative standard deviations of the MIP sensor test results were 1.25% and 2.12%, which were both less than 5.00%, indicating that it has good stability.
Sample Nph added (g/L) Nph detected (g/L) Recovery (%) RSD% (n=3) Tap water 1 10–4 1.024×10−4 102.4 0.16 Tap water 2 10–5 0.98×10−5 98.4 0.21 Tap water 3 10–6 0.935×10−6 93.5 0.19 Table S2. Determination of Nph solution of different concentration in tap water samples
In conclusion, a sensitive and selective MIP/PATP-AuNPs sensor for the detection of Nph was fabricated by electropolymerization of PATP-AuNPs in the presence of Nph as the template molecule in the gold electrode. The developed MIP sensor showed a wide linear range between 10−7 g/L and 10−3 g/L with a detection limit of 1.52 × 10−8 g/L, lower than that reported for other Nph sensors. The developed MIP sensor can be easily fabricated and conveniently operated and has high sensitivity and good selectivity. The MIP/PATP-AuNPs membrane is simple and robust and is an ideal choice for the development of the components of sensing devices. Moreover, the feasibility of its practical application has been proven in biological fluids. Furthermore, its potential has been demonstrated by electrochemical analysis.
Contributors KOU Jing and LU Li Li designed this study; GUO Zhen Zhong and ZHANG Xiu supervised all the experiments. KOU Jing, FAN Zhao Yu, HAN Yi Qun, ZHOU Run Zhe, XIA Ying Zhao, and LV Fang Zhi acquired the data. ZHOU Run Zhe, XIA Ying Zhao, and LYU Fang Zhi drafted the manuscript. FAN Zhao Yu and HAN Yi Qun critically revised the manuscript for important intellectual content. KOU Jing and HAN Yi Qun performed the data analysis. Jaffrezic-Renault NICOLE provided technical guidance in the experiments and polishied the manuscript.
Acknowledgment This paper thanks the Wuhan University of Science and Technology undergraduate innovation fund research project 2017–2018 (No. 17ZRA070) support. Z.G. thanks the ‘ChuTian Scholar’ Project Award support, Hubei Province, P.R. China. Nicole Jaffrezic-Renault thanks for International research network (IRN # 0876) France-China ‘New nanostructured materials and biomaterials for renewable electrical energy sources’ ‘MAREES’ (2019–2022) support.
The authors declare no conflict of interest.
A Molecularly Imprinted Polymer Sensor Based on the Electropolymerization of p-Aminothiophenol-Functionalized Au Nanoparticles Electrode for the Detection of Nonylphenol
doi: 10.3967/bes2020.122
- Received Date: 2020-03-01
- Accepted Date: 2020-07-08
| Citation: | KOU Jing, LU Li Li, FAN Zhao Yu, HAN Yi Qun, ZHOU Run Zhe, XIA Ying Zhao, LYU Fang Zhi, ZHANG Xiu, Jaffrezic-Renault NICOLE, GUO Zhen Zhong. A Molecularly Imprinted Polymer Sensor Based on the Electropolymerization of p-Aminothiophenol-Functionalized Au Nanoparticles Electrode for the Detection of Nonylphenol[J]. Biomedical and Environmental Sciences, 2020, 33(11): 887-891. doi: 10.3967/bes2020.122 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: